The intestinal microbiota and its interactions with host immunity have been intensely studied in many disease states. This knowledge could ultimately modify clinical management of allogeneic haematopoietic stem cell transplantation, which is accompanied by dramatic immunological and microbiota perturbations.
In recent years, the importance of the microbiota in many aspects of health and disease has become increasingly clear, and it is no surprise that the clinicaltrials.gov registry includes over two-thousand human studies related to microbiota. In our opinion, understanding the impact that gut microbiota have on patients, and the alterations that physicians could make accordingly to therapeutic approaches, will improve the clinical outcomes of complex human diseases. Using allogeneic haematopoietic stem cell transplantation (allo-HSCT) as an example, we will illustrate how the study of intestinal microbiota can inform clinical care and research.
Patients undergoing allo-HSCT are subject to the most dramatic immunological and microbiota perturbations that have been described. Most commonly, the haematopoietic system of a patient with a haematological disease is ablated and replaced with a donor haematopoietic stem cell graft. Many patients receive prophylactic antibiotics that affect the gut flora, and fevers often require treatment with additional broad-spectrum antibiotics. A further insult to the microbiota originates from compromised oral nutritional intake as a side effect of the chemotherapy and radiation that accompany the transplant.
The carefully timed and closely monitored sequence of an allo-HSCT provides a unique opportunity to observe changes in the intestinal flora and their relationship with environmental exposures and patient outcomes, reminiscent of a well-controlled experimental setting. Diet, antibiotic use, infections, immune reactions, malignant relapse and many other variables are readily evaluable. Studies in both mouse and human have indicated that many of the critical clinical outcomes after allo-HSCT, including overall survival, infections, and graft versus host disease (GVHD), are closely linked to changes in the intestinal flora1,2,3,4. GVHD — the most important manifestation of which is gastrointestinal inflammation — is of particular interest, as it is a major life-threatening complication of allo-HSCT.
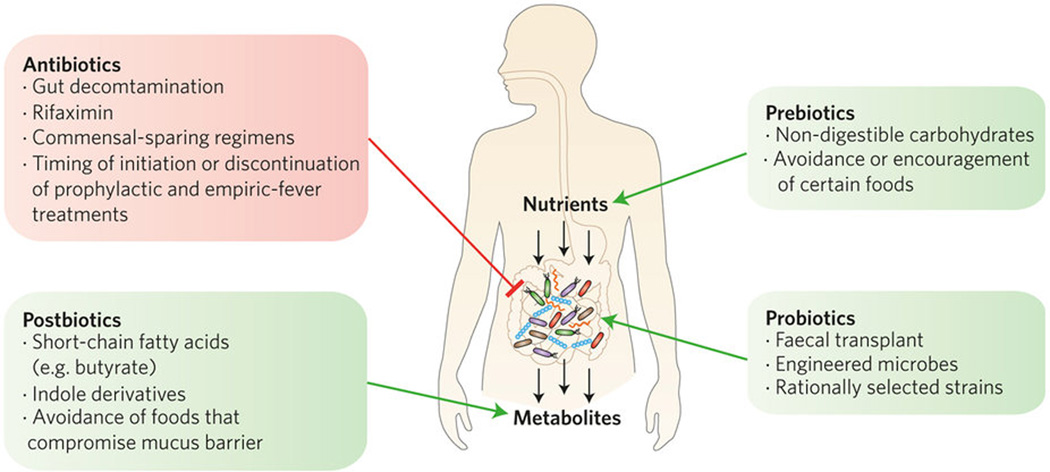
We envisage five broad types of approach (antibiotic, probiotic, prebiotic, postbiotic and diagnostic) to manipulate and assess microbiota–host interactions (Fig. 1). These approaches can be applied to any disease state, and we provide examples from the field of allo-HSCT below.
Figure 1.
The intestinal microbiota obtain nutrients from food consumed by the host, as well as from bile acids excreted into the intestinal tract. Metabolites produced by the microbiota can be absorbed by the gut and modulate mucosal and systemic immunity. Entry points for clinical intervention are depicted.
A probiotic strategy would directly introduce organisms that confer a benefit. In the weeks following allo-HSCT, a decrease in commensal intestinal bacterial diversity occurs in most patients. This is often accompanied by dominance of certain bacteria and is associated with significantly decreased overall survival3. Domination events, in which pathogens such as Enterococcus spp., Streptococci, and Proteobacteria account for 30% or more of faecal 16S ribosomal sequences, are important clinically. As many as one-half of bacteraemia episodes after allo-HSCT are preceded by intestinal domination by a corresponding organism2,4. A clinical trial to restore diversity and prevent post-transplant infections with Clostridium difficile is currently underway5. In this study, allo-HSCT patients with sufficient intestinal flora diversity before allo-HSCT and a post-transplant loss of the protective phylum Bacteroidetes receive their own pre-transplant flora. Probiotic approaches could be designed around Lactobacilli, which protect mice from experimental GVHD (refs 6,7). In patients receiving an allo-HSCT, a decrease in the abundance of the genus Blautia occurs frequently after allo-HSCT and is associated with increased mortality from GVHD (ref.1). Finally, one might introduce probiotic bacteria engineered to produce certain metabolites or antimicrobial peptides that mediate a benefit to the host.
Antibiotic strategies can be designed to minimize microbiota injury while still treating infections. Ever since the transplantation pioneer van Bekkum observed that experimental GVHD was largely ameliorated in germ-free mice8, human allo-HSCT has been performed with various degrees of intestinal flora manipulation, ranging from complete gut decontamination and laminar-flow isolation9 to the modern widespread use of prophylactic antibiotics and HEPA-filtered, positive-pressure hospital rooms. These manoeuvres have primarily been instituted to prevent infections, but they also affect the development of GVHD. One infection–prophylaxis regimen includes a fluoroquinolone and metronidazole, a combination that was found in a randomized study to reduce GVHD better than a fluoroquinolone alone10. This has influenced clinical practice for decades, and many centres employ this combined regimen. One interpretation of this randomized trial would be that the anaerobes targeted by metronidazole are responsible for causing GVHD. But another possibility is raised by retrospective evidence that complete gut decontamination confers significant protection from GVHD (refs 4,11). Whereas germ-free experimental conditions and successful clinical decontamination are protective of GVHD, perhaps attempts that achieve only partial decontamination render vacant a niche for one or more noxious bacteria? We speculate that preservation of a diverse microbiota, in which competition and colonization resistance suppress overgrowth of pathogenic strains, is preferable over a severely disrupted intestinal flora. Can toxicity of antibiotics be reduced without compromising patient safety? This question is currently being tested at a number of transplant centres to determine which regimens are the optimal ones to bring into practice. In 2012, for example, one of our centres switched antibacterial prophylaxis regimens from the combination of metronidazole and a fluoroquinolone to rifaximin. Rifaximin is a broad-spectrum antibiotic that dampens intestinal inflammation through unclear mechanisms; in mice it can preserve Lactobacillus spp., which can reduce the severity of experimental GVHD (ref. 7). After this switch, there was a reduction in transplant-related mortality observed in conjunction with several indicators of microbiota health12.
A prebiotic approach involves administration of certain foods or food components that are rich in poorly digested or poorly absorbed carbohydrates and fibres to confer a competitive advantage to beneficial commensal bacteria that are capable of metabolizing these substrates. Alternatively, administration of prebiotics may augment the production of metabolic products that result from their fermentation. Inulin-type fructans and arabinoxylans have been proposed as categories of prebiotics to evaluate.
In a postbiotic intervention, one would bypass the bacteria altogether and administer a bacterial metabolic product that mediates some benefit. Several Clostridial metabolites, including indole and its derivatives can negatively regulate the growth of potential pathogens such as Gram-negative bacteria and Candida13. Such approaches offer attractive safety considerations in a vulnerable immunocompromised patient population. Butyrate or other short-chain fatty acids would be candidates given their ability to induce regulatory T cells and improve GVHD in animal models14. Another possibility would be introduction of secondary bile acids, which are a product of conjugation of primary bile acids by the microbiota and can suppress Clostridium difficile15.
Finally, the microbiota offer diagnostic or prognostic opportunities. For example, certain subsets of the intestinal microbiota express tryptophanase, which converts dietary l-tryptophan to indole, which is then absorbed and further metabolized to 3-indoxyl sulfate and eliminated through the urine. Urinary concentrations of 3-indoxyl sulfate correlate with microbiota health as well as reduced intestinal GVHD and improved overall survival4. Important contributors to the production of 3-indoxyl sulfate include members of the Firmicute families Lachnospiraceae and Ruminoccoacceae, which have been linked to reduced intestinal inflammation13. While measurement of a urinary metabolite lacks the resolution of deep sequencing, the scalability and rapid turnaround of this assay could be used to design clinical trials in which patients with low levels of 3-indoxyl sulfate are offered microbiota-restoring interventions or additional GVHD prophylaxis strategies.
Recent studies have indicated that the murine flora plays a role in anti-tumour immune responses, including those triggered by chemotherapy, immune checkpoint-blockade drugs and radiation16. Since donor grafts can mediate antitumour activity, these studies raise a question of whether the intestinal microbiota has any impact on relapse of primary malignancy. This is an important question, since relapse remains the major hurdle to survival after allo-HSCT.
For any disease or treatment whose outcome depends upon the microbiota, the major outstanding question is to elucidate the mechanisms by which bacteria can exert beneficial or detrimental effects on the transplanted host. For example, does the ability of the microbiota to modulate immune responses occur via the adaptive immune system (that is, antigen-specific responses against floral antigens), or alternatively via the innate immune system (that is, pattern recognition receptors and innate immune cell populations)? These questions bear relevance not only for allo-HSCT but on other complex diseases. The novel approaches described here could readily be applied to patients with inflammatory bowel diseases, since these autoimmune syndromes share many similarities with gastrointestinal GVHD. Similarly, clinical protocols in the context of solid-organ transplantation (such as kidney, heart and liver) may also be influenced by a better understanding of the microbiota–host relationship, as outcomes after solid-organ transplantation also depend on the regulation of alloreactivity.
References
- 1.Jenq RR, et al. Biol. Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taur Y, et al. Clin. Infect. Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taur Y, et al. Blood. 2014;124:1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holler E, et al. Biol. Blood Marrow Transplant. 2014;20:640–645. doi: 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taur Y. Autologous fecal microbiota transplantation (auto-FMT) for prophylaxis of Clostridium difficile infection in recipients of allogeneic hematopoietic stem cell transplantation. Clinical trial NCT02269150. 2014 http://go.nature.com/Gtmmlt. [Google Scholar]
- 6.Gerbitz A, et al. Blood. 2004;103:4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 7.Jenq RR, et al. J. Exp. Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij DJ. Natl Cancer Inst. 1974;52:401–404. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- 9.Storb R, et al. N. Engl. J. Med. 1983;308:302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 10.Beelen DW, et al. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 11.Vossen JM, et al. PLoS ONE. 2014;9:e105706. doi: 10.1371/journal.pone.0105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber D, et al. Bone Marrow Transplant. 2016 http://dx.doi.org/10.1038/bmt.2016.66. [Google Scholar]
- 13.Weber D, et al. Blood. 2015;126:1723–1728. doi: 10.1182/blood-2015-04-638858. [DOI] [PubMed] [Google Scholar]
- 14.Mathewson ND, et al. Nature Immunol. 2016 http://dx.doi.org/10.1038/ni.3400. [Google Scholar]
- 15.Buffie CG, et al. Nature. 2014;517:205–208. [Google Scholar]
- 16.Zitvogel L, et al. Sci. Transl. Med. 2015;7:271ps1. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]