Abstract
Background
Evidence supports the role of feedback in reinforcing motivation for behavior change. Feedback that provides reinforcement has the potential to increase dietary self-monitoring and enhance attainment of recommended dietary intake.
Objective
To examine the impact of daily feedback (DFB) messages, delivered remotely, on changes in dietary intake.
Methods
A secondary analysis of the SMART trial, a single-center, 24-month randomized clinical trial of behavioral treatment for weight loss. Participants included 210 obese adults (mean body mass index=34.0 kg/m2) who were randomized to either a paper diary (PD), personal digital assistant (PDA), or PDA plus daily, tailored feedback messages (PDA+FB). To determine the role of daily tailored feedback in dietary intake, we compared the self-monitoring with daily feedback group (DFB, n=70) to the self-monitoring without daily feedback group (No-DFB, n=140). All participants received a standard behavioral intervention for weight loss. Self-reported changes in dietary intake were compared between the DFB and No-DFB groups and were measured at baseline, 6, 12, 18, and 24 months. Linear mixed modeling was used to examine percent changes in dietary intake from baseline.
Results
Compared to the No-DFB group, the DFB group achieved a larger reduction in energy (−22.8% vs. −14.0%, p=0.02) and saturated fat (−11.3% vs. −0.5%, p=0.03) intake, and a trend toward a greater decrease in total fat intake (−10.4% vs. −4.7%, p=0.09). There were significant improvements over time in carbohydrate intake and total fat intake for both groups (p’s<0.05).
Conclusion
Daily, tailored feedback messages, designed to target energy and fat intake and delivered remotely in real-time using mobile devices, may play an important role in the reduction of energy and fat intake.
INTRODUCTION
Lifestyle modification, which consists of a combination of reduced energy intake, increased physical activity and behavioral treatment, is the standard approach to weight loss treatment1,2. At the center of this approach is self-monitoring, defined as the systematic observation and recording of dietary intake for the purpose of increasing one’s awareness of eating behaviors3–7. This strategy forms the central core of behavioral treatment for weight management1,8,9.
The evidence supporting the significant role of consistent and timely self-monitoring in successful weight loss and weight loss maintenance continues to amass10–13. Traditionally, an individual turns in a diary on a regular basis to the interventionist, and the interventionist reviews it and provides written feedback to the participant. This feedback may be delivered one or more weeks after the diary was submitted, depending on the interval between face-to-face treatment sessions. However, ample data demonstrate inadequate sustained self-monitoring over time6. The reduced self-monitoring not only mitigates the benefit of increasing self-awareness of one’s behavior, but also eliminates the supportive and directive feedback an individual can receive.
Evidence supports the role of feedback in reinforcing motivation for behavior change, particularly when delivered in relation to progress toward goal attainment14,15. An earlier study demonstrated the effectiveness of delivering a feedback message in response to self-monitoring diaries16. Given the evidence supporting feedback messaging, the next logical step is addressing the timing of message delivery. Behavioral theory supports the premise that the more proximal the delivery of the reinforcement to the desired behavior, the more likely the desired behavior will increase17. Thus, informational and reinforcing feedback has the potential to increase self-monitoring and enhance goal attainment18.
Another component to consider is tailoring the feedback messages for each participant. Tailored feedback messages have been shown to be more effective in changing behavior than generic feedback19,20. It is possible that adding daily, remotely-delivered feedback messages, tailored to an individual’s self-monitoring entries, can have a positive effect on dietary intake above and beyond the delayed feedback that is given to the participant using a paper self-monitoring diary. Thus, the primary aim of this investigation was to examine the effect of receiving daily, tailored feedback messages, delivered remotely and in real-time to a mobile device, on changes in dietary intake during the 24-month SMART Trial. An earlier paper21 reported on the effects of self-monitoring using mHealth on weight outcomes at 24 months, where the mean percent weight loss from baseline to 24 months was 2.3% among those using a personal digital assistant (PDA) and received daily tailored feedback (PDA+FB group), 1.9% among the paper diary (PD) group, and 1.4% among those who used a PDA without receiving daily tailored feedback (PDA group). In the current investigation, we focused on the role that daily, tailored feedback messages played in changing dietary intake. We hypothesized that the addition of daily feedback messages, tailored to the participant’s self-monitoring entries for energy and fat intake and delivered remotely in real-time, would have a positive effect on limiting energy and fat intake.
METHODS
Study Design
The current investigation was a secondary analysis examining the effect of tailored, daily feedback messages on dietary intake in the SMART Trial (NCT00277771), a single-center, 24-month clinical trial of behavioral treatment for weight loss18. The parent study used a three-group design to determine the effect of different methods of self-monitoring on short- and long-term weight loss and on self-monitoring adherence. Participants were randomly assigned with equal allocation to use the PD, PDA, or PDA+FB for self-monitoring dietary intake. Because the focus of this report is on daily feedback, we combined the two groups that did not receive daily tailored feedback messages delivered in real-time (PD and PDA; the “No-DFB” group) and compared outcomes to the group that did receive daily feedback messages in real-time (PDA+FB; the “DFB” group). All participants received the same 24-month standard behavioral weight loss treatment (Figure 1).
Figure 1.
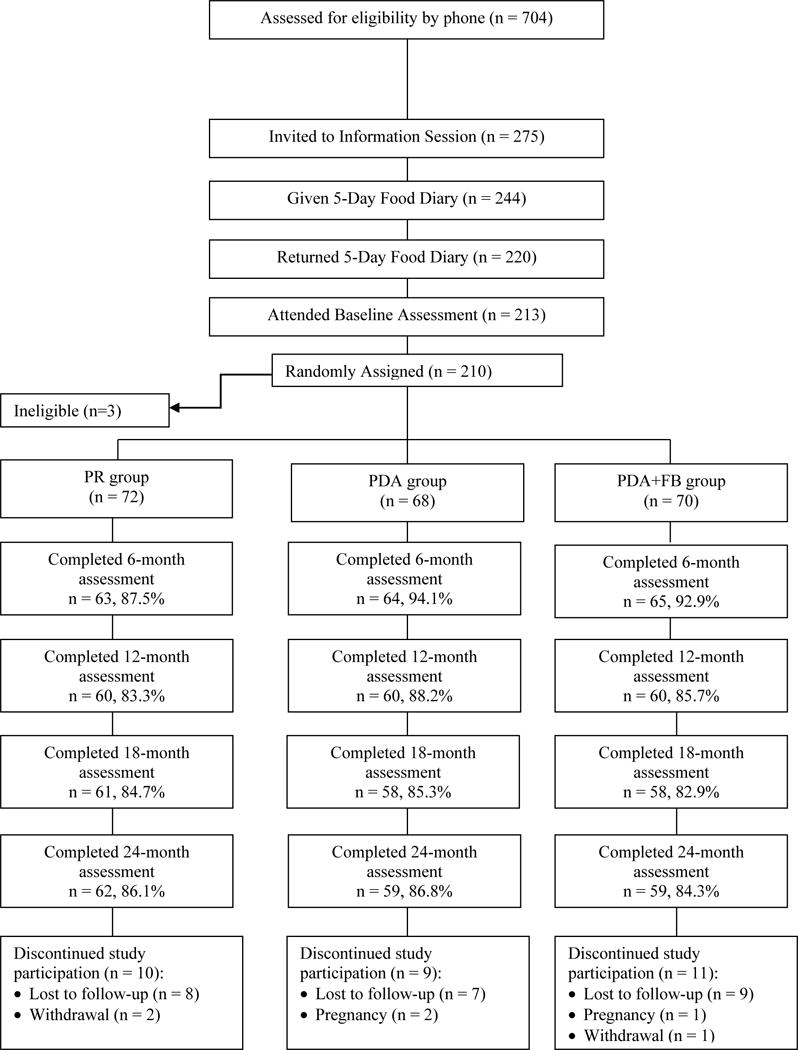
CONSORT Diagram
Participant Characteristics
Participants were recruited from the general population in three annual cohorts from 2006–2008. Eligible individuals were between the ages of 18 and 59 years, had BMIs ranging from 27 to 43 kg/m2, and were able to complete a 5-day food diary at screening. Individuals were excluded if they had physical limitations that prevented them from exercising, required medical supervision of their diet, participated in a weight loss program in the previous six months, or planned an extended absence or relocation within the next 24 months. Of the 704 individuals screened for eligibility, 494 were excluded for the following reasons: lack of interest in the study, did not pass the phone screening, did not return a completed screening packet, or did not complete the 5-day food diary at screening18. Overall, 210 participants were enrolled in the trial. All participants provided written informed consent and the study protocol was approved by the University of Pittsburgh Institutional Review Board.
Intervention
There were three main components to the standard behavioral intervention: 1) group sessions, 2) dietary and exercise goals 3) self-monitoring. The DFB group participants received the standard behavioral intervention as well as feedback messages.
Group sessions
There were 39 in-person group sessions, occurring once a week for months 1–4, bi-weekly for months 5–12, and once a month for months 13–18. One session was held in the 21st month and focused on weight maintenance. Sessions included instruction in developing healthy eating and physical activity habits, group problem-solving exercises and use of behavioral change strategies.
Dietary and exercise goals
Each participant received a daily energy and fat gram goal based on her/his gender and baseline weight, consistent with standard behavioral weight loss treatment9. The daily energy intake goal was between 1,200 and 1,500 calories for females and between 1,500 and 1,800 calories for males; the fat allowance was 25% of total calories for all participants. They were instructed to gradually develop an exercise program and aim to reach a weekly goal of 150 minutes of moderate intensity exercise by the 6th week; the goal was increased by 30 minutes every six months. Because of its convenience and low cost, walking was recommended, as well as bicycling and swimming.
Self-monitoring
Participants in the PD group were given standard paper diaries and instructed to record all foods eaten, energy and fat content, as well as minutes of exercise. PDA and PDA+FB group participants were provided a PDA that contained dietary self-monitoring software that tracked energy and fat intake and displayed current intake related to daily goals; the software included a USDA-based, 6,000-item food database (DietMate Pro©22,23). At each session, PD participants submitted their diaries and received new ones to use until the next session. The interventionist reviewed those diaries, provided written feedback, and returned the diaries at the next session. The PDA and PDA+FB participants turned in their PDAs at the beginning of the session. The self-monitoring data were then uploaded to the study computer and the PDAs were returned to participants at the end of the session. The interventionists received printed reports that appeared similar to the standard paper diaries for their review. The reports with the interventionists’ written comments were returned to the participants at the next group session, which was one to four weeks later depending on session frequency at that time. Once the sessions were more than one week apart, the written feedback on the diary was provided only for the most recent week’s diary.
Feedback messages for the daily feedback group
Participants in the DFB group also received daily messages delivered remotely and in real-time, which were displayed on the screen of the mobile device. These messages were focused specifically on energy and fat intake, as well as physical activity. For the purpose of this paper, we examined the effect of the messages administered in response to recorded dietary intake. The DFB messages were responsive to the information each participant had entered at that point in the day. If a participant had not made an entry, she/he was reminded to record food intake. A customized feedback program was designed for this study, and delivery of the messages in the DFB group was regulated by an investigator-developed algorithm. The algorithm was written in four time-related categories corresponding to the time of day the message would be delivered, and there were five sections in each category corresponding to goal attainment (e.g. did not self-monitor; calories and fat too high; calories too high, fat OK). The program randomly selected a time of day to deliver the messages, such that the message was received at different times once per day. Messages included both positive reinforcement about the participant’s progress and guidance to direct behaviors to stay within daily dietary goals. For example, if someone did not record any food for breakfast, a message might be sent late in the morning stating, “Hope you can find some time to record your breakfast.” If the person was at risk of exceeding the energy goal but was within limits of the fat goal, a message could be delivered that read, “Nice job limiting fats; might want to limit sweets/candy this afternoon.” Extensive details of the algorithm and feedback messages are published elsewhere18.
Measurements
All measurements were conducted at baseline, 6, 12, 18, and 24 months. Dietary intake was measured using two unannounced 24-hour dietary recalls that were guided by the Nutrition Data System for Research software program24,25. Data for the recalls were collected for one weekday and one weekend day. The endpoints for the current investigation were percent changes in dietary intake from baseline to 24 months.
Data Analysis
Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC). Statistical significance was set at p<0.05 for two-sided hypothesis testing. Descriptive statistics were reported as mean (SD) or frequency count (%). Linear mixed modeling was used to exam treatment group differences (PD vs. PDA; PD vs. PDA+FB; PDA vs. PDA+FB) of percent change relative to baseline for dietary intake. Based on these longitudinal results, we then combined the PD and PDA groups into one group (No-DFB), which was compared to the PDA+FB group (DFB). Baseline characteristics were compared between DFB and No-DFB groups using analysis of variance and the chi-square test of independence. Linear mixed modeling was used to assess the main effects of time and treatment groups and their interactions on percent change relative to baseline for dietary intake. Percent change relative to baseline for dietary intake at 24 months was compared within each treatment group using linear contrasts of linear mixed modeling. Missing data were handled through the linear mixed modeling, assuming data missing at random. Sensitivity analyses were conducted for possible influential cases identified as outliers through graphical methods. When the identified outliers were omitted via sensitivity analyses, our conclusions did not change, thus supporting the robustness of our findings.
RESULTS
Sample Characteristics
A total of 210 individuals were enrolled in the study, of which 70 were in the DFB group and 140 were in the No-DFB group. As detailed in Table 1, the majority of participants were female (84.8%) and White (78.1%) with a mean age and education of 46.8 years and 15.7 years, respectively. At the baseline assessment, participants weighed on average 93.8 kg and had a mean BMI of 34.0 kg/m2. The mean energy intake was 2090 kcal; approximately 47.6% of calories were from carbohydrates and 33.7% were from fat (Table 1). The treatment groups were similar with respect to socio-demographic and adiposity measures and their reported dietary intake at study entry. The retention at 24 months was 85.7%, with no differential attrition. Compared to those who did not complete the study, participants who completed the study tended to be older (47.7 vs. 41.8 years, p<0.0001) and have a lower BMI (33.7 vs. 35.6 kg/m2, p=0.04).
Table 1.
Baseline sociodemographic and dietary characteristics for the total sample (N=210) and by group from the SMART trial
| Characteristic | Feedback (n=70) Mean (SD) or % |
No Feedback (n=140) Mean (SD) or % |
Total (N=210) | Pa |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age (years) | 46.4 (9.4) | 47.0 (8.8) | 46.8 (9.0) | 0.60 |
| Female (%) | 84.3 | 85 | 84.8 | 0.89 |
| White (%) | 77.1 | 78.6 | 78.1 | 0.81 |
| Currently married/living with partner (%) | 67.1 | 69.3 | 68.6 | 0.58 |
| Education (years) | 15.5 (3.0) | 15.7 (3.0) | 15.7 (3.0) | 0.65 |
| Gross household income (%) | 0.70 | |||
| <$10,000–$30,000 | 19.1 | 14.6 | 16.1 | |
| >$30,000–$50,000 | 23.5 | 24.1 | 23.9 | |
| >$50,000 | 57.4 | 61.3 | 60 | |
| Full-time employment (%) | 77.1 | 85.7 | 82.9 | 0.12 |
| Dietary | ||||
| Energy intake (kcal) | 2113 (685) | 2078 (683) | 2090 (682) | 0.73 |
| Carbohydrate (% kcal) | 48.0 (9.1) | 47.5 (8.4) | 47.6 (8.6) | 0.69 |
| Protein (% kcal) | 16.4 (5.4) | 17.2 (4.1) | 16.9 (4.6) | 0.25 |
| Fat (% kcal) | 33.8 (7.4) | 33.6 (7.1) | 33.7 (7.2) | 0.90 |
| Saturated fat (% kcal) | 11.6 (3.5) | 11.3 (3.4) | 11.4 (3.4) | 0.53 |
| Sodium (mg) | 3343 (1161) | 3537 (1446) | 3472 (1358) | 0.33 |
| Total fiber (g) | 18.9 (7.6) | 19.7 (9.0) | 19.4 (8.6) | 0.51 |
| Added sugar (g) | 79.5 (57.7) | 74.9 (51.3) | 76.4 (53.4) | 0.56 |
Abbreviations: SD=standard deviation; Feedback=PDA+FB; No Feedback=PDA and PD combined groups; kcal=kilocalories; mg=milligrams; g=grams;
p-values are from chi-square test of independence (categorical) or ANOVA F test (continuous)
Percent Change in Dietary Measures between Groups
When examining percent change in dietary intake variables by time and feedback group, mixed model analysis revealed significant within-group decreases in energy (DFB: −22.8%, p<0.0001; No-DFB: −14.0%, p<0.0001) and total fat (DFB: −10.4%, p=0.0002; No-DFB: −4.7%, p=0.02) for both the DFB and No-DFB groups over time. In addition, the DFB group had a significant within-group decrease in saturated fat intake (−11.3%, p=0.005) over time. As displayed in Figure 2, both groups increased their energy and fat intake by 12 months, with the DFB group having the steepest increase. After 12 months, energy and total fat intake decreased in the DFB group, while saturated fat intake remained the same. In the No-DFB group, energy intake plateaued, while total and saturated fat intake continued to increase. The overall pattern revealed a larger percent decrease in the DFB group for energy and both fat outcomes compared to the No-DFB group. There were significant time effects for carbohydrate (p=0.003), total fat (p=0.01), and saturated fat intake (p=0.03).
Figure 2.
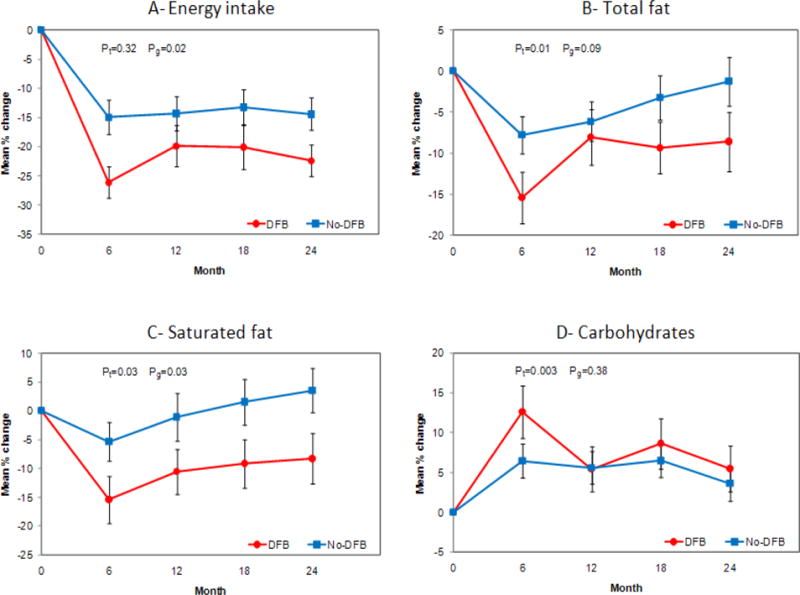
A: Description of percent change in energy intake over time by treatment group. B: Description of percent change in total fat over time by treatment group, C: Description of percent change in saturated fat over time by treatment group, D: Description of percent change in carbohydrate over time by treatment group.
Pt represents p-values for changes over time. Pg represents p-values for differences between treatment groups.
No significant group-by-time interactions were found (Figure 2). However, there were significant group effects for energy (p=0.02) and saturated fat (p=0.03) intake. Compared to the No-DFB group, the DFB group achieved a larger mean reduction in energy intake, mainly at 6 (−26.5% vs. −15.0%, p=0.01) and 24 (−23.4% vs. −14.1%, p=0.03) months, a larger reduction in saturated fat intake at 24 months (−9.3% vs. 3.4%, p=0.04), and a trend toward a significantly larger decrease in total fat intake at six months (−15.6% vs. −8.2%, p=0.06). Although there was no difference in added sugar intake between the DFB and the No-DFB groups, there was a significant within-group difference; the DFB group significantly reduced their added sugar intake from baseline to 24 months (−25.8%, p=0.01), whereas reductions for the No-DFB group did not reach statistical significance (−11.5%, p=0.10).
DISCUSSION
This study was the first to investigate the effect of remotely delivered, daily, tailored feedback messages on changes in dietary intake over 24 months. The main findings were that the group receiving the daily feedback messages targeting energy and fat intake (DFB group) had greater reductions in the amount of energy and saturated fat consumed than the group that did not receive daily feedback messages (No-DFB group). Although a marginally significant finding, the DFB group also achieved a greater decrease in total fat intake compared to the No-DFB group. There were no significant differences in other dietary measures between the DFB and No-DFB groups. We were powered to detect differences in weight, not dietary variables, which may explain why we did not detect statistically significant differences in more dietary variables. These findings support our premise that feedback messages tailored to the diary entries and delivered remotely in real-time to a mobile device can enhance motivation to better achieve the dietary goals. The once-per-day message may not have impacted the intake of other macronutrients since the messages were focused on only energy and fat intake. However, improvement in added sugar intake within the DFB group suggests messages that target one set of nutrients may also improve the intake of other nutrients.
The current study adds to the literature on self-monitoring and the use of feedback messages delivered to mobile devices. Our findings suggest that remotely delivered real-time feedback messages that are responsive to the self-monitoring entries may enhance motivation and provide guidance in reducing daily energy and fat intake. Compared to the 6-month intake, the increase in energy and total fat consumed that was observed at 12 months may have occurred because the participants had become desensitized to the messages, which had not been changed since the first month. A new library of messages was installed after the 12-month assessment, and the subsequent assessments demonstrated a reduction in the amount consumed, particularly in total fat. These findings suggest that feedback messages need to be refreshed at more frequent intervals to limit desensitization.
Previous studies using mobile devices (e.g. PDAs) for self-monitoring of dietary intake revealed improved adherence to self-monitoring23 and reductions in fat intake26; however, these studies were very brief in duration and did not deliver feedback messages23,26. More recently, Spring et al. reported the results of a study that used a PDA to provide remote coaching to improve behaviors related to diet and physical activity27. Results demonstrated remote coaching has potential for improving the adoption of healthy behaviors28. The findings of our 24-month study indicate that the changes observed may be sustainable in the long-term. Moreover, the advances in technology since the beginning of our study may increase adherence and improve the effectiveness of interventions delivered with the currently available mobile devices.
A systematic review of studies using prompts to promote healthy behaviors reported that effectiveness was enhanced when the prompts were frequent29. A study comparing weekly telephone calls to calls delivered every three weeks, designed to target increased walking among adults, indicated those who received the weekly prompts maintained the walking habit for a significantly longer time within the 6-month follow-up period30. These findings support the delivery of more frequent messages. The main difference between this and our study is that our messages were not generic prompts but were messages tailored to what had been entered in a food diary and related to daily dietary goals; moreover, they were delivered daily and without interrupting the individual as a telephone call would, considering the message we delivered was displayed on the screen of the mobile device.
With the advances in technology and the proliferation of mobile devices, opportunities to remotely deliver prompts or tailored feedback messages to an individual are rapidly increasing. In an earlier weight loss study using the Internet, those who received a weekly e-mail counseling message from the interventionist did significantly better at 6 months than those who received computer-automated feedback16. Our study used an investigator-developed algorithm to determine which message was sent in response to the diary entry. Specific information that was obtained in a discussion group following the trial indicated that the participants felt very positive about the messages and would have appreciated receiving them more often.
Although other studies have used mobile devices to encourage behavior change, tailored messages have not been provided to self-monitoring entries. Two recent feasibility studies evaluated the use of cell phones to deliver text messages as a means to promote weight loss or maintenance31,32. Patrick and colleagues conducted a 16-week randomized clinical trial comparing receipt of monthly printed material to personalized text messages; participants used the mobile phone to report their weight once a week. At 16 weeks, the intervention group lost 3.2% and the comparison group lost 1.0% of their baseline weight31. Gerber et al.32 used text messaging to support weight loss maintenance as interventionist contact frequency decreased. Participants received an average of three messages per week; 96% of the group reported reading the messages, and 79% indicated that the messages helped them attain their weight loss goals. These preliminary studies illustrate the potential of mobile devices in delivering messages to participants. However, neither of these studies incorporated self-monitoring as is usually conducted in SBT for weight loss; therefore, the content of the messages was not responsive to what the person was reporting at that specific time.
There are limitations that need to be addressed. There were relatively few males in our study; however, individuals who seek treatment for weight loss tend to be female and thus our sample was representative of those seeking treatment33. Because we included healthy individuals within specific ages and BMIs, the results may not be generalizable to populations outside of our age and BMI range. As with all studies examining dietary intake, we had to rely on self-reported intake; however, we did conduct 24-hour recalls using the Nutrition Data System for Research program, which is considered the gold standard for assessing dietary data.
Our study has several strengths. Unlike other studies that investigated the role of tailored feedback that was delivered less frequently and not in real-time16, our messages were delivered daily in real-time and in response to what had been recorded in the self-monitoring diary at that time. Additionally, we used a randomized design, followed participants for two years with an excellent retention rate, and had an ethnically diverse sample. We also tested a feedback intervention that was automated and thus the feedback did not require the time of the interventionist, which has implications for the future expansion of this feedback system. The feedback algorithm could also be expanded to identify improved patterns in behaviors over multiple days, even when daily goals are not met. There is significant potential in further development of algorithms that can read the diary entries and deliver feedback that is not only tailored to the person’s performance but also timely in providing reinforcement for attainment of behavior goals. As noted in a recent editorial, the new technologies available today to monitor food intake and physical activity give individuals tools that are important for weight management, and moreover, support the potential for increased interaction with providers through distance-based programs34,35. An important next step is to draw upon the technology that is available for the delivery of automated feedback messages but also to establish the evidence base for the proliferation of the downloadable, weight-loss mobile applications since that is what all individuals engaging in self-monitoring are using36
CONCLUSION
Our findings revealed that individuals who received a daily tailored feedback message reported reducing their energy and saturated fat intake to a greater degree than those who did not receive the messages. The current investigation demonstrated that tailored feedback messages delivered daily in real-time and in response to diary entries resulted in participants making significant improvements in dietary intake.
With the persistently high prevalence of obesity in adults, it is apparent there is potential benefit in providing support and guidance remotely to individuals who are engaged in weight management. mHealth interventions have shown an overall reduction in weight37, and there may be value in implementing additional strategies other than delayed and generic feedback messages using mHealth. The results of this study demonstrate that delivering personalized feedback messages in real-time may be an important tool for promoting self-regulation in weight management. Moreover, future studies may benefit from the use of mobile technology to deliver tailored feedback messages in a timelier manner. Using this mode of communication may permit more exquisite tailoring of messages and may potentially address additional facets of lifestyle change (e.g., sleep hygiene). The feedback algorithm could also be expanded to identify improved patterns in behaviors over multiple days, even when daily goals are not met. As noted in a recent editorial, the new technologies available today to monitor food intake and physical activity give individuals tools that are important for weight management and support the potential for increased interaction with providers through distance-based programs35. An important next step is to establish the evidence base for the proliferation of the downloadable, weight-loss mobile applications38.
Acknowledgments
We gratefully acknowledge the participants in this study who so willingly, gave of their time to complete the assessments. This study was supported, by National Institutes of Health grants #RO1-DK71817 and partial support, for L.E.B. by NIH K24 Award, NR010742. The conduct of the study was, also supported by the Data Management Core of the Center for Research, in Chronic Disorders NIH-NINR #P30-NR03924 and the General Clinical, Research Center, NIH-NCRR-GCRC #5MO1-RR00056 and the Clinical, Translational Research Center, NIH/NCRR/CTSA Grant UL1 RR024153 at, the University of Pittsburgh.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
Authors have no conflicts of interest to disclose.
Contributor Information
Erica J. Ambeba, Email: ejc25@pitt.edu, Departments of Health and Community Systems, and Epidemiology, University of Pittsburgh School of Nursing and Graduate School of Public Health, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA, 15261, Phone: (619) 806-7271, Fax: (412) 383-7293.
Lei Ye, Email: ley9@pitt.edu, Doctoral Student, Departments of Health and Community Systems, and Biostatistics, University of Pittsburgh School of Nursing and Graduate School of Public Health, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA, 15261, Phone: (415) 367-5270, Fax: (412) 383-7293.
Susan M. Sereika, Email: ssereika@pitt.edu, Professor, Departments of Health and Community Systems, and Biostatistics, University of Pittsburgh School of Nursing and Graduate School of Public Health, 360 Victoria Building, 3500 Victoria Street, Pittsburgh, PA 15261, USA, Phone: (412) 624-0799, Fax: (412) 624-1201.
Mindi A. Styn, Email: mimst31@pitt.edu, Research Assistant Professor, Departments of Health and Community Systems, and Epidemiology, University of Pittsburgh School of Nursing and Graduate School of Public Health, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA, 15261, Phone: (412) 624-0966, Fax: (412) 383-7293.
Sushama D. Acharya, Email: vgk3@cdc.gov, Research Fellow, Community Guide Branch, Office of Surveillance, Epidemiology and Laboratory Services, Epidemiology and Analysis Program Office, Centers for Disease Control and Prevention, 1600 Clifton Road, MS E-69, Atlanta, GA 30329, USA, Phone: (404) 498-0554, Fax: 404-498-0554.
Mary Ann Sevick, Email: sevick@pitt.edu, Associate Professor, Veteran Affairs Pittsburgh Healthcare System and Department of Medicine, University of Pittsburgh School of Medicine, Center for Research on Healthcare, 230 McKee Place, Pittsburgh, PA 15213, Phone: (412) 586-9788, Fax: (412) 647-0632.
Linda J. Ewing, Email: ewinglj@upmc.edu, Assistant Professor, Department of Psychiatry, University of Pittsburgh School of Medicine, 3811 O’Hara Street, Pittsburgh, PA 15213, USA, Phone: (412) 647-3089, Fax: (412) 647-4252.
Molly B. Conroy, Email: conroymb@upmc.edu, Assistant Professor, Departments of Medicine and Epidemiology, University of Pittsburgh Schools of Medicine and Graduate School of Public Health, Center for Research on Healthcare, 230 McKee Place, Suite 600, Pittsburgh, PA, 15206, Phone: (412) 692-4870, Fax: (412) 692-4838.
Karen Glanz, Email: kglanz@upenn.edu, Professor, Schools of Nursing and Medicine, University of Pennsylvania, 423 Guardian Drive, 801 Blockley Hall, Philadelphia, PA 19104, USA, Phone: (215) 898-0613, Fax: (215) 573-5315.
Yaguang Zheng, Email: yaz40@pitt.edu, Department of Health and Community Systems, University of Pittsburgh School of Nursing, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA 15261, USA, Phone: (412) 320-9103, Fax: (412) 383-7293.
Rachel W. Goode, Email: rlw22@pitt.edu, University of Pittsburgh School of Social Work and Graduate School of Public Health, 2117 Cathedral of Learning, Pittsburgh, PA 15260, USA, Phone: (412) 624-2229; Fax: (412) 383-7293.
Meghan Mattos, Email: mkm65@pitt.edu, Department of Health and Community Systems, University of Pittsburgh School of Nursing, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA 15261, USA, Phone: (412) 624-2469, Fax: (412) 383-7293.
Lora E. Burke, Email: lbu100@pitt.edu, Professor, University of Pittsburgh School of Nursing and Graduate School of Public Health, 415 Victoria Building, 3500 Victoria Street, Pittsburgh, PA 15261, USA, Phone: (412) 624-2305, Fax: (412) 383-7293.
References
- 1.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007 May;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009 Feb 17;150(4):255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mossavar-Rahmani Y, Henry H, Rodabough R, et al. Additional self-monitoring tools in the dietary modification component of The Women’s Health Initiative. J Am Diet Assoc. 2004 Jan;104(1):76–85. doi: 10.1016/j.jada.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 5.Burke LE, Warziski M, Starrett T, et al. Self-monitoring dietary intake: current and future practices. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2005 Jul;15(3):281–290. doi: 10.1016/j.jrn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011 Jan;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acharya SD, Elci OU, Sereika SM, Styn MA, Burke LE. Using a personal digital assistant for self-monitoring influences diet quality in comparison to a standard paper record among overweight/obese adults. J Am Diet Assoc. 2011 Apr;111(4):583–588. doi: 10.1016/j.jada.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Heart Lung and Blood Institute. Guidelines on Overweight and Obesity: Electronic Textbook-Appendix VIII. Glossary of Terms. 2007 Dec 12; Available at http://www.nhlbi.nih.gov/guidelines/obesity/e_txtbk/appndx/apndx8.htm:accessed.
- 9.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity: Clinical applications. 2nd. New York: Marcel Dekker; 2004. pp. 147–167. [Google Scholar]
- 10.Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone AA. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemporary Clinical Trials. 2008;29(2):182–193. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011 Feb;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy M, Yang K, Elci O, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART Trial. Medicine and Science in Sports and Exercise. 2011;43(8):1568–1574. doi: 10.1249/MSS.0b013e31820b9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant L, Evans A. Principles of behavioral analysis. New York: HarperCollins College Publishers; 1994. [Google Scholar]
- 15.Patterson R. The new focus: Integrating behavioral science into disease management. In: Patterson R, editor. Changing patient behavior: Improving outcomes in health and disease management. San Francisco: Jossey-Bass; 2001. pp. 1–21. [Google Scholar]
- 16.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Archives of internal medicine. 2006 Aug 14–28;166(15):1620–1625. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 17.Powers RG, Osborn JG. Fundamentals of Behavior. New York: West Publishing Company; 1976. [Google Scholar]
- 18.Burke LE, Styn MA, Glanz K, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemporary Clinical Trials. 2009 Nov;30(6):540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiClemente CC, Marinilli AS, Singh M, Bellino LE. The role of feedback in the process of health behavior change. Am J Health Behav. 2001 May-Jun;25(3):217–227. doi: 10.5993/ajhb.25.3.8. [DOI] [PubMed] [Google Scholar]
- 20.Kazdin AE. Behavior Modification in Applied Settings. 6th. Belmont, CA: Wadsworth/Thomas Learning; 2011. [Google Scholar]
- 21.Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. American journal of preventive medicine. 2012 Jul;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beasley J, Riley WT, Jean-Mary J. Accuracy of a PDA-based dietary assessment program. Nutrition. 2005 Jun;21(6):672–677. doi: 10.1016/j.nut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr. 2008 Apr;27(2):280–286. doi: 10.1080/07315724.2008.10719701. [DOI] [PubMed] [Google Scholar]
- 24.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. Journal of Nutrition. 2006 Oct;136(10):2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 25.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. American Journal of Clincal Nutrition. 2003 May;77(5):1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 26.Glanz K, Murphy S, Moylan J, Evensen D, Curb JD. Improving dietary self-monitoring and adherence with hand-held computers: a pilot study. Am J Health Promot. 2006 Jan-Feb;20(3):165–170. doi: 10.4278/0890-1171-20.3.165. [DOI] [PubMed] [Google Scholar]
- 27.Spring B. Guiding adherence to exercise and diet with E-Networks. Paper presented at: AHA Scientific Sessions; 2012; Los Angeles, CA. [Google Scholar]
- 28.Spring B, Schneider K, McFadden HG, et al. Multiple Behavior Changes in Diet and Activity: A Randomized Controlled Trial Using Mobile TechnologyBehavior Changes in Diet and Activity. Arch Intern Med. 2012 May 28;172(10):789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11(2):e16. doi: 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombard DN, Lombard TN, Winett RA. Walking to meet health guidelines: the effect of prompting frequency and prompt structure. Health Psychol. 1995 Mar;14(2):164–170. doi: 10.1037//0278-6133.14.2.164. [DOI] [PubMed] [Google Scholar]
- 31.Patrick K, Raab F, Adams MA, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber BS, Stolley MR, Thompson AL, Sharp LK, Fitzgibbon ML. Mobile phone text messaging to promote healthy behaviors and weight loss maintenance: a feasibility study. Health Informatics J. 2009 Mar;15(1):17–25. doi: 10.1177/1460458208099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring) 2012 Jun;20(6):1234–1239. doi: 10.1038/oby.2011.140. [DOI] [PubMed] [Google Scholar]
- 34.Rao G, Kirley K. The Future of Obesity Treatment: Accessible, Inexpensive, and Technology-Based? Arch Intern Med. 2013;173(2):111–112. doi: 10.1001/jamainternmed.2013.1232. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr. 2012;96(5):949–950. doi: 10.3945/ajcn.112.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azar KMJ, Lesser LI, Laing BY, et al. An Evaluation of Weight Management Apps for use in Clinical Practice: Room for Improvement. Am J Prev Med. 2013 in press(Novermber) [Google Scholar]
- 37.Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013 Jul-Aug;28(4):320–329. doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breton ER, Fuemmeler BF, Abroms LC. Weight loss—there is an app for that! But does it adhere to evidence-informed practices? Translational Behavioral Medicine. 2011;1:523–529. doi: 10.1007/s13142-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


