Abstract
Background
Salmonella enterica serovar Typhimurium (S. Typhimurium) causes gastroenteritis in humans and paratyphoid disease in some animals. Given the emergence of antibiotic resistance, vaccines are more effective than chemotherapy in disease control.
Objectives
The aim of this experimental study was to evaluate the immunogenicity of diphtheria toxoid (DT) conjugated with S. Typhimurium -derived OPS (O side chain isolation) in mice to determine its potential as a vaccine candidate against salmonellosis.
Materials and Methods
Lipopolysaccharide (LPS) was extracted from the bacterial strain. After isolation of the O side chain of LPS, detoxification, and conjugation of the detoxified OPS samples with DT, pyrogenicity, toxicity, and sterility tests were performed. To vaccination, four groups of female Balb/c mice were used in an immunization test. Antibody responses were measured by the ELISA method. Challenging processes were performed to analyze the efficacy of the OPS-DT compound.
Results
Two weeks after the first vaccination dose, there was no significant difference in the antibody titers of the OPS and OPS-DT groups. However, after the second and third doses, the antibody titers of the OPS-DT group increased significantly compared with those of the control groups (P < 0.001). The induction of anti-OPS antibodies was as follows: OPS-DT>OPS. The most anti-OPS IgG antibody was IgG1. Challenging procedure showed successful protective characteristics in clinical examinations.
Conclusions
The results indicated that DT increased anti-OPS antibodies against the OPS-DT compound. The antibody response to OPS-DT was greater than that to OPS alone. We conclude that OPS-DT is an appropriate and acceptable vaccine candidate against salmonellosis.
Keywords: Vaccine Candidate, OPS, DT, ELISA, Salmonella Typhimurium
1. Background
Salmonella enterica serovar Typhimurium is a gram-negative enteric motile bacterium and a member of the Enterobacteriaceae family. S. Typhimurium can infect humans and animals, leading to the development of gastroenteritis. After entering intestinal epithelial cells, the bacteria damage the microvilli on the surface of the cells. The pathogenicity of S. enterica infection depends on its ability to invade tissues and survive in macrophages (1). In addition to phagosomal killing resistance via the production of reactive oxygen species (2), the emergence of antibiotic resistance has become a serious problem in salmonellosis control and treatment (3). Although new antibiotics and medicinal plants have recently been introduced that are effective against infectious disease (4, 5), vaccination is the best way to combat resistance. Vaccination has economic aspects, in addition to hygiene promotion (6, 7).
Several oral vaccines are available that killed cellular parts of the bacterium or contain attenuated bacterium. Some vaccines have been produced that include outer membrane proteins (purines) of S. Typhimurium. However, these vaccines have not been approved because of their hazardous side effects. Next-generation vaccines have also been developed that utilize polysaccharides as a subunit vaccine, but these polysaccharides are T-cell independent antigens and do not incite memory responses. Consequently, researchers have focused on bivalent subunit conjugate vaccines, which bind the polysaccharide to a proteinaceous macro-carrier as a carrier element in a hapten-carrier model (8-11). Diphtheria toxoid (DT) is a common carrier with a molecular weight of 70 kDa and antigenic properties that are used in subunit vaccines (12).
2. Objectives
In this study, the immunogenicity of OPS (O side chain isolation) and a DT-conjugated compound was investigated in a mouse model to determine its efficacy as a vaccine candidate.
3. Materials and Methods
This was an experimental study performed in the department of microbiology, Zanjan branch, islamic azad university, Zanjan, IR Iran.
S. Typhimurium: Lyophilized S. Typhimurium SH4809 and S. Typhimurium SH2201 were purchased (biological research center of islamic azad university of Zanjan) and cultured on nutrient agar medium (Conda S.A., Madrid, Spain). S. Typhimurium SH4809 was used for lipopolysaccharide (LPS) preparation, and S. Typhimurium SH2201 was used in the oral challenge.
LPS extraction: Bacterial LPS was extracted according to published protocols (13, 14).
LPS concentration: The tube was centrifuged at 4° C at 2500 rpm for 20 minutes to deplete protein, RNA, and DNA, and then the volume of the supernatant was measured. A mixture of 96% ethanol (Merck Co., Germany) and NaCl (3:1) was added to the supernatant and then placed in a refrigerator for 24 hours until halo formation. The next day, the mixture was centrifuged at 4° C, 2500 rpm for 60 minutes. The supernatant was then discarded, and the residue containing the LPS was stored (15).
Isolation of OPS: The tube containing the LPS precipitate was refrigerated, and then 4 mL of distilled water were added, and the tube was shaken. To dissolve the precipitate, 12 mL of distilled water were added, drop by drop, followed by 200 µL of 2% acetic acid (Merck Co., Germany) in distilled water. It was sealed, placed in a beaker and then autoclaved at 120° C for 15 minutes. Next, the tube was centrifuged at 2500 rpm for 30 minutes at 4° C, followed by the addition of 2 grams of trichloroacetic acid (Merck Co., Germany). The tube was then shaken and centrifuged at 2500 rpm for 30 minutes at 4° C again. Finally, the OPS-containing supernatant was isolated, and 96% ethanol in a volume of 3:1 was added and refrigerated for 2 hours to allow precipitation (15).
Gel filtration for purification of the O side chains: The OPS-containing supernatants were purified, as described previously (16). After gel filtration was completed, the light absorbance of all the fractions was read at 210 nm (Figure 1). Afterward, the absorption spectrum of each tube was measured at 100 and 300 nm, and the contents of tubes with higher absorption were merged with each other. The contents were then lyophilized and sterilized by filtration through a 0.22 μm filter.
Figure 1. Light Absorption of Fractions at 210 nm.
pH alignment: The pH of the solution was adjusted to 8.5 by adding HCl or NaOH (Merck Co., Germany) and incubated in a refrigerator for 24 hours.
Endotoxin level of the D-LPS samples: The endotoxicity of the antigens was measured using the LAL method, as described previously. The permissible amount of endotoxin for humans is lower than 5 EU/mL (15).
Protein assay: The Bradford protein assay method was used to assay the DT. The admissible amount of protein in LPS is 10 mg, according to the WHO protocol (17, 18).
Total carbohydrate assay: This assay was performed using phenol, as described previously (19).
Nucleic acid assay: UV spectrophotometry at 260 nm was performed to check for contamination by DNA (17).
Detoxification of LPS: This was done by the alkaline method, as reported previously (20).
Conjugation of detoxified OPS samples with DT: Conjugation was performed via the amidation reaction using cyanogen bromide, sodium bicarbonate, adipic acid dihydrazide, and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide (EDAC) (Sigma Co., UK) (15, 17).
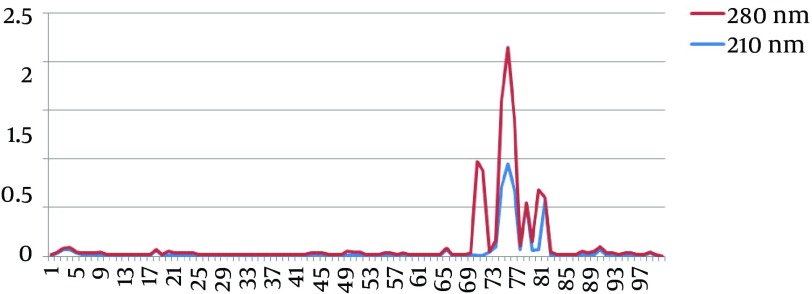
To purify OPS-DT conjugation crossed through a sepharose Cl-2B chromatography column using the gel filtration method (16). The light absorption of the fractions was then measured at 210 and 280 nm (Figure 2). The molecules were conjugated when the peaks of light absorption of the curves overlapped at 210 and 280 nm.
Figure 2. Measurement of the light absorption of the OPS-DT fractions on a sepharose Cl-2B chromatography column at 210 and 280 nm. The peaks of highest absorption overlapped at both 210 and 280 nm. The 70-72 fractions at 210 nm are conjugated molecules.
Rabbit pyrogenicity test: To analyze the pyrogenicity of the prepared conjugations, rabbits were chosen in three groups, and their body temperatures were checked. After first temperature recording by putting the thermometer into the rabbit’s anal, next measurements were done each 15 minutes over a 1-hour period. The last recorded temperature before the administration was considered as first temperature. The inoculation was done by injecting through ear marginal vein, and the body temperature was recorded each 15 minutes over a 3-hour period. The average body temperature of each rabbit was calculated. Body temperature changes lower than 0.5° C for each rabbit was considered as apyrogenic inoculum (21).
Toxicity test: To determine whether the inoculum was toxic, 10 µg (equals one human dosage) were injected intraperitoneally into three mice. The animals were then monitored for 7 days to determine weight reductions and mortality (21).
Sterility test: In this assay, prepared conjugations were cultured on Tioglicolate, nutrient agar, blood agar, Mac Conkey agar, and Saboraud dextrose agar (Conda S.A., Madrid, Spain). The media was maintained at both aerobic and anaerobic conditions, and the results were analyzed after an adequate time (22).
Vaccination: Sixty female Balb/c mice were purchased from the Research Institution of Pasteur (Karaj, IR Iran). Before the experiment, the mice were housed in standard stainless cages at 23-25° C, 60-70% humidity, and a 12-hours light/dark cycle for a week. The mice were given free access to a standard diet and water. The animals were divided into four groups, with 15 mice in each group. The first group was vaccinated with OPS, and the second group was vaccinated with OPS-DT. The dosage of each vaccine was 10 µg. The third and fourth groups were vaccinated with DT and normal saline, respectively, as control groups. The vaccine was administered intraperitoneally three times (0.5 cc each time) for 2 weeks. Two weeks after each injection, peripheral blood was collected from five mice in each group. Each time, the blood was centrifuged at 3000 rpm for 5 minutes to obtain serum and stored at -20° C.
Enzyme-linked immunosorbent assay (ELISA): An indirect ELISA was performed to determine the levels of IgA, IgM, IgG3, IgG2b, IgG2a, IgG1, and total IgG antibodies in the titers, as published previously (15). This ELISA assay was run in triplicate, using an Anthos ELISA reader.
Challenge infection: To attain a satisfying profile of the OPS-DT compound, the challenge strain of S. Typhimurium SH2201 was cultured on nutrient agar (Conda S.A., Madrid, Spain) for 24 hours first. Afterward, the colonies were suspended in PBS, and the dosage was determined according to the agar plate count technique. Each group (OPS, OPS-DT, and control groups) was orally administered a 50% lethal dose (LD50) of S. Typhimurium 21 days after the vaccination doses. To analyze the results of the oral challenge test, all the mice were examined three times daily after 3 days of the challenge for 3 weeks. To determine the clinical status of the animals, the following clinical responses to infection were recorded by direct observation: weight, feeding, activity, vitality, appearance, ataxia, and tremor. The clinical status was scored, with scores for each factor ranging from 0.0 to 5.0. The average score of the three daily measurements was determined as the final daily score.
All the challenge procedures were done according to the principles of laboratory animal care (23), and they were approved by the ethical committee of islamic azad university, Zanjan (EC 92-308).
3.1. Statistical Analysis
After running the ELISA in triplicate, the average titration of each antibody was calculated. Differences between the sera titrations of the OPS and OPS-DT groups with those of the control groups were analyzed by SPSS 18.0 software using a t-test, and P < 0.05 was considered a significant difference.
4. Results
According to the Bradford protein assay method, the protein content was 0.2 mg per 1 kg of dried LPS. The nucleic acid content was lower than 0.9 µg per 1 kg of LPS.
As shown in Figure 2, the light absorption of the OPS-DT conjugations indicated that the OPS polysaccharide antigens were conjugated to DT.
The rabbit pyrogen test and animal survival indicated that the prepared conjugations did not contain any pyrogen substance and were not toxic.
The cultures results of the sterility test were negative.
4.1. Antibody Titers in Each Group
4.1.1. IgA
The IgA titration analysis after the first injection indicated little variation in the IgA levels of the OPS and OPS-DT groups. However, the IgA titer of the OPS-DT group was higher than that of the OPS group 2 weeks after the second and third injections. The IgA titer in the OPS-DT group increased from 20 IU/mL to 29.2 IU/mL after the third injection in comparison to the first. The IgA titer increased significantly 2 weeks after the administration of the vaccine, as compared to that of the control groups (P < 0.001) (Table 1).
Table 1. Anti-OPS IgA Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 17* | 23* | 24/6* |
| OPS-DT-vaccinated | 20** | 25** | 29/2** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. Control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001).
4.1.2. IgM
The IgM titer of the OPS-DT group increased from 97 IU/mL to 826 IU/mL after the third injection in comparison to after the first injection. The increase in the IgM titer of the OPS and OPS-DT groups after 2 weeks was significant in comparison to that of the control groups (P < 0.001) (Table 2).
Table 2. Anti-OPS IgM Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 83* | 150* | 172* |
| OPS-DT-vaccinated | 97** | 270** | 826** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001).
4.1.3. Total IgG and Its Subclasses
The total IgG in the titers of the groups vaccinated with the conjugate vaccine increased from 371 IU/mL after the first injection to 1870 IU/mL after the third injection (Table 3). The IgG subclasses in the titers of the groups vaccinated with the conjugate vaccine are listed in Tables 4-7. 4-7. The titers of total IgG and those of all the IgG subclasses increased significantly after 2 weeks in the OPS and OPS-DT groups in comparison to those of the control groups (P < 0.001).
Table 3. Total Anti-OPS IgG Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 80/6* | 319* | 337* |
| OPS-DT-vaccinated | 371** | 1292** | 1870** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001)
Table 4. Anti-OPS IgG1 Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 69* | 109* | 150/2* |
| OPS-DT-vaccinated | 100/2** | 400/2** | 1100** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001)
Table 7. Anti-OPS IgG3 Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 57/2 * | 85 * | 111/8 * |
| OPS-DT-vaccinated | 99/8 ** | 370 ** | 1000/2 ** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001).
Table 5. Anti-OPS IgG2a Titers in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 18 * | 39 * | 56 * |
| OPS-DT-vaccinated | 33 ** | 63 ** | 100/2 ** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001).
Table 6. Anti-OPS IgG2b Titration in the Sera of the Four Groups of Mice 2 Weeks After the First, Second, and Third Vaccinationsa.
| Group | Titer of Sera (OD Unit) (Mean ± SD) | ||
|---|---|---|---|
| 2 Weeks After the First Vaccination | 2 Weeks After the Second Vaccination | 2 Weeks After the Third Vaccination | |
| OPS-vaccinated | 16/8 * | 18/6 * | 23 * |
| OPS-DT-vaccinated | 18/6 ** | 68 ** | 100/8 ** |
| DT-vaccinated (control I) | 0 | 0 | 0 |
| Neg. control (control II) | 0 | 0 | 0 |
a* and ** denotes a significant difference in comparison to the control groups (P < 0.001).
4.2. Oral Challenge of Mice After Vaccination
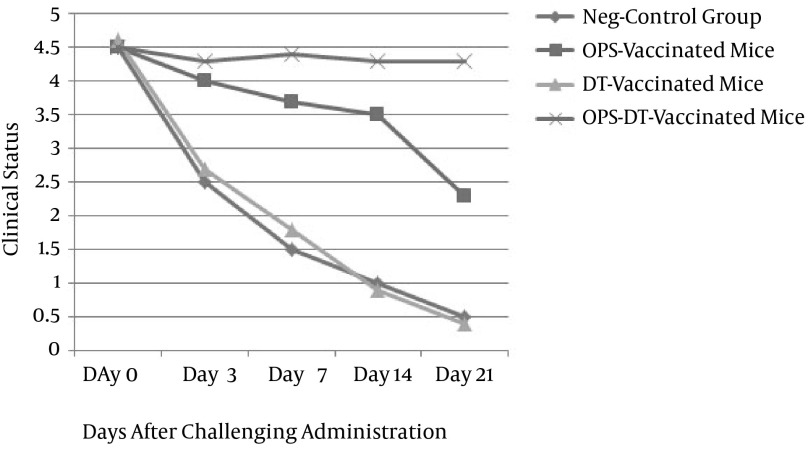
According to the antibody titration results, the oral challenge did not affect the clinical status of the OPS-DT group. This group showed strong protection 21 days after exposure to the challenge strain of S. Typhimurium. In contrast, the OPS-only group showed little protection against the oral challenge, and the clinical status of the mice declined 2 weeks after the administration of the challenge. The two control groups showed no protection against the challenge strain, and their clinical status decreased all during 3 weeks evaluation (Figure 3).
Figure 3. Clinical Status of the Four Groups of Mice After the Oral Challenge.
5. Discussion
Nontyphoidal S. enterica infections are an important problem for global public health. Diseases caused by S. Typhimurium range from gastroenteritis to systemic infections. At present, no vaccine is available that can induce protective immunity (24). In the present study, the immunogenicity of the S. Typhimurium-derived OPS conjugated with DT (as the carrier protein) was evaluated to determine its efficacy as a vaccine candidate.
In various studies, live attenuated S. Typhimurium. vaccines were utilized against nontyphoidal infections (25, 26). However, these vaccines may be unusable in immunosuppressed patients. Therefore, in the present study, OPS, a component of the cell wall component, was used.
Many previous studies have tested the potential of bacterial LPS as a vaccine candidate antigen. These studies reported inadequate responses to nontyphoidal S. enterica infections because S. enterica is a facultative intracellular pathogen, and both B- and T-cell responses are required for successful clearance (24). Polysaccharides, such as LPS, are thymus-independent antigens and cannot trigger T cells; consequently, they cannot elicit an efficient memory response. Furthermore, some bacterial polysaccharides are similar to the host’s glycolipids and glycoproteins and therefore have poor immunogenicity (27, 28). In addition, as LPS is a toxic component, it is not a suitable candidate for use in a vaccine. To eliminate this problem, the toxic portion (lipid A) of LPS is removed, and the remaining polysaccharide is used in the vaccine. However, the remaining polysaccharide side chain has low immunogenicity and is thus unable to generate a vigorous immune response, as mentioned above. To increase the immunogenicity of polysaccharides, they are bound to an immunogenic protein, resulting in the conversion of a thymus-independent antigen to a thymus-dependent antigen (28).
In the present study, the vaccine candidate was conjugated by DT as a carrier component. In previous studies, various peptides were utilized as carrier proteins (29-31). The results of the present study indicated that the antibody response to OPS-DT was comparable to that reported in those studies. In other research, an acceptable LPS-DT conjugate vaccine against Pseudomonas aeruginosa was introduced (15). Thus, DT seems to be a suitable carrier. In addition, in the present study, the antibody responses to the OPS-DT compound were greater than those to OPS. Therefore, the results showed that DT has favorable adjuvant activity.
Long-term and effective immunity are crucial requirements of any vaccine (32). In the present study, increases in the titers of all the antibodies were noticeable after OPS-DT vaccination. The predominant antibody was IgG1, which was similar to that reported in an earlier study (15). It seems that IgG1 has the most protective activity. According to a previous study, mucosal and serum antibodies (IgA and IgG), as well as cell-mediated immunity, provided protection against Salmonella infections. Enhanced levels of secretory IgA antibodies and serum IgG and IgM antibodies were also reported to be essential to the efficacy of a vaccine candidate (33). In the present study, according to the antibody titers and the results of the oral challenge, OPS-DT seemed to provide adequate protection.
In this basic research study, the OPS-DT compound induced a favorable response to S. Typhimurium infection in mice. These results may be helpful in the design of future vaccine and immunization studies against other bacterial infections.
In conclusion, the antibody response of the OPS-DT group was higher than that of the OPS group. The levels of all types of antibodies increased after vaccination, and the predominant antibody was IgG1. Therefore, OPS-DT elicited a T-dependent antibody response, which enables isotype switching. The OPS-DT group had adequate protection against the oral challenge.
Footnotes
Authors’ Contribution:Study concept and design: Vahid Amini; acquisition of data: Hossein Kazemian, Jalil Kardan Yamchi, and Seifu Gizaw Feeyisa; analysis and interpretation of the data: Saeed Aslani, Aref Shavalipour, and Hamidreza Houri; drafting of the manuscript: Hamid Heidari; critical revision of the manuscript: Seifu Gizaw Feeyisa and Mehrdad Halaji; statistical analysis: Mohammadneshvan Hoorijani.
References
- 1.Thiennimitr P, Winter SE, Baumler AJ. Salmonella, the host and its microbiota. Curr Opin Microbiol. 2012;15(1):108–14. doi: 10.1016/j.mib.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 3.Wong MH, Yan M, Chan EW, Biao K, Chen S. Emergence of clinical Salmonella enterica serovar Typhimurium isolates with concurrent resistance to ciprofloxacin, ceftriaxone, and azithromycin. Antimicrob Agents Chemother. 2014;58(7):3752–6. doi: 10.1128/AAC.02770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazemian H, Ghafourian S, Heidari H, Amiri P, Yamchi JK, Shavalipour A, et al. Antibacterial, anti-swarming and anti-biofilm formation activities of Chamaemelum nobile against Pseudomonas aeruginosa. Rev Soc Bras Med Trop. 2015;48(4):432–6. doi: 10.1590/0037-8682-0065-2015. [DOI] [PubMed] [Google Scholar]
- 5.Kazemian H, Haeili M, Kardan Yamchi J, Rezaei F, Gizaw Feyisa S, Zahednamazi F, et al. Antimycobacterial activity of linezolid against multidrug-resistant and extensively drug-resistant strains of Mycobacterium tuberculosis in Iran. Int J Antimicrob Agents. 2015;45(6):668–70. doi: 10.1016/j.ijantimicag.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Rahimi S, Shiraz ZM, Salehi TZ, Torshizi MAK, Grimes JL. Prevention of salmonella infection in poultry by specific egg-derived antibody. Int J Poult Sci. 2007;6(4):230–5. doi: 10.3923/ijps.2007.230.235. [DOI] [Google Scholar]
- 7.Zhao S, Qaiyumi S, Friedman S, Singh R, Foley SL, White DG, et al. Characterization of Salmonella enterica serotype newport isolated from humans and food animals. J Clin Microbiol. 2003;41(12):5366–71. doi: 10.1128/JCM.41.12.5366-5371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler BA. A commercially available siderophore-receptor and porin-based vaccine reduced the prevalence of escherichia coli o157: H7 in the feces of beef cattle under field conditions in 10 commercial feedlots [Dissertation]. Kansas, USA: Kansas State University; 2012. [Google Scholar]
- 9.Ledesma MA, Ochoa SA, Cruz A, Rocha-Ramirez LM, Mas-Oliva J, Eslava CA, et al. The hemorrhagic coli pilus (HCP) of Escherichia coli O157:H7 is an inducer of proinflammatory cytokine secretion in intestinal epithelial cells. PLoS One. 2010;5(8):12127. doi: 10.1371/journal.pone.0012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res. 2012;135:161–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 13.Cui C, Carbis R, An SJ, Jang H, Czerkinsky C, Szu SC, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010;17(1):73–9. doi: 10.1128/CVI.00266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo L, Abitiu N, Coderch N, Hita B, Merino S, Gavin R, et al. The inner-core lipopolysaccharide biosynthetic waaE gene: function and genetic distribution among some Enterobacteriaceae. Microbiology. 2002;148(Pt 11):3485–96. doi: 10.1099/00221287-148-11-3485. [DOI] [PubMed] [Google Scholar]
- 15.Najafzadeh F, Shapouri R, Rahnema M, Rokhsartalab Azar S, Kianmehr A. Pseudomonas aeruginosa PAO-1 Lipopolysaccharide-Diphtheria Toxoid Conjugate Vaccine: Preparation, Characterization and Immunogenicity. Jundishapur J Microbiol. 2015;8(6):17712. doi: 10.5812/jjm.8(5)2015.17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother. 2012;8(4):494–8. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theilacker C, Coleman FT, Mueschenborn S, Llosa N, Grout M, Pier GB. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect Immun. 2003;71(7):3875–84. doi: 10.1128/IAI.71.7.3875-3884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun. 2008;76(4):1702–8. doi: 10.1128/IAI.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol. 2007;98(3):560–4. doi: 10.1016/j.biortech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Rauber RH, Perlin VJ, Fin CD, Mallmann AL, Miranda DP, Giacomini LZ, et al. Interference of salmonella typhimurium lipopolysaccharide on performance and biological parameters of broiler chickens. Rev Bras Cienc Avic. 2014;16(1):77–81. doi: 10.1590/s1516-635x2014000100011. [DOI] [Google Scholar]
- 21.Park CY, Jung SH, Bak JP, Lee SS, Rhee DK. Comparison of the rabbit pyrogen test and Limulus amoebocyte lysate (LAL) assay for endotoxin in hepatitis B vaccines and the effect of aluminum hydroxide. Biologicals. 2005;33(3):145–51. doi: 10.1016/j.biologicals.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Moldenhauer J, Sutton SV. Towards an improved sterility test. PDA J Pharm Sci Technol. 2004;58(6):284–6. [PubMed] [Google Scholar]
- 23.Gorska P. Principles in laboratory animal research for experimental purposes. Med Sci Monit. 2000;6(1):171–80. [PubMed] [Google Scholar]
- 24.Ferreira RB, Valdez Y, Coombes BK, Sad S, Gouw JW, Brown EM, et al. A Highly Effective Component Vaccine against Nontyphoidal Salmonella enterica Infections. MBio. 2015;6(5):01421–15. doi: 10.1128/mBio.01421-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Ridder L, Maes D, Dewulf J, Butaye P, Pasmans F, Boyen F, et al. Use of a live attenuated Salmonella enterica serovar Typhimurium vaccine on farrow-to-finish pig farms. Vet J. 2014;202(2):303–8. doi: 10.1016/j.tvjl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Periaswamy B, Maier L, Vishwakarma V, Slack E, Kremer M, Andrews-Polymenis HL, et al. Live attenuated S. Typhimurium vaccine with improved safety in immuno-compromised mice. PLoS One. 2012;7(9):45433. doi: 10.1371/journal.pone.0045433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson A, Cranage MP, Hudson MJ. Vaccine protocols. 2 ed. Vol. 87. Totowa, NJ: Humana Press; 2003. [DOI] [Google Scholar]
- 28.Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr Res. 2003;338(23):2539–47. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Faezi S, Safarloo M, Amirmozafari N, Nikokar I, Siadat SD, Holder IA, et al. Protective efficacy of Pseudomonas aeruginosa type-A flagellin in the murine burn wound model of infection. APMIS. 2014;122(2):115–27. doi: 10.1111/apm.12101. [DOI] [PubMed] [Google Scholar]
- 30.Izgi K, Iskender B, Sakalar C, Arslanhan A, Saraymen B, Canatan H. Evaluation of two different adjuvants with immunogenic uroplakin 3A-derived peptide for their ability to evoke an immune response in mice. Eur Cytokine Netw. 2015;26(2):46–56. doi: 10.1684/ecn.2015.0365. [DOI] [PubMed] [Google Scholar]
- 31.Lanzilao L, Stefanetti G, Saul A, MacLennan CA, Micoli F, Rondini S. Strain Selection for Generation of O-Antigen-Based Glycoconjugate Vaccines against Invasive Nontyphoidal Salmonella Disease. PLoS One. 2015;10(10):0139847. doi: 10.1371/journal.pone.0139847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song L, Xiong D, Kang X, Yang Y, Wang J, Guo Y, et al. An avian influenza A (H7N9) virus vaccine candidate based on the fusion protein of hemagglutinin globular head and Salmonella typhimurium flagellin. BMC Biotechnol. 2015;15:79. doi: 10.1186/s12896-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allam US, Krishna MG, Lahiri A, Joy O, Chakravortty D. Salmonella enterica serovar Typhimurium lacking hfq gene confers protective immunity against murine typhoid. PLoS One. 2011;6(2):16667. doi: 10.1371/journal.pone.0016667. [DOI] [PMC free article] [PubMed] [Google Scholar]