Summary
Acinic cell carcinoma of breast is a rare subtype of triple-negative breast carcinoma and demonstrates extensive morphologic overlap with acinic cell carcinoma of the salivary gland. In this study, we perform a detailed morphologic and immunohistochemical description of 2 cases of this rare entity and undertake a comprehensive review of all reported cases of breast acinic cell carcinoma in the English language literature to date. One-third of reported cases of breast acinic cell carcinoma have been associated with the presence of a ductal carcinoma not otherwise specified component, which is frequently poorly differentiated. Breast acinic cell carcinoma can demonstrate focal morphologic features similar to microglandular adenosis; these areas are frequently negative for collagen IV and laminin on immunohistochemistry. The true relationship between these 2 entities remains unclear, but we advocate that microglandular adenosis–like areas at the periphery of a breast acinic cell carcinoma should be considered part of the carcinomatous process and re-excised if this process extends to the initial surgical margins.
Keywords: Acinic cell carcinoma of breast, Triple-negative breast cancer, Morphology, Immunohistochemistry, Molecular pathology
1. Introduction
Acinic cell carcinoma (AcCC) of breast was first described by Roncaroli et al [1] in 1996 and is recognized as a subtype of triple-negative breast carcinoma (TNBC) in the current World Health Organization classification [2]. It is one of several rare subtypes of breast carcinoma that demonstrate morphologic overlap with the repertoire of tumors seen in the salivary glands [3]. Although most breast carcinomas are “ductal” in appearance and show no evidence of acinar or “secretory”-type differentiation, rare cases of invasive ductal carcinoma (IDC) can demonstrate S-100– or lysozyme-positive cells with granular cytoplasm [4], including carcinomas with apocrine morphology, or those that arise in microglandular adenosis (MGA) [5]. Although the term secretory carcinoma is currently used exclusively to describe breast carcinomas associated with the presence of the ETV6-NTRK3 translocation, it may be said that there is a larger subcategory of breast carcinomas, including rare entities such as AcCC and cystic hypersecretory carcinoma, which recapitulate the prosecretory phenotype of the lactating breast. In this article, we report 2 recent cases of breast AcCC diagnosed at our institution, and we review what is currently known about this rare entity in terms of morphology, immunohistochemistry, and molecular pathology.
2. Materials and methods
Two cases of breast AcCC were identified from the departmental pathologic database, and the clinical, radiologic, and pathologic details of both cases were reviewed. Immunohistochemistry was undertaken as part of the diagnostic workup in both cases, and the antibodies and dilutions used are summarized in Table 1. One of the cases underwent molecular analysis using the Memorial Sloan Kettering Integrated Mutation Profiling for Actionable Cancer Targets platform, a next-generation sequencing bait-capture platform, which assesses for mutations, copy number variations, and fusions in 341 genes that are known to be oncogenic drivers [6]. A literature review of breast AcCC was also undertaken and included all prior reports of breast AcCC in the English language literature to date. This study was conducted in accordance with institutional research board guidelines.
Table 1.
Immunohistochemical antibodies used
| Antibody | Clone | Vendor | Working dilution |
|---|---|---|---|
| ER | SP1 | Ventana | RTU |
| PR | 1E2 | Ventana | RTU |
| HER2 | 4B5 | Ventana | RTU |
| EMA | E29 | Ventana | RTU |
| CK7 | OV-TL 12/30 | Dako | 1:1600 |
| S100 | pAb (Rabbit) | Dako | 1:8000 |
| Collagen IV | CIV22 | Ventana | RTU |
| Laminin | pAb (Rabbit) | Biogenex | 1:100 |
| α-1-Antitrypsin | pAb (Rabbit) | Ventana | RTU |
| α-1-Antichymotrypsin | pAb (Rabbit) | Ventana | RTU |
| GCDFP-15 | D6 | Covance | 1:1000 |
| Synaptophysin | SNP88 | Biogenex | 1:2000 |
| Chromogranin | LK2H10 | Ventana | RTU |
| Mammaglobin | 31A5 | Ventana | RTU |
| Lysozyme | pAb (Rabbit) | Ventana | RTU |
| Amylase | pAb (Rabbit) | Nordic Immunology | 1:5000 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; EMA, epithelial membrane antigen; GCDFP-15, gross cystic disease fluid protein 15; RTU, ready to use.
3. Results
3.1. Case report 1
3.1.1. Clinical history
A 47-year-old woman presented with a palpable mass in the lower outer quadrant of right breast, which was 2.8 cm in maximum dimension on sonographic examination and mammographically occult. The patient had no personal or family history of breast cancer. Core needle biopsy (CNB) of the mass was diagnosed as “poorly differentiated carcinoma with apocrine features.” A separate area of microcalcifications in the right upper outer quadrant (UOQ) was also biopsied and showed sclerosing adenosis with apocrine change and microcalcifications. At lumpectomy, a 2.3-cm “poorly differentiated carcinoma with apocrine features” was diagnosed. The tumor was estrogen receptor (ER) negative and human epidermal growth factor receptor 2 (HER2) negative and showed focal weak progesterone receptor (PR) positivity (β5% of cells). A separate 0.5-cm ER-positive, PR-positive, HER2-negative moderately differentiated carcinoma was identified in one of the extra margins excised around the main tumor. Final surgical margins were negative. One of 18 right axillary lymph nodes contained metastatic poorly differentiated carcinoma. The patient underwent systemic chemotherapy, followed by radiotherapy and hormone therapy. Four years later, a 0.7-cm sonographically detected mass in the right UOQ was identified, and bilateral mastectomy was performed after the presence of recurrent carcinoma was confirmed on biopsy. A diagnosis of multifocal invasive carcinoma with AcCC-like features was made on the right mastectomy. Carcinoma involved all 4 quadrants including postsurgical scar tissue in the UOQ and lower outer quadrant; the largest single tumor mass was 3.2 cm. Retrospective review of the prior lumpectomy and preceding CNB lead to the recognition that all of the tumors shared features of breast AcCC. The contralateral mastectomy was entirely benign, but 1 of 2 left axillary lymph nodes showed metastatic carcinoma. The patient underwent further chemotherapy but developed another metastasis involving a right internal mammary lymph node within 1 year. She is currently alive with disease, 6 years after her initial diagnosis.
3.1.2. Morphology
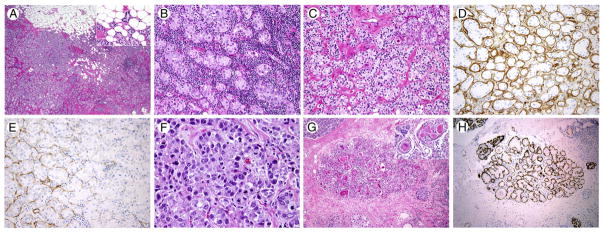
Histologic analysis of the first right breast lumpectomy specimen demonstrated a spectrum of architectural appearances, ranging from large nodular areas of carcinoma with a solid and nested appearance, through acinar-like areas with a discrete pseudolobular growth pattern, to scattered tubular or microglandular structures and single cells that infiltrated widely into adjacent fibroadipose tissue (Fig. 1A–C and F). Microglandular structures frequently contained a central globule of colloid-like eosinophilic material, similar in appearance to MGA (Fig. 1C, inset). A multifocal lymphoplasmacytic infiltrate was present, particularly in association with areas of acinar-like architecture (Fig. 1B). This spectrum of architectural features was associated with a parallel cytologic spectrum, where the solid, nested carcinoma was composed of mitotically active cells with high-grade nuclei of moderate-to-severe pleomorphism, coarse chromatin, and a prominent single nucleolus (Fig. 1F), whereas the well-differentiated acinar-like structures demonstrated smaller, monomorphic eccentric nuclei with fine chromatin, a single small nucleolus, and less mitotic activity (Fig. 1C). In the middle of the cytologic spectrum, infiltrative, less well-developed tubules and scattered cell nests demonstrated intermediate-type nuclei (Fig. 1B). The constituent cells of the acinar-type structures contained finely granular cytoplasm, which looked “clear” at low power, whereas the most poorly differentiated component of the carcinoma showed more uniform basophilic granular cytoplasm, typical of high-grade IDC not otherwise specified (NOS). Areas of the tumor that occupied the center of the morphologic and cytologic spectrum showed much more variation in the tinctorial qualities of their cytoplasm, ranging from finely granular to coarsely granular and including the presence of large, brightly eosinophilic globules. Other focal features identified included the involvement of a normal breast terminal duct lobular unit by an AcCC-like process, which may represent either lobular cancerization or an intraepithelial form of AcCC (AcCC ductal carcinoma in situ [AcCC DCIS]) (Fig. 1G and H). Pseudolactational change was also seen focally within the tumor. Lymphovascular invasion (LVI) by pleomorphic high-grade carcinoma cells was readily identifiable.
Fig. 1.
Representative images of breast acinic cell carcinoma (AcCC) (case 1). A, Infiltrative margin, infiltrating fat (inset) (hematoxylin and eosin [H&E], original magnification, ×20). B, Prominent lymphoplasmacytic infiltrate (H&E, ×100). C, Clear cell morphology, with colloid-like intraluminal material (inset) (H&E, ×100). D, Collagen IV immunohistochemistry (IHC) in tubular areas (collagen IV, ×100). E, Collagen IV IHC in solid nested areas (collagen IV, ×200). F, Poorly differentiated tumor component (H&E, ×400). G, In situ AcCC component (H&E, ×40). H, Calponin IHC in in situ component (calponin, ×40).
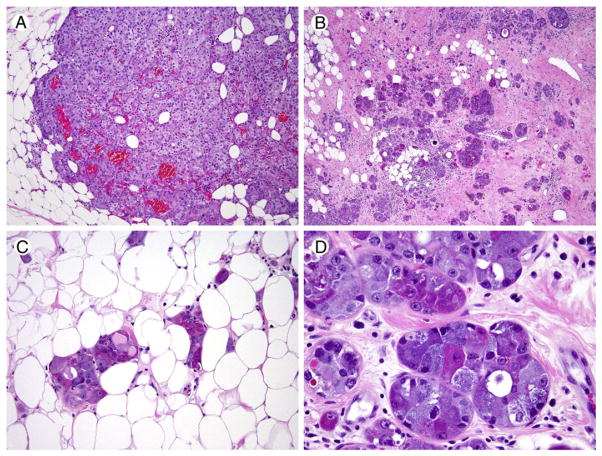
The recurrent carcinoma present in the mastectomy specimen shared many of the same features; however, the most striking difference was the relative absence of the differentiated “clear” acinar-like component. The recurrent tumor was predominantly composed of solid areas, nests, and tubules composed of a mixed cell population of brightly eosinophilic and basophilic cells, often within the same tubular structure, with fine-to-coarse cytoplasmic granularity (Fig. 2). Of note, scattered rounded solid nests of the AcCC-like cells were present throughout the recurrent tumor, simulating the appearance of a solid DCIS component.
Fig. 2.
Representative images of the recurrent breast acinic cell carcinoma (case 1). A, Solid area (H&E, ×40). B, Infiltrative tubular areas (H&E, ×20). C, Subtle infiltration of adipose tissue (H&E, ×200). D, Variably eosinophilic and basophilic granular cytoplasm in tubular cells, imparting a variegated appearance (H&E, ×400).
3.1.3. Immunohistochemistry
Immunohistochemical (IHC) staining of the original tumor demonstrated that it was focally PR positive (β5% of cells) and negative for ER and HER2. The MGA-like tubular areas were positive for PR (focal), epithelial membrane antigen (EMA), CK7, and S-100. Both tubular and solid nested areas of the tumor showed variable positivity for the basement membrane proteins collagen IV and laminin (Fig. 1D and E). Both the original and recurrent tumors showed positivity for α-1-antichymo-trypsin (A1-ACT), S-100, EMA, lysozyme, and gross cystic disease fluid protein (GCDFP). Very focal synaptophysin and mammaglobin positivity were also seen, whereas α-1-antitrypsin and chromogranin were negative. Cells with eosinophilic granules and globules were strongly periodic acid–Schiff (PAS) (diastase resistant) positive. The recurrent tumor was negative for ER, PR, and HER2 and also negative for androgen receptor (AR). The presence of a focus of intraepithelial AcCC was confirmed with p63 and calponin IHC, which demonstrated the presence of a myoepithelial cell layer in the involved lobule (Fig. 1H). Myoepithelial markers (p63 and calponin) were entirely negative in the remainder of the tumor.
3.1.4. Molecular analysis
Targeted next-generation sequencing of the recurrent carcinoma demonstrated the presence of a TP53 frameshift mutation, an MLL3 splicing mutation, and a TSC2 missense mutation (A289V). All 3 mutations were found at similar allelic frequencies. There were also missense mutations with lower allelic frequencies found in MED12 (D1204E), MLL (K1225N), MLL2 (T2017S), and TP63 (R594L). Copy number analysis revealed a 2-fold increase in FOXA1.
3.2. Case report 2
3.2.1. Clinical history
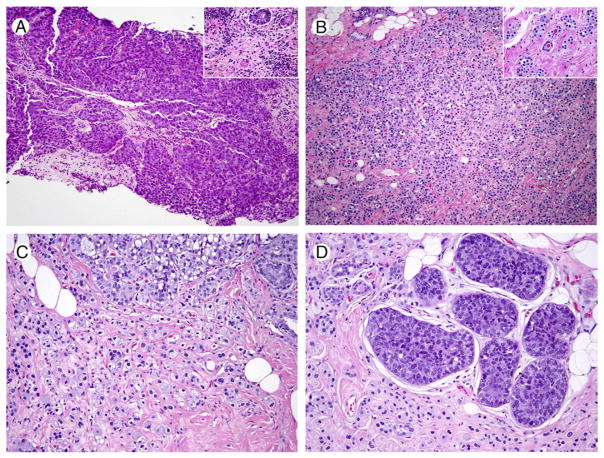
A 49-year-old woman presented with a palpable mass in the right breast. On radiologic assessment, a right UOQ ill-defined solid mass associated with innumerable anechoic cysts was seen. Ultrasound-guided core biopsy showed poorly differentiated carcinoma (Fig. 3A). The patient underwent neoadjuvant chemotherapy and subsequently underwent lumpectomy with additional margins. The pathologic diagnosis was well-differentiated breast AcCC, which was extensively infiltrative and involved multiple margins. LVI was identified, but 2 sentinel lymph nodes were benign. No residual solid poorly differentiated carcinoma was identified. At mastectomy, a residual 1.1-cm focus of breast AcCC was identified. The patient is alive with no evidence of disease 18 months postmastectomy.
Fig. 3.
Representative images of breast acinic cell carcinoma (case 2). A, Poorly differentiated carcinoma (H&E, ×40), with scattered adjacent tubules with eosinophilic granular cytoplasm (inset, H&E, ×200). B, Diffuse, infiltrative residual carcinoma, post–neoadjuvant chemotherapy (H&E, ×40). C, Tubules and single infiltrative carcinoma cells with clear and basophilic cytoplasm (H&E, ×100). D, Residual in situ carcinoma (H&E, ×100).
3.2.2. Morphology
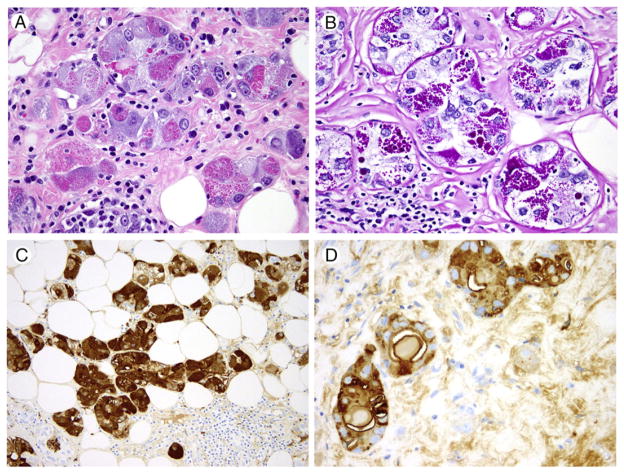
The original biopsy was predominantly composed of a solid, poorly differentiated, triple-negative IDC NOS. However, at the periphery of the solid tumor, small moderately to poorly formed microglandular structures composed of cells with variably eosinophilic and amphophilic cytoplasm were identified, infiltrating normal breast stroma in a haphazard manner, and associated with a lymphocytic infiltrate (Fig. 3A, inset). Post–neoadjuvant chemotherapy, no residual poorly differentiated IDC NOS was identified, and the predominant carcinoma cell type was a relatively monomorphic cell with abundant amphophilic, finely granular cytoplasm, which was dispersed in single cells, clusters, tubules, and sheets (Fig. 3B and C). In sheet-like areas, the tumor developed a histiocytoid appearance, with particularly voluminous cytoplasm. Focally, a component of high-grade DCIS NOS was identified (Fig. 3D). A scattered secondary population of tubules and solid nodules composed of cells with brightly eosinophilic, coarsely granular cytoplasm was also present, very similar in appearance to the tumor morphologyseen in the first case (Fig. 4A). Rare foci of LVI were seen.
Fig. 4.
Representative images of breast acinic cell carcinoma (case 2). Variegated tubules composed of cells with eosinophilic or basophilic granular cytoplasm and focal calcification, positive for PAS diastase, lysozyme, α-1-antitrypsin (H&E, ×400 [A]; PAS diastase, ×400 [B]; lysozyme IHC, ×200 [C]; α-1-antichymotrypsin IHC, ×400 [D]).
3.2.3. Immunohistochemistry
IHC demonstrated that tumor cells were positive for lysozyme, A1-ACT (Fig. 4C and D), and S-100 and negative for amylase, α-1-antitrypsin, synaptophysin, chromogranin, ER, PR, and HER2. p63 staining confirmed the presence of a residual solid poorly differentiated in situ component. Areas with eosinophilic granules and globules were strongly PAS diastase positive (Fig. 4B).
3.3. Literature review of breast AcCC
There have been 47 cases of breast AcCC reported in the English language literature, including the 2 new cases reported in this article [1,4,7–27]. A detailed morphologic description was provided in 31 of these cases [1,4,7–25]. Shimao et al [9] reported the only case of breast AcCC to date occurring in a male patient. The mean patient age at diagnosis was 51 years (median age, 49; range, 23–80 years), whereas the mean tumor size was 3.2 cm (median, 3 cm; range, 1.3–5.5 cm). Follow-up data were available on 25 cases, with a median follow-up duration of 28 months (range, 6–185 months); 19 (76%) of the 25 cases had experienced no tumor recurrence at last review. Six patients developed metastasis to sites such as bone, liver, and lung [4,7,8,13], and 2 died of their disease [4,8].
Review of prior reports demonstrates the remarkable consistency in the morphologic appearance of this tumor type. Table 2 summarizes the reported relative frequency of each of the individual characteristic morphologic features of breast AcCC in the subset of reported cases with detailed morphologic review (n = 31), whereas Table 3 is a summary of the IHC profile of the breast AcCCs reported to date. Most reported cases of breast AcCC describe variation in the tumor architecture, with solid and nested components admixed with areas of tubular and microglandular morphology. The phenomenon of tubules with central luminal extracellular “colloid-like” material was described as “MGA-like” in several articles [4,7,13], whereas other articles interpreted the appearance as suggestive of true MGA [22]. Interestingly, in 11 cases where collagen IV and laminin immunohistochemistry was performed, these MGA or MGA-like areas were entirely negative for these basement membrane markers in 8 cases [7,13,19,24]. In the remaining 3 cases [12] (including the 2 cases reported in this article), very focal positivity was noted. The cytologic appearance of tumor cells, with coarse eosinophilic granules and globules, was labeled “Paneth cell like” in 11 cases [4,7,16,19,22,24]. Seven cases also reported a population of cells with basophilic cytoplasm [4,11,19]; in 4 of these cases [4] (including case 2 from this article), cells with basophilic and eosinophilic cytoplasmic granules were located together within the same tubular structure, giving a variegated appearance (Fig. 2).
Table 2.
Summary of morphologic features of breast AcCC
| Morphologic features of breast AcCC | |
|---|---|
| Architecture (n = 25) | |
| Solid component | 84% (21/25) |
| Tubular component | 92% (23/25) |
| Colloid-like luminal material in tubules | 92% (23/25) |
| Infiltrative growth pattern | 80% (20/25) |
| Cytoplasm (n = 25) | |
| Prominent eosinophilic cytoplasmic granularity (fine to coarse) | 96% (24/25) |
| Large cytoplasmic eosinophilic globules | 68% (17/25) |
| Cells with clear cytoplasm | 52% (13/25) |
| Cells with basophilic cytoplasm | 28% (7/25) |
| Nuclei (n = 21) | |
| Round-to-oval, single nucleolus, low-intermediate grade | 71% (15/21) |
| Range of appearance present, from low to high grade | 29% (6/21) |
Table 3.
Summary of reported immunohistochemical results in breast AcCC
| Immunohistochemical features of breast AcCC | % Positivity |
|---|---|
| PAS (diastase resistant) | 100% (19/19) |
| S-100 | 85% (23/27) |
| Lysozyme | 100% (22/22) |
| EMA | 100% (19/19) |
| Amylase | 94% (17/18) |
| α-1-Antitrypsin (A1AT) | 56% (5/9) |
| α-1-Antichymotrypsin (A1ACT) | 91% (10/11) |
| CK7 | 100% (7/7) |
| Neuroendocrine markers (synaptophysin) | 16% (2/12) |
| GCDFP | 44% (7/16) |
| Hormone markers | |
| ER | 12% (3/25) |
| PR | 20% (5/25) |
| AR | 11% (1/9) |
| HER2 | 0% (0/19) |
| Triple-negative carcinoma (ER/PR/HER2 negative) | 74% (14/19) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; AR, androgen receptor; HER2, human epidermal growth factor receptor 2; GCDFP-15, gross cystic disease fluid protein 15.
Of the 47 breast AcCC cases reported, 15 patients (32%) had an associated IDC NOS; of these, 10 had a solid, poorly differentiated carcinoma, 2 had a well-differentiated tubular/microglandular carcinoma, 1 had a moderately differentiated carcinoma, whereas 1 metaplastic carcinoma has also been reported [4,8,11,23,27]. Tumor grade was not detailed in the remaining cases. In 1 case [4], the poorly differentiated IDC NOS component was ER positive, whereas in the first case described in this article, the original ER-negative tumor showed weak PR positivity in less than 5% of cells. The remaining cases with an IDC NOS component were reportedly triple negative (ER/PR/HER2 negative). Three cases (10%) had solid high-grade DCIS NOS identified in association with breast AcCC [4,15] including the second case reported in this article. LVI was identified in 7 of 13 cases where that parameter was explicitly assessed, and lymph node metastases were identified in 8 of 27 cases, ranging from 1 to 10 positive lymph nodes. Of the 8 cases with lymph node metastasis, 4 had a poorly differentiated IDC NOS component to their main tumor [4,7].
Seven cases of breast AcCC have had ultrastructural analysis [1,7–10,22]. In all 7 cases, the dominant feature was the presence of multiple, variably sized cytoplasmic granules, whereas ribosomal endoplasmic reticulum was prominent in 5 cases, and abundant mitochondria were noted in 3 cases.
4. Discussion
The breast is embryologically, morphologically, and functionally related to secretory glands of other sites, including the salivary glands. It is, therefore, unsurprising that breast carcinomas may recapitulate the appearance of tumors more commonly seen in the salivary glands, including adenoid cystic carcinoma, pleomorphic adenoma, adenomyoepithelioma, myoepithelioma, oncocytic carcinoma, and mucoepidermoid carcinoma [3,28]. Conversely, ETV6-NTRK3 translocation–associated secretory carcinoma of the breast (T-SC) has a rare analogous counterpart in the salivary gland, mammary analog salivary gland carcinoma, which bears the same translocation [29]. However, T-SC is only one of a number of breast carcinoma subtypes that can demonstrate a “secretion-rich” phenotype, with variably sized cysts and pools of extracellular secretions and/or prominent cytoplasmic zymogen-like granules. These entities may together constitute a spectrum of morphologies in which the degree of development of the “secretory” phenotype varies from focal to predominant. Cystic hypersecretory carcinoma, with its dominant secretion-rich morphology, may be thought of as representing one end of this spectrum, whereas ductal carcinoma with focal eosin-ophilic granular cells [4] or pseudolactational features may represent the opposite pole of this morphologic range. The middle ground in this morphologic spectrum is represented by AcCC of breast.
It is clear from the 47 cases of breast AcCC that have now been described that this entity has a wide morphologic spectrum of appearances in terms of architectural and cytologic features. Extensive intratumoral morphologic variation can be seen in a single tumor, highlighting the diagnostic challenges associated with core biopsy of these lesions.
The IHC profile of breast AcCC shares many features with AcCC of salivary gland, with frequent expression of S-100, lysozyme, amylase, and A1-ACT and PAS positivity in addition. Although most cases have been negative for hormone receptors (including AR) and all have been negative for HER2, it is useful to note that rare cases have shown some expression of ER and PR. Where myoepithelial markers such as calponin and p63 have been tested in breast AcCC, they are consistently negative, confirming the true invasiveness of this tumor type. Interestingly, the basement membrane–associated proteins collagen IV and laminin have also been negative in most breast AcCC cases where they have been reported (Table 3). However, as demonstrated in the first case described in this article, these markers are not always entirely negative in breast AcCC.
The true nature of the relationship between MGA and breast AcCC is unclear. There is extensive morphologic overlap between the well-differentiated tubular component of breast AcCC and MGA, whereby both entities are characterized by a widely infiltrative growth pattern. Furthermore, both express S-100 and are negative for myoepithelial markers and hormone receptors. The question of whether the 2 lesions are related has been previously highlighted [12], and some authors have interpreted the presence of infiltrative microglandular structures at the periphery of a breast AcCC tumor as evidence that the carcinoma arose on the background of an MGA substrate. Because IHC for laminin and collagen IV is thought to be positive in most MGA [5], in contrast to the results of such IHC in most tested cases of breast AcCC, it might appear that these markers could be used to differentiate these lesions. However, the first case reported in this article highlights the need for caution in adopting this strategy, as this tumor showed significant variation in basement membrane protein expression on IHC, ranging from entirely negative to strongly positive. Therefore, a CNB of this lesion may demonstrate variable collagen IV and laminin IHC results, depending on the area sampled. At present, one can suggest that the absence of collagen IV and laminin expression may represent evidence against the presence of true MGA, but this cannot be stated definitively due to known variation in staining patterns. When the MGA-like process extends to surgical margins in the setting of breast AcCC, re-excision is advised.
The presence of a TP53 mutation in the molecular analysis of the first case described in this article is in keeping with the findings of Ripamonti et al [19], who reported the presence of a TP53 mutation in a breast AcCC that occurred in a BRCA1 mutation carrier, and Piscuoglio et al [27], who recently described TP53 mutations in 80% (8/10) of breast AcCCs tested. TP53 mutation was found in 37% of all breast carcinomas tested in The Cancer Genome Atlas breast carcinoma study [30] and is the most common mutation in breast cancer. In contrast, Piscuoglio et al [27] did not detect any mutations in TP53 in 20 salivary AcCCs, leading those authors to conclude that from a molecular pathology standpoint, breast AcCCs have far more in common with TNBC than salivary AcCCs. In keeping with these results, Guerini-Rocco et al [31] recently performed massively parallel sequencing analysis of the same cohort of breast AcCCs as Piscuoglio et al [27] and demonstrated a pattern of complex copy number aberrations that also mirrors that seen in TNBC. MLL3, another likely oncogenic driver in our case, was also mutated in 7% of cases in the breast carcinoma/The Cancer Genome Atlas study [30], making it one of the top five most frequently mutated genes. Finally, the amplification of FOXA1, a transcription factor important for ER binding to DNA, is predicted to lead to cellular reprogramming to establish an estrogen-dependent cell phenotype [30]. FOXA1 has also been shown to repress the transcription of a subset of basal-type breast cancer–associated genes [32] and, therefore, may play a role in the maintenance of a luminal phenotype in breast AcCC tumor cells.
Many early case reports suggested, based on very small case series, that breast AcCC is likely to have a good prognosis [1,7]. However, it is clear that poorly differentiated TNBC can frequently be a component of breast AcCC and that recurrences and death from this disease do occur. It seems probable that prognosis is largely driven by the presence of the poorly differentiated component. Furthermore, there is limited evidence from cases involving surgical excision after neoadjuvant chemotherapy (including case 2 of this study) that although the poorly differentiated tumor component may be comparatively chemosensitive, the better differentiated acinar and microglandular carcinoma may be more chemoresistant [8,23]. However, this data analysis is provisional, pending future larger studies of this rare tumor entity.
In summary, breast AcCC is a rare subtype of breast carcinoma that is usually triple negative on immunohistochemistry and has a wide morphologic spectrum that can include a high-grade, poorly differentiated component. The relationship between breast AcCC and MGA remains unclear, but it is apparent that MGA-like areas at the periphery of breast AcCC should be considered a part of the carcinomatous process and re-excised if they extend to the initial surgical margins. Recognition of the entity of breast AcCC is also important in avoiding the potential diagnostic pitfall of interpreting well-differentiated areas of AcCC as MGA on biopsy. The key to making the diagnosis is the combination of knowledge of the entity and recognition of the characteristic morphology and IHC profile (as detailed in Tables 2 and 3). Recent molecular analysis of this tumor subtype suggests that the most common mutations (TP53, PIK3CA) seen in IDC NOS also occur in breast AcCC. Thus, in contrast to other analogous breast and salivary gland tumor types, such as ETV6-NTRK translocation–related tumors or MYB-NFIB translocation–related adenoid cystic carcinomas of both sites, AcCC of breast and salivary gland appear to be genetically distinct, despite their morphologic similarities.
Footnotes
Disclosures: The author(s) have no conflicts of interest or funding to disclose. This work has no specific funding.
References
- 1.Roncaroli F, Lamovec J, Zidar A, Eusebi V. Acinic cell–like carcinoma of the breast. Virchows Arch. 1996;429:69–74. doi: 10.1007/BF00196823. [DOI] [PubMed] [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, et al., editors. WHO Classification of Tumors of the Breast. Lyon: IARC; 2012. [Google Scholar]
- 3.Pia-Foschini M, Reis-Filho JS, Eusebi V, Lakhani SR. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol. 2003;56:497–506. doi: 10.1136/jcp.56.7.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo L, Bell D, Qui H, Sahin A, Wu Y, Sneige N. Paneth-cell–like eosinophilic cytoplasmic granules in breast carcinoma. Ann Diagn Pathol. 2011;15:84–92. doi: 10.1016/j.anndiagpath.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli F. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000;8:303–15. doi: 10.1177/106689690000800409. [DOI] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan-Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damiani S, Pasquinelli G, Lamovec J, Peterse JL, Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000;437:74–81. doi: 10.1007/s004280000206. [DOI] [PubMed] [Google Scholar]
- 8.Coyne JD, Dervan PA. Primary acinic cell carcinoma of the breast. J Clin Pathol. 2002;55:545–7. doi: 10.1136/jcp.55.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimao K, Haga S, Shimizu T, et al. Acinic cell adenocarcinoma arising in the breast of a male: a clinicopathological, immunohistochemical and ultrastructural study. Breast Cancer. 1998;5:77–81. doi: 10.1007/BF02967419. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt FC, Riberio CA, Alvarenga S, Lopes JM. Primary acinic cell-like carcinoma of the breast—a variant with good prognosis? Histopathology. 2000;36:286–9. doi: 10.1046/j.1365-2559.2000.0872f.x. [DOI] [PubMed] [Google Scholar]
- 11.Elster EA, Markusic J, Ball R, et al. Primary acinic cell carcinoma of the breast. Am Surg. 2002;68:993–5. [PubMed] [Google Scholar]
- 12.Kahn R, Holtveg H, Nissen F, Holck S. Are acinic cell carcinoma and microglandular carcinoma of the breast related lesions? Histopathology. 2003;42:195–203. doi: 10.1046/j.1365-2559.2003.01532_1.x. [DOI] [PubMed] [Google Scholar]
- 13.Peintinger F, Leibl S, Reitsamer R, Moinfar F. Primary acinic cell carcinoma of the breast: a case report with long-term follow-up and review of the literature. Histopathology. 2004;45:642–56. doi: 10.1111/j.1365-2559.2004.01957.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanahashi C, Yabuki S, Akamine N, Yatabe Y, Ichihara S. Pure acinic cell carcinoma of the breast in an 80 year old Japanese woman. Pathol Int. 2007;57:43–6. doi: 10.1111/j.1440-1827.2007.02055.x. [DOI] [PubMed] [Google Scholar]
- 15.Stolnicu S, Dohan M, Preda O, Puscasiu L, Garcia-Galvis OF, Nogales FF. Primary acinic cell carcinoma of the breast associated with an intraductal acinic cell component. Patologia. 2010;48:204–7. [Google Scholar]
- 16.Chang ED, Lee EJ, Lee AW, Kim JS, Kang CS. Primary acinic cell carcinoma of the breast: a case report with an immunohistochemical and ultrastructural studies. J Breast Cancer. 2001;14:160–1. doi: 10.4048/jbc.2011.14.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choh CTP, Komar V, Courtney SP. Primary acinic cell carcinoma of the breast: a rare lesion with a good prognosis. Breast J. 2012;18:610–1. doi: 10.1111/tbj.12013. [DOI] [PubMed] [Google Scholar]
- 18.Shingu K, Ito T, Kaneko G, Itoh N. Primary acinic cell carcinoma of the breast: a clinicopathological and immunohistochemical study. Case Rep Oncol Med 2013. 2013;2013:372947. doi: 10.1155/2013/372947. ID 372947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripamonti CB, Colombo M, Mondini P, et al. First description of an acinic cell carcinoma of the breast in a BRCA1 mutation carrier: a case report. BMC Cancer. 2013;13:46. doi: 10.1186/1471-2407-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osaka T, Takeuchi K, Horii R, Iwase T, Akiyama F. Secretory carcinoma of the breast and its histopathological mimics: value of markers for differential diagnosis. Histopathology. 2013;63:509–19. doi: 10.1111/his.12172. [DOI] [PubMed] [Google Scholar]
- 21.Sakuma T, Mimura A, Tanigawa N, Takamizu R. Fine needle aspiration cytology of acinic cell carcinoma of the breast. Cytopathology. 2013;24:403–5. doi: 10.1111/j.1365-2303.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 22.Falleti J, Coletti G, Rispoli E, et al. Acinic cell carcinoma of the breast arising in microglandular adenosis. Case Rep Pathol. 2013;2013:736048. doi: 10.1155/2013/736048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkler N, Morrell G, Factor R. Invasive carcinoma with acinic cell–like features of the breast. Breast J. 2013;19:334–5. doi: 10.1111/tbj.12119. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Li W, Lang R, et al. Primary acinic cell carcinoma of the breast; a case report and review of the literature. Int J Surg Pathol. 2014;22:177–81. doi: 10.1177/1066896913483898. [DOI] [PubMed] [Google Scholar]
- 25.Limite G, Di Micco R, Esposito E, et al. The first case of acinic cell carcinoma of the breast within a fibroadenoma: case report. Int J Surg. 2014;12:S232–5. doi: 10.1016/j.ijsu.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Reis-Filho JS, Natrajan R, Vatcheva R, et al. Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6 “split apart” probes. Histopathology. 2008;52:840–6. doi: 10.1111/j.1365-2559.2008.03046.x. [DOI] [PubMed] [Google Scholar]
- 27.Piscuoglio S, Hodi Z, Katabi N, et al. Are acinic cell carcinomas of the breast and salivary glands distinct diseases? Histopathology. 2015;67:529–37. doi: 10.1111/his.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foschini MP, Krausz T. Salivary gland-type tumors of the breast: a spectrum of benign and malignant tumors including “triple negative carcinomas” of low malignant potential. Semin Diagn Pathol. 2010;27:77–90. doi: 10.1053/j.semdp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 30.Ma CX, Ellis MJ. The Cancer Genome Atlas: clinical applications for breast cancer. Oncology. 2013;27:1263–1269. 12749. [PubMed] [Google Scholar]
- 31.Guerini-Rocco E, Hodi Z, Piscuogloio S, et al. Massively parallel sequencing analysis of acinic cell carcinoma of the breast. J Pathol. 2015;237:166–78. doi: 10.1002/path.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernardo GM, Bebek G, Ginther CL, et al. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene. 2013;32:554–63. doi: 10.1038/onc.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]