Abstract
Background
Platelet-rich plasma (PRP) is a human plasma product enriched by platelets, growth factors, and fibrinogen with high hemostatic and healing properties.
Objectives
The aim of this study was to evaluate the effect of autologous PRP on wound healing in high-risk women undergoing cesarean sections.
Patients and Methods
In this balanced, randomized, and controlled trial, 140 patients were admitted to Arash women’s hospital, Tehran, Iran from May of 2013 to November of 2014 for elective cesarean surgery. The patients were randomly assigned into two groups. The intervention group received PRP after surgery, whereas the control group received the usual care. All patients were evaluated at baseline, five days, and eight weeks after the cesarean section. The primary endpoint used the REEDA scale for assessing the changes in wound healing. The secondary outcome measures used were the Vancouver scar scale (VSS) and the visual analog scale (VAS). All scale scores were analyzed using a repeated measures test for variance.
Results
At the end of study, the PRP group showed a greater reduction in the edema ecchymosed discharge approximation (REEDA) score compared to the control group (85.5% reduction in the PRP group; 72% in the control group) (P < 0.001). Compared with the control group, the PRP group had a significantly greater reduction in the VAN score, beginning on the fifth day after the cesarean section (-0.7, 38% reduction in PRP group; -0.8, 33% in control group) (P < 0.001), and this trend was stable at the end of the eighth week (-0.6, 54% reduction in PRP group; -0.3, 18% in control group). Furthermore, patients treated with PRP experienced a 93% reduction in the VAS score at the end of follow-up, but the control group only observed a 79% reduction (P < 0.001).
Conclusions
It seems that applying PRP is an effective therapeutic approach for wound healing, and faster wound healing is expected due to the presence of more platelets and growth factors.
Keywords: Platelet-Rich Plasma, Caesarean Section, Wound Healing
1. Background
Caesarean deliveries are used for 15% of births around the world, and this rate has continued to increase (1). These deliveries have become a major concern in developing countries such as Iran, with a high rate of 47.9% for caesarean sections (2). The postpartum period is a difficult and challenging time for mothers, especially due to the requirements of caring for a newborn baby. Surgical site complications such as infection, hematoma, seroma, dehiscence, and pain may occur in the puerperal period. These complications are associated with substantial morbidity and mortality, prolonged hospital stay, and increased cost. Therefore, reduction in the incidence of these morbidities would cut down on medical expenses and improve maternal and neonatal care (3). Several risk factors affect the wound healing process in caesarean sections, including: (1) twin birth, (2) chronic systemic disease (diabetes, hypertension, and immune deficiencies), (3) surgery duration > 90 minutes, obesity, (4) previous incision, (5) corticosteroid therapy, (6) immunosuppression treatment, and (7) anemia (4, 5).
Surgical wounds heal through an orderly sequence of several distinct physiological and biological events that include hemostasis, inflammation, proliferation, epithelialization, fibroplasia, and maturation (6, 7). This process is initiated immediately upon injury. A platelet plaque develops which is composed of platelets and fibrin, and the platelets release granules containing multiple growth factors and thromboxane A2, the latter of which acts as a potent vasoconstrictor. Transforming growth factor beta (TGF-β) is the key growth factor that plays a central role in wound healing. In a wound site, following the clot formation, platelets and mesenchymal stem cells release their complex contents in the wound healing process. The maximum tensile strength of the tissue is reached approximately eight weeks after injury (6). In vitro studies on the proliferation of mesenchymal stem cells (MSC) confirmed that PRP improves MSC proliferation and differentiation, suggesting a high regenerative potential of PRP (8). Platelet-rich plasma (PRP) has been used for more than a decade in injectable or gel form (9), and many studies have demonstrated that PRP stimulates regeneration of the soft tissues (fat, skin, and mucosa) (10, 11) as well as the hard tissues (tendons and bones) (12, 13). However, there are many clinical studies that have been published with a wide range of results, and the evaluation of the influence of PRP on wound healing and pain remains variable. Although most studies have concluded that PRP is safe with positive effects, others have indicated conflicting results (10-14).
2. Objectives
In this study we attempted to evaluate the efficacy of topical application of autologous PRP in treatment for the wound healing process and the perception of pain in high risk patients who underwent cesarean sections.
3. Patients and Methods
3.1. Trial Design
This was a balanced, randomized, and controlled trial that was performed on 140 candidates for cesarean delivery who were admitted to Arash women’s hospital in Tehran, Iran between May of 2013 and November of 2014. Arash women’s hospital is a general women’s hospital located in Tehranpars, an eastern suburb of Tehran. It is an educational, research, and treatment center for gynecology and obstetrics.
A research protocol including methods, considered outcomes, sample size calculation, and ethical considerations was produced prior to the initiation of the trial. The study protocol was approved by the institutional review board of Tehran University of Medical Sciences. Informed consent was obtained from all participants and the study was registered in Iranian registry of clinical trials (www.IRCT.ir) under the number IRCT2014111618866N3.
3.2. Participants
All cesarean sections were carried out in a training hospital and under the supervision of senior residents, and all of the patients received preoperative intravenous cephalothin (2 gr). The inclusion criteria were body mass index (BMI) > 25, prior cesarean section, diabetes or gestational diabetes, twin pregnancy, use of corticosteroid medication, and anemia. The exclusion criteria were chronic pain disorders, hepatitis, hemoglobin (Hb) < 9 mmol/L, coagulation disorders, and platelet levels < 150 × 106/L.
3.3. Interventions
In the operating room before the start of each procedure, approximately 55 cc of whole blood was drawn from the uninvolved arm of each patient in the intervention group (group A) into a 60 ml sterile syringe containing citrate for anticoagulation. The blood was immediately centrifuged at 3200 PRM. Following 15 minutes of centrifugation, 4 - 5 mL of PRP was obtained. Then, the PRP was buffered by using sodium bicarbonate. All of the procedures were conducted in an operation room with the purpose of safe guarding sterilization. After closure of the fascia and prior to skin closure, PRP was directly applied to the subcutaneous tissue of the wound site by using a sterile syringe. In the control group (group B), the patients received no topical treatment and the subcutaneous tissue was cleaned with normal saline before skin closure. For all participants, no drains were used and the skin was closed with intracutaneous Monocryl 2-0 according to the procedures of routine care. After skin closure, a wound dressing with a compressed bandage was applied. The patients were examined by the physicians who were blind to group allocation of the patients on day 1, and then five days and eight weeks after the procedure. Pain was evaluated by the visual analog scoring system (VAS). The wound healing was evaluated by using the Vancouver scar scale (VSS) and the edema ecchymosed discharge approximation (REEDA) scale.
3.4. Outcomes
The primary endpoint used the REEDA scale for assessing the changes in wound healing. REEDA as a descriptive scale has 4 points in a categorical score that measures 5 items of healing: redness (hyperaemia), edema, ecchymosis, discharge, and approximation of the wound edges (coaptation). Each item is rated on a scale of 0 to 3, and total scores may range from 0 to 15. A lower score indicates better healing (15).
The secondary outcomes were measured by VSS and VAS. VSS was used to detect formation of keloids or hypertrophic scars. It assesses 4 subjective variables: vascularity, height/thickness, pliability, and pigmentation within a possible range of 0 - 14 for the total score (16). The VAS assesses pain via a continuous measurement instrument that is operationally comprised of a horizontal line, anchored at each end by verbal descriptors such as no pain and the worst pain imaginable. The subject is asked to indicate a spot on the scale that best represents her degree of pain. The score is determined by measuring the distance (mm) between the no pain anchor to the point that the patient marks, providing a range of scores from 0 - 100. A higher score indicates greater pain intensity (17).
3.5. Sample Size
Based on an expected mean of 7.3 and standard deviations of 1.5 and 6.5, and 1.4 of the primary endpoint (REEDA) in the control and intervention groups, respectively, we determined that we would need a sample size of 70 patients in each group for 80% reliability in detecting a significant difference between the treatment and control groups (with two-sided type 1 error of 5%).
3.6. Randomizations
Patients were randomly assigned into two groups using a random number sequence generated by statistical software and stratified with a 1: 1 allocation using random block sizes of 6. A randomization list prepared by an epidemiologist was inserted into a set of numbered sealed envelopes. Whenever a patient was found to have qualified and had consented to participate in the trial, the numbered envelope was opened to determine the intervention technique. Participants and outcome assessors did not know which group they had been assigned to for the duration of the study.
3.7. Statistical Methods
Statistical analysis was performed by using computer software SPSS 18 for windows. We compared the difference between the baseline characteristics of patients after randomization into the two groups with a chi-square test for the different categories and with a student’s t-test for continuous variables. Values of P = 0.05 were considered statistically significant. The endpoint mean of the REEDA, VSS, and VAS scores were analyzed using repeated measures analysis of variance. The model included treatment as a fixed factor along with age, platelet count, hemoglobin count, body mass index (BMI), REEDA score, VSS score, and VAS score at baseline as covariates.
4. Results
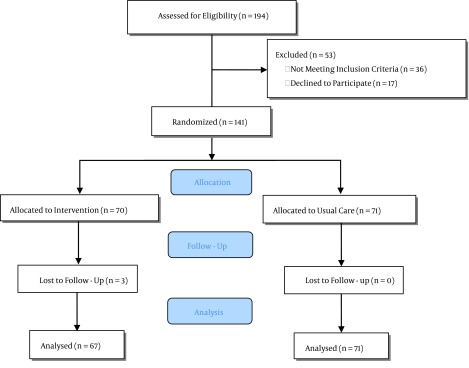
Between May of 2013 and November of 2014, 194 patients were enrolled in the study. Of the 138 eligible patients, 67 patients were assigned to the treatment group and 71 to the control group. The analysis was determined by intention-to-treat and included all patients who were randomly assigned. Figure 1 shows the trial profile. In both groups, almost half of the patients were primigravida, and there was no difference between the two groups in terms of the gravidity. Also there was no significant difference between the two groups regarding their hemodynamic indices or blood groups. The groups were well matched at the baseline and the characteristics of the patients did not differ dramatically. The baseline demographic and clinical characteristics are summarized in Table 1.
Figure 1. Flowchart Showing Participants and Group Disposition.
Table 1. Clinical Characteristics of the Patients by Either Platelet-Rich Plasma (PRP) Treatment or the Control Groupa.
| Characteristics | PRP (n = 67) | Control (n = 71) | P Value |
|---|---|---|---|
| Maternal age in years, mean ± SD | 29.55 ± 5.45 | 28.05 ± 5.25 | 0.057 |
| Gravidity, mean ± SD | 1.57 ± 0.64 | 1.69 ± 0.89 | 0.383 |
| Previous cesarean scar | 17 (25.37) | 16 (22.53) | 0.843 |
| History of immunodeficiency | 1 (1.49) | 0 (0) | 0.49 |
| History of diabetes | 5 (7.46) | 10 (14.08) | 0.2 |
| History of anemia | 9 (13.43) | 13 (18.3) | 0.3 |
| Corticosteroid usage | 8 (11.94) | 11 (15.49) | 0.4 |
aValues re expressed as No. (%) unless otherwise indicated.
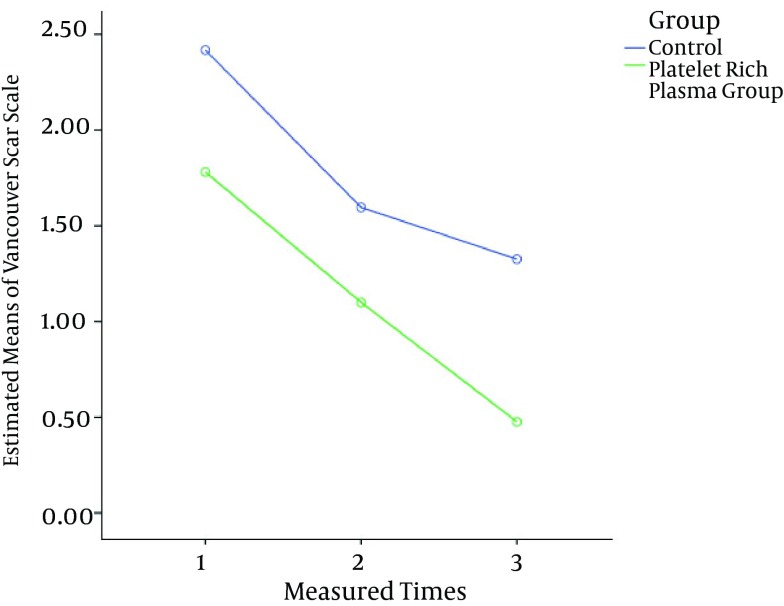
Mauchly’s sphericity test indicated that the assumption of sphericity had been established as χ2 (2) = 0.223, P = 0.89. The means of the primary and secondary outcomes for three measured times for both groups are shown in Table 2. In the analysis of the overtime scores, the PRP group had lower scores compared to control group for all measures. After five days, the reduction in the REEDA score for the PRP group was -1.03, a 43% reduction. This trend was constant overtime and the results after eight weeks showed a decrease of -0.57, a 42.5% reduction. A similar trend was observed in the control group: after five days, the reduction in the REEDA score was -0.64, a 25% reduction, and after eight weeks, there was a -0.87 decrease, or a 47% reduction (Figure 2).
Table 2. Means of REEDA, VAS, and VAS Scores for the Two Groups During the Studya.
| Groups | Time | P Value | ||
|---|---|---|---|---|
| Day 1 | Day 5 | Week 8 | ||
| REEDA score | P < 0.001 | |||
| PRP | 2.37 ± (0.71) | 1.34 ± (0.59) | 0.77 ± (0.51) | |
| Control | 2.49 ± (0.58) | 1.85 ± (0.61) | 0.98 ± (0.52) | |
| VAN score | P < 0.001 | |||
| PRP | 1.8 ± (0.6) | 1.1 ± (0.65) | 0.5 ± (0.56) | |
| Control | 2.39 ± (0.9) | 1.59 ± (0.64) | 1.29 ± (0.7) | |
| VAS score | P < 0.001 | |||
| PRP | 3.98 ± 0.63) | 2.28 ± (0.79) | 1.11 ± (0.84) | |
| Control | 4.83 ± (0.95) | 3.3 ± (1.26) | 1.7 ± (0.74) | |
aValues are expressed as mean ± SD.
Figure 2. Improvement From the Baseline in the Means of the VSS Scores Over Time.
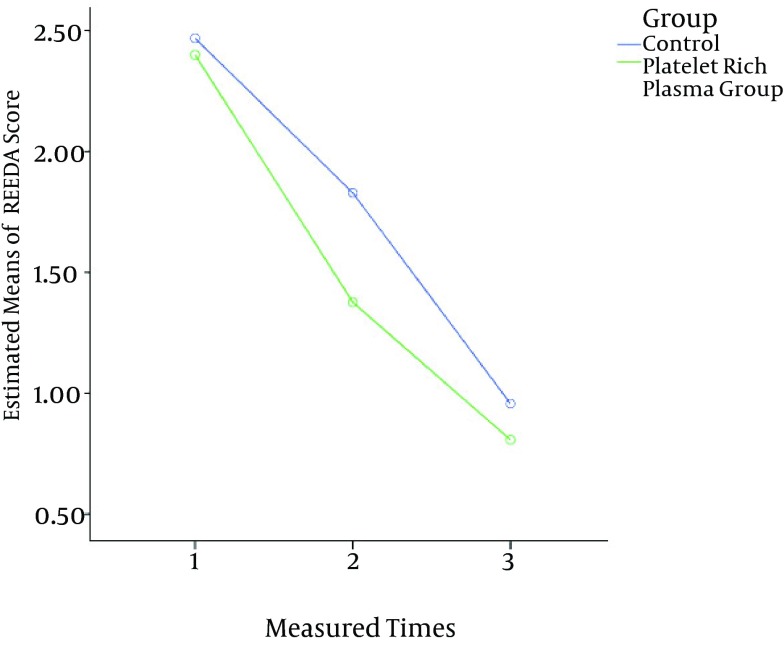
The general analysis with repeated measures showed that treatment with PRP had a significant effect on reducing the REEDA score compared with the treatment of the control group (F (1, 132) = 7.28, P = 0.008). For the secondary end point (VAN score), the results showed that treatment with PRP had a significant effect on reducing the score compared with the treatment of the control group (F (1, 132) = 50.55, P < 0.001). Compared with the control group, the PRP group had a significantly greater reduction in the VAN score beginning on the fifth day (-0.7, 38% reduction in the PRP group; -0.8, 33% in the control group) and this trend was stable at the end of the eighth week (-0.6, 54% reduction in the PRP group; -0.3, 18% in the control group) (Figure 3)
Figure 3. Improvement From the Baseline in the Means of the REEDA Scores Over Time.
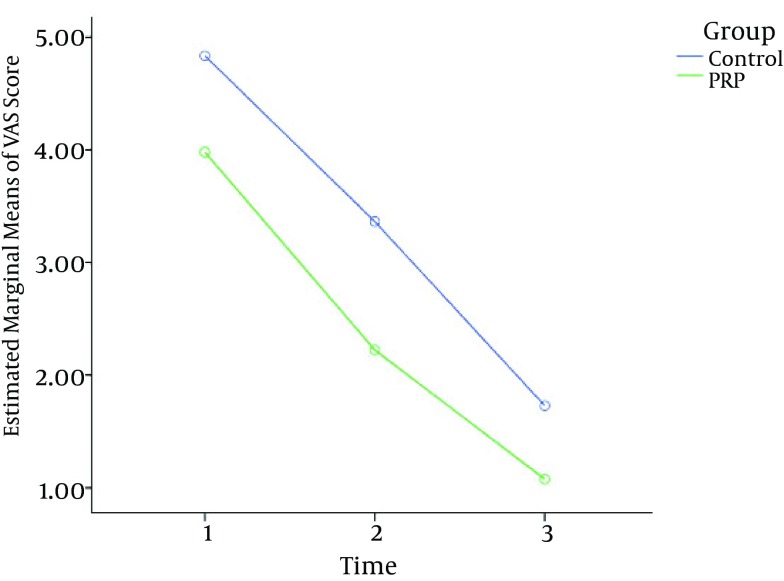
The changes in the mean of the VAS score over time are illustrated in Figure 4. Patients treated with PRP experienced a -1.7 decrease, or a 42% reduction, in the VAS score at 5 days, and after 8 weeks of the study, the observed decline was -1.17, a 51% reduction. A similar trend was observed in the control group: after 5 days, there was a decrease of -1.53, a 31% reduction, and after eight weeks, there was a -1.6 decrease, or a 48% reduction (Figure 4). Repeated measures analysis showed a significant effect on the VAS score in favor of PRP (F (1, 132) = 80.15, P < 0.001).
Figure 4. Improvement From the Baseline in the Means of the VAS Scores Over Time.
5. Discussion
Complications of the wounds in cesarean sections is an unresolved problem and remains one of the common causes of puerperal morbidity. Topical application of PRP as a novel method with the potential to prevent post-operative wound infection, enhance the wound healing process, and reduce pain and other adverse events has been acknowledged by the surgical community. PRP is a volume fraction of blood having a high concentration of platelets above the baseline that markedly improves the adhesive properties and the process of wound healing (18, 19). After application of PRP, the tissue-healing substances are released. The supra-physiological concentration of platelets at the wound site accelerate the healing process and protect the wound against the infection (18-21).
Because of its physiological role in wound healing, PRP is being used more often for a variety of clinical applications, and it is now popular to apply it as a part of routine treatment. However, it still remains uncertain whether the impact of topical application of PRP is a fact or a fiction (22). We found that patients who were treated with topical autologous PRP (group A) had a significant reduction in pain, keloids, and hypertrophic scar formation, and they experienced better wound healing after surgery in comparison with the control group (group B) (P < 0.0001). None of 67 patients in group A developed adverse events. Similar results were found by Fanning and colleagues (3). This study was performed on 55 patients undergoing gynecologic surgeries such as hysterectomies and advanced urogynecological and laparoscopic procedures. A non-randomized clinical outpatient trial with a follow-up was conducted for 28 days, and the authors showed that there was a significant reduction in pain (P < 001). Also, narcotic use was reduced for nearly 50% in the treated patients compared with the control subjects.
Other studies similar to the one presented here have concluded that PRP improves the wound healing process (10, 22, 23). However, in contrast to the results of this study, some authors have shown the failure of PRP in promoting wound healing (13, 14, 24). One such study was a double- blind randomized and controlled trial which used autologues platelet gel after total knee arthroplasty on 102 patients with a 3-month follow up. The authors have concluded that there was no positive effect of the autologues platelet gel on wound healing. They have also indicated that it had no effect on pain or hemoglobin values (24).
Everts et al. (25) applied platelet-leukocyte gel on 40 patients who underwent open subacromial surgery and showed that the VAS for pain had been decreased. They also demonstrated a significant reduction in recovery time and analgesic usage during the 6-week follow-up. The VAS score was 2.0 ± 2.0 and 1.1 ± 0.3 in the control and treatment groups, respectively, which were the same as the present study, whereas the mean VAS in the PRP and the control group at the first day, fifth day, and eighth week after surgery were 0.6 ± 0.07, 0.96 ± 0.11, and 2.2 ± 0.79 vs. 3.3 ± 1.2, and 1.11 ± 0.84 vs. 1.7 ± 0.75, respectively. This study did not evaluate analgesic usage and dose. Furthermore, it is assumed that the serotonin released from the activated platelets may be responsible for pain reduction (16). This phenomenon was explained by Sprott et al. in a study claiming that serotonin is released from platelets after acupuncture therapy and causes pain reduction (26).
The present study had the following limitations: First, it was not double-blind, and this could have generated biases. Second, the pain was not measured by the use of analgesics. Third, it had a short-term follow-up period. Fourth, we did not investigate the effects of varying platelet concentrations. Currently, there are numerous studies with variable evidence. Variants can be related to many factors including health status, donor variability, centrifuge duration and speed, platelet concentration, or different types of wounds and tissues, among other factors. Clearly it is necessary to perform further multicentric, controlled, and double-blind clinical trials with similar and standard protocols to assess the potential influences of PRP until more conclusive evidence becomes available.
5.1. Conclusion
The present study is the first prospective, randomized, and controlled trial evaluating the efficacy of autologous PRP in cesarean section surgery, and it has demonstrated that PRP has positive effects on wound healing and pain reduction in high-risk patients undergoing cesarean section. There are plans to design and conduct a similar but more comprehensive study on women who will undergo gynecologic cancer surgery.
Acknowledgments
We would like to thank the nursing, administrative, and secretarial staff of the gynecology and obstetrics surgery department and clinic at Arash women’s hospital for their contribution to the maintenance of our patient records, without which this project would have been impossible. We would also like to thank the Arash research development center for their contributions in the process of preparing this article.
Footnotes
Authors’ Contribution:Study concept and design, Afsaneh Tehranain; acquisition of data, Bahareh Esfahani-Mehr; analysis and interpretation of data, Mahdi Sepidarkish, Negar Rezaei; drafting of the manuscript, Somaye Sadat Heidary; critical revision of the manuscript for important intellectual content, Afsaneh Tehranain; statistical analysis, Mahdi Sepidarkish; administrative, technical, and material support, Reihaneh Pirjani; study supervision, Afsaneh Tehranain.
Conflict of Interest:The authors have no conflicts of interest.
Funding/Support:Authors confirm that they received financial support from Tehran University of Medical Science.
Financial Disclosure:Dr. Afsaneh Tehranian received a financial grant from Tehran University of Medical Science.|
References
- 1.Betran AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, et al. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21(2):98–113. doi: 10.1111/j.1365-3016.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 2.Bahadori F, Hakimi S, Heidarzade M. The trend of caesarean delivery in the Islamic Republic of Iran. East Mediterr Health J. 2014;19 Suppl 3:S67–70. [PubMed] [Google Scholar]
- 3.Fanning J, Murrain L, Flora R, Hutchings T, Johnson JM, Fenton BW. Phase I/II prospective trial of autologous platelet tissue graft in gynecologic surgery. J Minim Invasive Gynecol. 2007;14(5):633–7. doi: 10.1016/j.jmig.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101(6):1183–9. doi: 10.1016/s0029-7844(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 5.Chaim W, Bashiri A, Bar-David J, Shoham-Vardi I, Mazor M. Prevalence and clinical significance of postpartum endometritis and wound infection. Infect Dis Obstet Gynecol. 2000;8(2):77–82. doi: 10.1155/S1064744900000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones HW, Rock JA, TeLinde RW. Te Linde's operative gynecology. Lippincott Williams and Wilkins; 2015. [Google Scholar]
- 7.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 8.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–5. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107(1):229–37. doi: 10.1097/00006534-200101000-00037. discussion 238-9. [DOI] [PubMed] [Google Scholar]
- 10.Balbo R, Avonto I, Marenchino D, Maddalena L, Menardi G, Peano G. Platelet gel for the treatment of traumatic loss of finger substance. Blood Transfus. 2010;8(4):255–9. doi: 10.2450/2009.0129-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen TM, Tsai JC, Burnouf T. A novel technique combining platelet gel, skin graft, and fibrin glue for healing recalcitrant lower extremity ulcers. Dermatol Surg. 2010;36(4):453–60. doi: 10.1111/j.1524-4725.2010.01480.x. [DOI] [PubMed] [Google Scholar]
- 12.Nacopoulos C, Dontas I, Lelovas P, Galanos A, Vesalas AM, Raptou P, et al. Enhancement of bone regeneration with the combination of platelet-rich fibrin and synthetic graft. J Craniofac Surg. 2014;25(6):2164–8. doi: 10.1097/SCS.0000000000001172. [DOI] [PubMed] [Google Scholar]
- 13.Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–65. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 14.Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676–82. doi: 10.1007/s00167-009-0762-8. [DOI] [PubMed] [Google Scholar]
- 15.Hill PD. Psychometric properties of the REEDA. J Nurse Midwifery. 1990;35(3):162–5. doi: 10.1016/0091-2182(90)90166-3. [DOI] [PubMed] [Google Scholar]
- 16.Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21(3):205–12. doi: 10.1067/mbc.2000.104750. [DOI] [PubMed] [Google Scholar]
- 17.Ismail AK, Abdul Ghafar MA, Shamsuddin NS, Roslan NA, Kaharuddin H, Nik Muhamad NA. The Assessment of Acute Pain in Pre-Hospital Care Using Verbal Numerical Rating and Visual Analogue Scales. J Emerg Med. 2015;49(3):287–93. doi: 10.1016/j.jemermed.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(8):987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;22(6):432–8. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- 20.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–54. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 21.Martinello T, Bronzini I, Perazzi A, Testoni S, De Benedictis GM, Negro A, et al. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res. 2013;31(2):306–14. doi: 10.1002/jor.22205. [DOI] [PubMed] [Google Scholar]
- 22.Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet-rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J. 2013;10(5):612–5. doi: 10.1111/iwj.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Caro A, Ausin P, Boada M. Platelet rich plasma improves the healing process after airway anastomosis. Interact Cardiovasc Thorac Surg. 2011;13(6):552–6. doi: 10.1510/icvts.2011.273995. [DOI] [PubMed] [Google Scholar]
- 24.Peerbooms JC, de Wolf GS, Colaris JW, Bruijn DJ, Verhaar JA. No positive effect of autologous platelet gel after total knee arthroplasty. Acta Orthop. 2009;80(5):557–62. doi: 10.3109/17453670903350081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everts PA, Devilee RJ, Brown Mahoney C, van Erp A, Oosterbos CJ, Stellenboom M, et al. Exogenous application of platelet-leukocyte gel during open subacromial decompression contributes to improved patient outcome. A prospective randomized double-blind study. Eur Surg Res. 2008;40(2):203–10. doi: 10.1159/000110862. [DOI] [PubMed] [Google Scholar]
- 26.Sprott H, Franke S, Kluge H, Hein G. Pain treatment of fibromyalgia by acupuncture. Rheumatol Int. 1998;18(1):35–6. doi: 10.1007/s002960050051. [DOI] [PubMed] [Google Scholar]