Abstract
HCT is a rapidly evolving field with active preclinical and clinical development of new strategies for patient assessment, graft selection and manipulation, and pre- and post-transplant drug and cell therapy. New strategies require evaluation in definitive clinical trials, however, HCT trials face unique challenges, including the relatively small number of transplantations performed at any single center, the diverse indications for HCT requiring dissimilar approaches, the complex nature of the intervention itself, the risk of multiple complications in the immediate post-transplant period, and the risk of important, though infrequent, late effects. The Blood and Marrow Transplant Clinical Research Network (BMT CTN) was established by the US National Heart Lung and Blood Institute and the National Cancer Institute to meet these challenges. In its 15 years as a Network, the BMT CTN has proven to be a successful infrastructure for planning, implementing and completing such trials, and providing definitive answers to questions leading to improvements in the understanding and practice of HCT. It has opened 37 trials, about half Phase II and half Phase III, enrolled more than 8,000 patients and published 57 papers addressing important issues in the treatment of patients with life-threatening malignant and non-malignant blood disorders. This review describes the Network’s accomplishments, key components of its success, lessons learned over the past 15 years and challenges for the future.
Keywords: Hematopoietic Cell Transplantation, Blood and Marrow Transplant Clinical Trials Network, Infrastructure, Review
Introduction
About 21,000 hematopoietic cell transplantations (HCTs) will be performed in the United States (US) in 2016, and 65,000 worldwide. HCT is a rapidly evolving field with active preclinical and clinical development of new approaches to patient assessment, graft selection and manipulation, and pre- and post-transplant drug and cell therapy. New diagnostic and therapeutic strategies require evaluation in definitive clinical trials as does the role of HCT versus other therapies. However, HCT trials face unique challenges, including the relatively small number of transplantations performed at any single center, the diverse indications for HCT requiring dissimilar approaches, the complex nature of the intervention itself, the risk of multiple complications in the immediate post-transplant period, and the risk of important, though infrequent, late effects. Although there is a longstanding mechanism for investigators to collaboratively conduct observational HCT studies in the US using data collected by the Center for International Blood and Marrow Transplant Research (CIBMTR), the ability to collaboratively develop and implement multicenter interventional HCT trials was long hampered by limited funding, by the low priority afforded to HCT trials both by networks focused on non-HCT cancer therapy and by pharmaceutical companies, and by lack of an effective multicenter HCT trials infrastructure (1,2).
To address this need, in 2001, the National Institute of Health’s (NIH’s) National Heart, Lung, and Blood Institute (NHLBI) and National Cancer Institute (NCI) issued a Request for Applications (RFA HLA-01-004) inviting participation in a Blood and Marrow Transplant Clinical Research Network (BMT CTN). The objective of the RFA was to establish and maintain the necessary infrastructure to conduct large, multi-institutional clinical trials to improve HCT outcomes. The mandate was to execute large Phase II and III trials with broad national participation. In its 15 years as a Network, the BMT CTN has proven to be a successful infrastructure for planning, implementing and completing such trials, providing definitive answers to questions that have led to improvements in the understanding and practice of HCT. It has opened 37 trials addressing important issues in the treatment of patients with life-threatening malignant and non-malignant blood disorders. These trials, about half Phase II and half Phase III, with median enrollment of 180 (range, 17– 1700), address issues of donor availability, engraftment, graft-versus-host disease (GVHD), post-transplant infection, disease control, organ toxicity, cost-effectiveness and quality of life. The goal is to make HCT a therapy that is more universally available (by expanding donor sources and allowing use in older, sicker patients), safer (by reducing regimen-related toxicity, life-threatening infection and GVHD) and effective (by various strategies to prevent relapse). Illustrative studies and findings are listed in Table 1.
Table 1.
Selected BMT CTN Studies with significant findings
| EVALUATING CONDITIONING REGIMENS |
| 0301 Phase I/II trial of fludarabine-based conditioning for allogeneic marrow transplantation from human leukocyte antigen (HLA)-compatible unrelated donors in severe aplastic anemia: Optimizing transplantation regimens for rare diseases is difficult and requires a multicenter effort. This study determined that fludarabine is not sufficiently immune suppressive to replace cyclophosphamide in conditioning regimens for unrelated donor transplantation for aplastic anemia. Additionally, it found excess toxicity with a commonly used dose of cyclophosphamide when combined with fludarabine. This unexpected finding is anticipated to change practice in many centers. (28,43) |
| 0401 Phase III trial comparing Rituxan/BEAM versus Bexxar/BEAM prior to autologous HCT for persistent or relapsed chemotherapy-sensitive diffuse large B cell non-Hodgkin lymphoma (DLBCL): Determined that addition of radioimmunotherapy to the standard conditioning regimen of BEAM provides no clinical benefit for patients undergoing autologous HCT for DLBCL. Although several small Phase II studies suggested that dose-intensification might decrease relapse, the primary cause of treatment failure after autologous HCT for DLBCL, this study failed to show an impact on relapse but did show increased toxicity. Future trials will focus on maintenance strategies and/or immune therapies after HCT to improve disease control, marking a significant change in direction for the field. (24) |
| 0601 Phase II trial of unrelated donor HCT for children with severe sickle cell disease using a reduced-intensity conditioning regimen: Determined that a reduced-intensity conditioning regimen of alemtuzumab, fludarabine, and melphalan, although effective for engraftment of bone marrow, was associated with unacceptably high levels of graft failure after cord blood transplantation in children with sickle cell anemia. This disappointing finding using cord blood indicates the need for novel strategies for the large number of sickle cell disease patients who cannot find an HLA-matched adult donor. (42) |
| GRAFT-VERSUS-HOST DISEASE (GVHD) PREVENTION AND TREATMENT |
| 0303: A single-arm, multicenter Phase II trial of transplants of HLA-matched, CD34+ enriched, T cell depleted peripheral blood stem cells isolated by the CliniMACS system in the treatment of patients with acute myeloid leukemia (AML) in first or second complete remission: Confirmed, in a multicenter setting, the feasibility and consistency of T cell depletion by CD34 selection, with results in AML that warranted development of a Phase III trial versus non-T cell depleted transplantation. These data were used by the Food and Drug Administration in its determination to approve, for the first time, a CD-34 selection column for clinical use in the US. A Phase III trial comparing outcomes of CD34-selected transplants using this approach with standard bone marrow transplants followed by calcineurin-inhibitor based GVHD prophylaxis (BMT CTN 1301 PROGRESS II) has recently opened in the BMT CTN. (11) |
| 0302: Initial systemic treatment of acute GVHD: a Phase II randomized trial evaluating etanercept, mycophenolate mofetil, denileukin diftitox, and pentostatin: Identified the most promising agent to move into a Phase III trial (see 0802 below). Data from this trial were also used to determine that GVHD biomarker panels can be used for early identification of patients at high or low risk for treatment non-responsiveness or death and that biomarker panels may provide opportunities for early intervention and improved survival following HCT. The wider use of biomarkers to identify patients at high risk of GVHD will allow us to tailor our therapies to better control this complication in these patients and to reduce toxicity in patients who are unlikely to benefit from intensive immune suppression. The BMT CTN is now incorporating biomarker-defined risk stratification into the design of its GVHD treatment trials. (14,18,21) |
| 0802: A Phase III randomized, double blind trial evaluating corticosteroids with mycophenolate mofetil versus corticosteroids with placebo as initial systemic treatment of acute GVHD: Found no benefit in GVHD-free survival when mycophenolate mofetil was added to corticosteroids for initial therapy of acute GVHD requiring system treatment. Although these results were discouraging, the BMT CTN has used these data to focus on a newer therapeutic agent, sirolimus, in the upcoming BMT CTN 1501 study using a biomarker risk stratification developed in BMT CTN 0302 and 0802 to identify patients with standard risk who might be able to avoid corticosteroid therapy. Other agents are being considered for testing in patients with high risk acute GVHD. The Network’s ability to conduct GVHD trials in a timely manner allows for definitive Phase III results to be quickly disseminated and promising agents to be efficiently tested. |
| 0402: A Phase III randomized, multicenter trial comparing sirolimus / tacrolimus with tacrolimus / methotrexate as GVHD prophylaxis after HLA-matched, related peripheral blood stem cell transplantation: Identified a high risk of toxicity when sirolimus is substituted for standard methotrexate for GVHD prophylaxis when the conditioning regimen includes busulfan, and no advantage in acute GVHD-free survival. Although this study showed a modest improvement in grade III–IV acute GVHD, the findings do not support substituting sirolimus for methotrexate since it may increase toxicity in patients who receive busulfan for conditioning, it was associated with higher risks of chronic GVHD, and it did not improve survival. Novel approaches to preventing GVHD are needed and are being explored in the accruing BMT CTN 1203 PROGRESS I and 1301 PROGRESS II studies. (25,35) |
| GRAFT SOURCES |
| 0501: Multicenter, open label, randomized trial comparing single versus double umbilical cord blood transplantation in pediatric patients with leukemia and myelodysplasia: Demonstrated no survival benefit and more acute GVHD for children receiving infusion of two umbilical cord blood units versus one umbilical cord blood unit after transplantation for hematologic malignancies. This collaborative study with the Children’s Oncology Group indicates, unexpectedly, that increasing cell dose beyond the accepted minimum by adding another cord blood unit does not improve survival after cord blood transplantation in children and increases the risk of acute GVHD. This has important implications for future strategies to improve hematopoietic recovery and decrease transplant-related mortality after cord blood HCT. (27) |
| 0201: A Phase III randomized, multicenter trial comparing G-CSF mobilized peripheral blood stem cell with marrow transplantation from HLA compatible unrelated donors: Found no difference in survival for recipients of unrelated donor peripheral blood versus bone marrow grafts, but an increased risk of chronic GVHD requiring prolonged immune suppression with peripheral blood grafts. Although peripheral blood has largely replaced bone marrow as a graft source for unrelated donor transplantation, this study suggests that this may not be appropriate in the myeloablative conditioning setting, which has important implications for clinical practice. This is the largest prospective study of unrelated donor transplantation ever performed. It would not have been possible without the infrastructure provided by the BMT CTN. An ancillary study shows that patients in this trial were representative of the larger population of patients receiving HCT during the time period. (10,57) |
| 0603/0604: Multicenter, Phase II trials of non-myeloablative conditioning and transplantation of partially HLA-mismatched bone marrow / umbilical cord blood from unrelated donors in patients with hematologic malignancies: Confirmed single-center results in a multicenter setting using reduced-intensity conditioning and haploidentical bone marrow transplantation or double cord blood transplantation in adults with hematologic malignancies, with data supporting a subsequent Phase III trial. Acceptable outcomes of double cord and haploidentical bone marrow transplantation suggest that many more adults should be offered HCT, even when an HLA-matched adult donor is not available. These approaches are now being compared in a randomized Phase III trial (BMT CTN 1101). (3,51) |
| DISEASE TREATMENT |
| 0704 (CALGB 100104): A Phase III, randomized, double-blind study of maintenance therapy with CC-5013 or placebo following autologous stem cell transplantation for multiple myeloma: Determined lenalidomide maintenance therapy dramatically improves progression-free survival and overall survival after autologous HCT for multiple myeloma. The BMT CTN was an important contributor to this study, which was led by Cancer and Leukemia Group B and used its Network and the CIBMTR database to devise an accrual plan that allowed the trial to successfully meet its enrollment target after initial accrual difficulties. The data have led to a major change in clinical practice, with most myeloma patients now receiving lenalidomide maintenance after transplantation. (5) |
| 0502 (CALGB 100103): Phase II study of allogeneic HCT for older patients with AML in first morphologic complete remission using a non-myeloablative preparative regimen: Demonstrated the feasibility and effectiveness of allogeneic HCT using reduced intensity conditioning in this first prospective US cooperative group trial conducted in a homogeneously treated group of older AML patients in first remission. The study demonstrates that, with reduced-intensity conditioning, patients older than 60 can benefit from the graft-versus-leukemia effects of allogeneic HCT with outcomes similar to younger patients. These data should increase the use of HCT in older AML patients and provides justification for extending the upper age range in allograft trials. (4) |
| 0701: Phase II trial of non-myeloablative allogeneic HCT for patients with relapsed follicular non-Hodgkin lymphoma beyond first complete response: Demonstrated that allogeneic HCT using a rituximab-containing reduced-intensity conditioning regimen confers high complete response rates, a low incidence of relapse / progression, and prolonged survival with acceptable toxicity in heavily pretreated follicular lymphoma patients.(68) This study provides justification for future trials comparing nontransplant with transplant salvage strategies in this disease, which has the potential to change practice. |
| SUPPORTIVE CARE |
| 0101: A randomized double-blind trial of fluconazole versus voriconazole for the prevention of invasive fungal infections in allogeneic blood and marrow transplant recipients: Demonstrated that fluconazole, a low-cost antifungal agent, has similar efficacy and is generally more cost-effective than the newer and more expensive drug, voriconazole, in preventing serious fungal infections in the first 6 months after HCT but that voriconazole may be cost-effective for those undergoing allogeneic HCT for AML. This Phase III comparison of fluconazole and voriconazole indicates that newer is not always better and that, for most patients, standard fluconazole is effective fungal prophylaxis. However, an ancillary study suggested there is a subset of patients for whom primary antifungal prophylaxis with voriconazole may be more appropriate, allowing more informed treatment planning by transplant centers. (22,47) Both of these findings inform general HCT practice. |
| QUALITY OF LIFE |
| 0902: A Phase III randomized, multicenter trial testing whether exercise or stress management improves functional status and symptoms of autologous and allogeneic recipients: Demonstrated no improvement in physical or mental quality of life with exercise training, stress management training, or combined stress management and exercise training compared to usual care. This trial tested modest, easily applied interventions in the early transplant period. While lack of an effect was disappointing, the trial enrolled more than 700 patients enrolled in 19 months, demonstrating that the BMT CTN has an effective infrastructure to conduct studies addressing quality of life issues. (20) |
Numerous advances substantially changed the landscape of HCT over the decade and a half since establishment of the BMT CTN and BMT CTN trials played a key role in developing many of them, building on preclinical and early clinical work being done in its member centers and elsewhere. In the early days of the BMT CTN, complications of HCT, especially GVHD, limited the effectiveness and availability of the procedure. Nearly half of the patients in need of HCT, including most patients in ethnic minority groups, particularly African-Americans, were denied the procedure because they did not have available matched donors. Older and less fit patients were deemed not candidates for HCT because of toxicity concerns. BMT CTN-led trials evaluating use of unrelated umbilical cord blood and related haploidentical donor transplantation after reduced intensity conditioning confirmed the safety and effectiveness of these alternative allograft sources in the multicenter setting, with results close to those seen with HLA-matched donors (3). The BMT CTN, working collaboratively with the NCI-supported cooperative group The Alliance for Clinical Trials in Oncology (previously CALGB), also validated single-center data that reduced intensity conditioning allows allogeneic HCT to be used effectively in AML patients older than 60 years, with either an HLA-identical sibling or unrelated donor (4). These studies helped expand applicability of HCT to many more patients with both malignant and non-malignant blood disorders, including patients into their 70s. Additionally, >30% of participants in the ongoing BMT CTN phase 3 trial randomizing between unrelated umbilical cord blood and related haploidentical allografts are from ethnic minority groups, and patients into their 70s are eligible.
The availability and effectiveness of HCT make it an important platform for incorporating novel therapies. HCT not only provides a state of minimal residual disease but, in the case of allografting, a new, non-tolerant immune system. The BMT CTN is at the forefront of trials combining HCT with novel therapeutics. Their collaboration with The Alliance to complete a randomized trial of lenalidomide maintenance after HCT for multiple myeloma, a trial almost closed for poor accrual prior to BMT CTN’s participation, proved practice-changing (5). A trial adding a dendritic cell vaccine to posttransplant maintenance in this setting is about to be launched. These and other studies represent a next generation of BMT CTN trials studying post-HCT strategies to improve response and reduce relapse (see below).
Network Structure
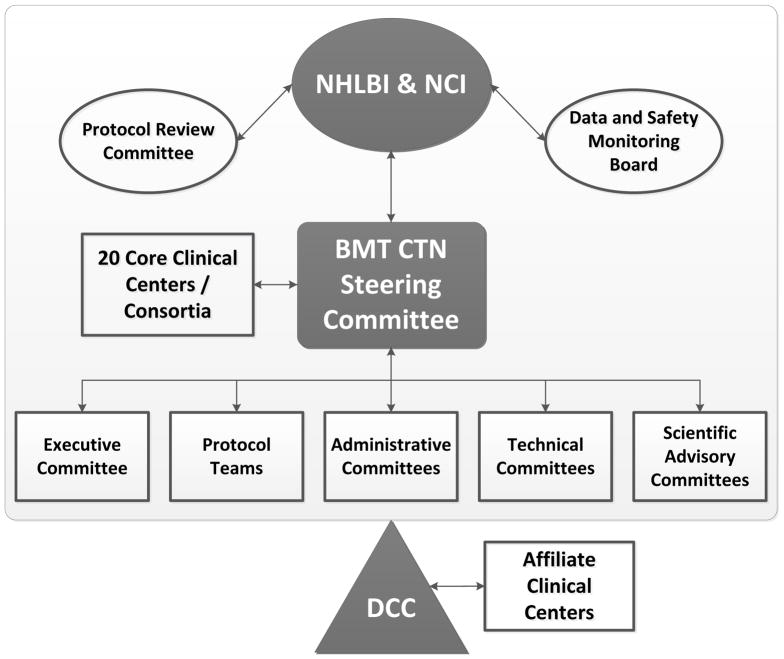
The initial structure funded 16 clinical Core Centers geographically distributed throughout the United States; several of these Core Centers were consortia of 2 or more institutions. The Data and Coordinating Center (DCC) is a consortium of three organizations, each with extensive experience in HCT: the CIBMTR, the Emmes Corporation, and the National Marrow Donor Program (NMDP)/Be The Match. The CIBMTR is a collaborative research program of the Medical College of Wisconsin, Milwaukee, and NMDP/Be The Match, Minneapolis, with offices on both campuses. Non-CIBMTR departments of NMDP/Be The Match handle contracts and finances for the Network. The Emmes Corporation is a contract research organization in Rockville, Maryland that managed two previous national HCT trials funded by NHLBI: The T-Cell Depletion Trial in Unrelated Donor Marrow Transplantation (6) and The Cord Blood Transplantation Trial (7). The DCC grant was awarded to the Medical College of Wisconsin with subcontracts to NMDP/Be The Match and Emmes. Today the BMT CTN is in its third grant cycle. Although subsequent cycles brought some changes, including increasing the number of Core Centers to 20, the basic structure is the same [Figure 1].
Figure 1.
BMT CTN Organizational Structure.
The BMT CTN Steering Committee sets the scientific agenda and oversees selection, design, execution, and analysis of all BMT CTN studies. The BMT CTN Steering Committee includes the Principal Investigator (PI) of each Core Center or Consortium and the DCC, the NHLBI Project Officer, the NCI Project Officer, a representative of each of the NCI-funded cooperative groups, and representatives of Affiliate Centers that meet standards for exemplary participation in BMT CTN trials.
Protocols are developed by Protocol Teams, each composed of 2 or more Protocol Co-Chairs, 5–7 other investigators, an NHLBI and an NCI representative, a DCC Protocol Officer who is an MD with clinical trials training and experience, a DCC Protocol Coordinator, a DCC statistician and an NHLBI statistician. Protocol development begins after a concept (presented at a very early stage of development) is accepted by the Steering Committee and is facilitated by weekly conference calls and, recently, by one or more in-person meetings of the Protocol Team.
Independent review committees appointed by NHLBI provide additional oversight for BMT CTN trials: 1) the Protocol Review Committee, which evaluates each study for scientific merit; and 2) two Data and Safety Monitoring Boards (DSMB), each responsible for about half of the Network portfolio. Each of these includes a Chair and Members with expertise in biostatistics, clinical trials, bioethics, HCT and specific disease areas of Network studies.
Accomplishments
Since launching its first trial in November 2003, the BMT CTN has an outstanding record of accomplishments. A few (current through October 2015) are listed below.
-
The Network opened 37 clinical trials addressing important issues in the treatment of patients with life-threatening blood disorders; to date, the Network has enrolled >8,300 participants from >120 centers on these trials.
BMT CTN was the lead group for 30 trials; 5 were developed in collaboration with other NIH-funded groups and 2 in collaboration with R01-funded investigators;
Twenty-four trials (19 led by BMT CTN) completed enrollment and patient follow-up; two trials completed enrollment but follow-up is ongoing; 11 are still enrolling patients.
-
BMT CTN trials have a high rate of accrual success and impact.
Only two BMT CTN-led trials were closed for poor accrual; of note, both led to peer-reviewed publications (8,9);
Sixteen of the other 19 BMT CTN-led trials with completed accrual finished within 6 months of projections;
BMT CTN 0201 was the largest randomized trial of unrelated donor transplantation and showed that using bone marrow versus peripheral blood grafts reduces chronic GVHD and improves long-term quality of life (10).
BMT CTN 0303, a study of CD34-selected allografts for AML, led to the first Food and Drug Agency approval of a cell-selection device for clinical use in the US (11)
BMT CTN 0603 was the first multicenter trial to show the safety and effectiveness of related haploidentical bone marrow transplantation, resulting in dramatic increases in use of this HCT approach (3).
BMT CTN 0502/CALGB 100701 demonstrated the efficacy of related and unrelated donor transplantation with reduced intensity conditioning in older patients with AML (4).
BMT CTN 0704/CALGB 100104 proved the activity of lenalidomide as post-transplant maintenance therapy for multiple myeloma, demonstrating a benefit in both progression-free and overall survival (5). An ongoing trial, BMT CTN 0702, builds on this trial to address the role of posttransplant consolidation and second transplantation in an era when almost all patients receive posttransplant maintenance.
The Network developed a research repository that currently houses >350,000 donor and recipient specimens; these specimens and linked data are made available to qualified investigators (www.bmtctn.net – resource materials). To date, there are eight ancillary publications resulting from analyses of these samples and data (12–19). One resulted in a novel biomarker for outcome of acute GVHD and is currently being used by the Network to stratify patients for intervention studies (14, 18). Ancillary studies are encouraged by Network RFAs that solicit concise concepts that are quickly reviewed and, where appropriate, awarded, with awardees monitored closely for productivity.
The Network incorporated standard assessment of patient-reported outcomes into its trials and has conducted one trial specifically assessing post-transplant quality of life (20).
-
The Network published 57 peer-reviewed manuscripts, including 16 primary results papers (3–5, 8–11, 20–28)) and 41 other papers addressing diverse issues of disease biology, defining and staging posttransplant complications, state of the science, patient-reported outcomes, cost-effectiveness and trial design (2, 12–19, 29–60).
-
Seventeen of 19 BMT CTN-led studies reaching primary endpoint are published or submitted for publication; two studies completed in the last year will be submitted soon.
The median time from reaching primary endpoint to having an analysis available for review by the protocol team was 27 days.
Eighty-one percent of completed studies had a primary manuscript submitted within a year of final data analysis; the median time to submission was 7.6 months.
In a study done by NHLBI to evaluate impact of Network publications versus other publications addressing similar issues, 63% of the Network papers published before 2013 (the most recent date for which this analysis was performed), had an impact score in the top 5th percentile; 86% had an impact score in the top 20th percentile.
-
The Network leveraged NIH support to develop public-private collaborations that have provided an additional $332,000,000 of in-kind and $36,000,000 of direct support for BMT CTN trials.
Keys to Success
In many ways, the structure and operations of the BMT CTN are similar to those of many other multicenter trials groups. Yet, its accrual and publication productivity are superior to that observed in several other Networks during the same time period (61). The Institute of Medicine noted that only 60% of the trials launched by the NCI-funded cooperative groups before 2010 achieved minimum accrual goals. A series of reports by Dilts, et al, described extremely long times from concept to activation for Phase III cancer trials ranging from 1.25 to almost 7 years, due to complex bureaucratic review processes involving multiple oversight bodies (62–65). These critiques led to a major reorganization of the cancer clinical trials infrastructure which aims to improve efficiency and productivity. A recent publication reported that results of less than two-thirds of NHLBI-funded cardiovascular trials were published within 30 months of completion (66). Another recent study of US academic medical centers found only 36% of completed trials with results published within 24 months of completion (67). In considering the history of the BMT CTN, there are unique features that helped the Network achieve its goal: completing important multicenter trials addressing critical issues in HCT.
Streamlined infrastructure
Although largely similar to other networks, there are a few structural differences. The BMT CTN resisted the temptation to have standing disease- or modality-focused committees with primary responsibility for setting the scientific agendas in their areas. Instead, this responsibility resides with the larger Steering Committee, guided by community input garnered, in large part, from periodic State of the Science Symposia (see below). Committees are formed ad hoc to plan the State of the Science Symposia, to act on the Symposia’s conclusions, and to address other issues as necessary (e.g., review the portfolio of studies in GVHD, consider conditioning regimen dose modifications for obese patients). The Steering Committee also considers input from other relevant sources such as the NHLBI’s recent strategic visioning initiative (http://www.nhlbi.nih.gov/about/documents/strategic-visioning). The Network’s five standing Technical Committees (Biomarkers, Special Populations, Toxicity and Supportive Care, Pharmacy and Clinical Research Associates Committee) have specific roles in protocol development and review, but not in setting the scientific agenda. The lack of standing committees avoids expectations to have a protocol in every topic area and brings a broader perspective to assigning priority. It also decreases the administrative burden of committee support for the DCC. It should be acknowledged that this is possible because HCT is already a relatively specialized area and the Steering Committee itself possesses a breadth of knowledge and expertise that allows intelligent discussion of most issues and of most diseases for which HCT is a potential treatment. The Committee is quick to invite additional expertise for those topics that require it.
Governance of the Steering Committee is also unique. The Chair position is rotated, with members electing individuals to a non-renewable two-year term, preceded by two-years as vice-chair and one year as chair elect and succeeded by a one year term as past-chair. Consequently, during its almost 15 year existence, the Network has had eight chairs from eight different institutions (Table 2). Each made unique contributions to the Network and each gained a deeper understanding of the issues faced in implementing the Network’s scientific agenda, augmenting their capacity for continued contributions on the Steering Committee. This rotating approach increases the sense of ownership of the membership, since leadership positions are not concentrated in just a few people from a restricted number of centers.
Table 2.
BMT CTN Steering Committee Chairs
| Chair | Institution | Term |
|---|---|---|
| John R. Wingard, MD | University of Florida, Gainesville | 2001–03 |
| Daniel J. Weisdorf, MD | University of Minnesota, Minneapolis | 2004–05 |
| James L. Ferrara, MD | University of Michigan, Ann Arbor1 | 2006–07 |
| Joseph H. Antin, MD | Dana Farber Cancer Institute, Boston, Massachusetts | 2008–09 |
| Sergio A. Giralt, MD | MD Anderson Cancer Center, Houston, Texas2 | 2010–11 |
| Ginna G. Laport, MD | Stanford University, Palo Alto, California | 2012–13 |
| Frederick R. Appelbaum, MD | Fred Hutchinson Cancer Center, Seattle, Washington | 2014–15 |
| Steven M. Devine, MD | The Ohio State University, Columbus | 2016–17 |
| Richard J. Jones, MD3 | The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland | 2018–19 |
Currently at Mount Sinai Medical Center, New York, New York
Currently at Memorial Sloan Kettering Cancer Center, New York, New York
Currently Vice-Chair
Inclusivity and Collaboration
BMT CTN Core Centers include large and medium-sized, adult and pediatric centers that are widely geographically distributed (Table 3). Seven of the 20 Core Centers are Consortia that comprise, in total, 22 centers. The Pediatric Blood and Marrow Transplant Consortium is also a BMT CTN Core Center and comprises more than 70 US pediatric HCT programs, 61 of which have enrolled patients on Network protocols. Additionally, the BMT CTN encourages and facilitates broad participation of the HCT community through its Affiliate Center system, whereby centers apply to participate in specific protocols through BMT CTN’s public website (bmtctn.net). About 20% of the BMT CTN’s overall accrual comes from Affiliate Centers. Additionally, investigators from Affiliate Centers are invited to serve on State of Science committees (see below) and may propose new studies using the same process as the members of Core Centers or Consortia, also found on the public website. The BMT CTN also encourages investigators from Affiliate Centers to serve on Network committees and protocol teams. BMT CTN Steering Committee meetings are open to investigators from Affiliate Centers and those that accrue at least 12 patients annually onto at least two studies are given voting membership on the Committee. The Network also encourages involvement of junior investigators, from both Core and Affiliate Centers, on protocol teams and other Network committees. Some of these early-mid career investigators recruited to participate in the Network subsequently become part of the Network leadership, including the current Steering Committee chair.
Table 3.
BMT CTN Core Centers and Principal Investigators
Baylor College of Medicine / Methodist Hospital (Consortium); PI: Helen Heslop, MD, DSc (Hon)
|
Case Western Reserve University Ireland Cancer Center (Consortium); PI: Hillard Lazarus, MD
|
| City of Hope National Medical Center, Duarte, California; PI: Ryo Nakamura, MD |
Dana Farber / Partners in Cancer Care (Consortium); PI: Joseph Antin, MD
|
| Duke University Medical Center, Durham, North Carolina; PI: Joanne Kurtzberg, MD |
| Fred Hutchinson Cancer Research Center, Seattle, Washington; PI: Frederick Appelbaum, MD |
| H. Lee Moffitt Cancer Center, Tampa, Florida; PI: Claudio Anasetti, MD |
| Johns Hopkins University Oncology Center, Baltimore, Maryland; PI: Richard Jones, MD |
| Memorial Sloan-Kettering Cancer Center, New York, New York; PI: Sergio Giralt, MD |
| Northside Hospital, Atlanta, Georgia; PI: Asad Bashey, MD, PhD |
Ohio State University Comprehensive Cancer Center (Consortium); PI: Steven Devine, MD
|
| Pediatric Blood & Marrow Transplant Consortium, 70 centers in the US and Canada; PI: Michael Pulsipher, MD |
| Stanford Hospital and Clinics, Palo Alto, California; PI: Robert Negrin, MD |
University of Florida College of Medicine (Consortium); PI: John Wingard, MD
|
University of Michigan Medical Center Consortium; PI: Gregory A. Yanik, MD
|
| University of Minnesota, Minneapolis; PI: Daniel Weisdorf, MD |
University of Nebraska Medical Center (Consortium); PI: Julie Vose, MD
|
| University of Pennsylvania Hospital, Philadelphia; PI: Edward Stadtmauer |
| University of Texas MD Anderson Cancer Center, Houston; PI: Amin Alousi, MD |
| Washington University, St. Louis, Missouri; PI: Peter Westervelt, MD, PhD |
The BMT CTN collaborates with other networks with overlapping missions, including the NCI cancer cooperative groups (the National Clinical Trials Network, or NCTN). Seven of the trials opened by the BMT CTN were led by an NCI cooperative group or were separately funded by NCI grant. One or more NCI cooperative groups participated in 6 BMT CTN-led trials. Additionally, the Network has collaborated with the AIDS Malignancy Consortium, the Canadian Blood and Marrow Transplant Group, the National Institute of Allergy and Infectious Diseases, the National Institute on Minority Health and Health Disparities, the NIH Office of Rare Diseases Research, and the Sickle Cell Disease Clinical Research Network for a variety of trials. The philosophy of the Network is that there are limited resources for multicenter trials; these are used most efficiently when the community collaborates rather than competes so that each trial accrues on schedule. For example, the Network delayed opening BMT CTN 0702 while it collaborated with The Alliance to complete CALGB 100104/BMT CTN 0704 (see above).
Inclusivity and collaboration also mark the processes for setting the scientific agenda. A diverse group of voices contribute to deliberations of the BMT CTN Steering Committee. This group has worked together to build a scientific agenda that is compelling and feasible and that considers proposals from individuals and groups outside the Network. The scientific agenda includes consideration of the need for a network to address a pressing problem, gap areas within the missions of NHLBI and NCI, patient availability and referral patterns, and competing studies. BMT CTN largely sets its longer-term scientific agenda at periodic State of the Science Symposia, which also solicits input from the broader scientific community (33,49,55). The structure for these Symposia involves constituting disease- and issue-specific committees with members from diverse HCT and non-HCT backgrounds. These committees deliberate for several months prior to an open, in-person forum, to identify the most promising areas for HCT trials and to develop compelling study proposals in those areas. Proposals are presented at the forum, with reviews provided by external reviewers (including international experts) and with opportunities for public comment. A planning committee comprising committee chairs, NIH representatives, and external reviewers then prioritizes the trial concepts. Three State of the Science Symposia were held in 2001, 2007 and, most recently, in 2014. Although the proceedings of the initial symposium in 2001 (which preceded establishment of the Network) were not published, the BMT CTN completed nine of its recommended studies. Trial prioritizations from the 2007 Symposium are well documented (33) as are the outcomes (49): 9 of the 11 trials recommended were undertaken with four completed and five ongoing. The 2014 State of the Science Symposium was the largest to date, with 13 committees including 112 committee members, 20 external reviewers, and more than 300 attendees at the open forum. Ultimately 12 concepts were prioritized (55). The BMT CTN is now developing many of these studies, with several scheduled to open in 2016.
Access to the CIBMTR Database
A key asset of the BMT CTN is access to comprehensive, current clinical data about the population for whom most of its trials are intended. The CIBMTR maintains, with separate support of the NIH and the Health Resources and Services Administration, and aided by a 2005 federal requirement for reporting HCT outcomes data, an observational database of information on almost all of the HCTs performed in the US (www.cibmtr.org). This database provides invaluable information that allows the BMT CTN to assess the feasibility of study proposals, to consider potential efficacy of new agents, to design protocols with appropriate inclusion criteria and outcome estimates, and to recruit centers likely to accrue. Once trials are opened, the database is used to promptly and effectively address accrual barriers by assessing characteristics of persons who do and those who do not enroll on the trial. Data collected by the CIBMTR also complement data collected through the BMT CTN’s clinical trials system, thereby easing the data-reporting burden for centers, though requiring additional coordination and technical interfaces between the two data systems. Since the CIBMTR follows transplant recipients long-term, a separate long-term follow-up system is not necessary for patients on BMT CTN trials. The value of having CIBMTR data to design, conduct and monitor studies that can successfully accrue cannot be under-estimated and is perhaps the most unique and important among the features contributing to the Network’s success. Innovative uses of the CIBMTR database to complement trials are being further explored, including using CIBMTR registry data to dramatically minimize data collection for quality of life trials (BMT CTN 0902) and prospective enrollment of controls for comparison to data in Phase II trials (BMT CTN 1203).
Accountability
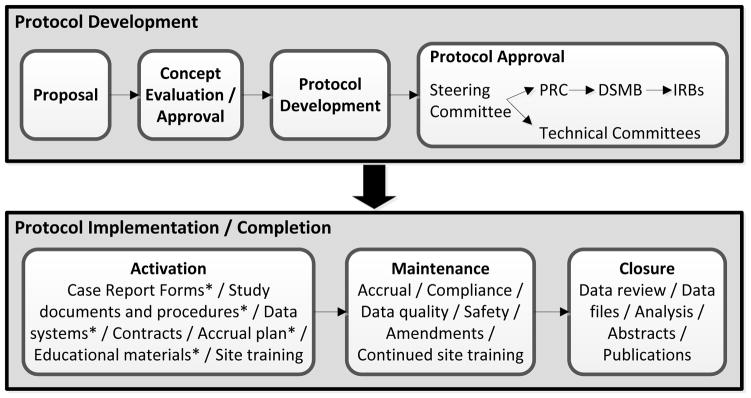
The BMT CTN has well-defined metrics for all aspects of protocol development, implementation and completion. These are used to focus individual Protocol Teams and the Network on ensuring the success of each study, to quickly identify when obstacles to success arise and to implement effective remedies. Protocol development and completion (Figure 2). Concepts for protocols are accepted for review at a very early stage of development, generally before a well-defined statistical or treatment plan is developed. (This contrasts to the current requirement for concepts submitted to the NCI disease-specific steering committees for implementation in the NCTN, which require a higher degree of development.) Consequently, careful oversight of the development process is necessary to avoid long delays to implementation. Once the Steering Committee approves a concept to be developed into a protocol, a protocol team is assembled (see above); the benchmark for having protocol documents ready for submission to the Protocol Review Committee is six months. The median time has decreased from 8.5 to 4.6 months over the life of the Network and most protocols now meet the benchmark, though a few have been delayed by need for FDA approval or contract negotiations with pharmaceutical or biotechnology companies.
Figure 2.
Schema for protocol development and implementation.
* Work on these documents / tasks begins during the protocol development phase and is generally close to completed by the time the first Institutional Review Board approvals are available.
The two-step review process by the Protocol Review Committee and DSMB is generally efficient, taking a median of 2.6 months. Review by the BMT CTN’s Technical Committees occurs in parallel. The benchmark for activation once a protocol is released to centers is five months; the current median is 4.7 months.
Each protocol is accompanied by an accrual plan that includes projected quarterly accrual numbers and a detailed blueprint for ensuring that projections are met. This plan can include recruitment of specific affiliate centers, development of patient and physician educational materials, outreach to patient advocacy groups, and targeted presentations at transplant and referral centers and key conferences. Actual versus projected accrual rates are available on the BMT CTN’s private website and updated nightly. They are reviewed monthly by the Protocol Teams with early institution of actions to address less-than-expected accrual. As noted above, these are assisted by analyses of the CIBMTR database.
A plan for reviewing endpoint data is also part of protocol development. Usually this begins early in the life of the trial to avoid lengthy delays for data review after the last patient reaches primary endpoint. In general, a data set is available for review by the protocol team within one month of the latter event (median time 27 days). The Network benchmark for submitting a manuscript after receiving the final data set is nine months; first authorship can be reassigned from investigators that fail to meet this benchmark. The median time is 7.6 months (range, 1.8 to 15.7). As noted above, 81% were submitted less than a year after final data analysis. Protocol teams are also encouraged to plan ancillary papers early in the protocol development process and to consider additional ideas for secondary analyses as data become available. Current and historical times for steps of the protocol development and completion process are shown in Figure 3.
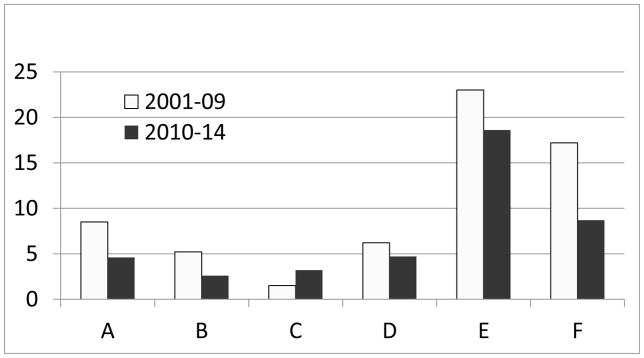
Figure 3.
Timelines for Steps in the Protocol Development and Activation Process: A. Time from Protocol Team Formation to Submission of Protocol to Protocol Review Committee (PRC); B. Time from Submission to the PRC to Approval by the Data Safety and Monitoring Board (DSMB); C. Time from DSMB Approval to Activation; D. Time from Protocol Team Formation to Activation; E. Time from Reaching Primary Endpoint to Manuscript Submission. All times are in months.
Development of Robust Data Collection Processes and Efficient Safety Monitoring Procedures
The first tasks of the Network were aimed at developing tools for the efficient collection of data from inherently difficult studies. Committees made up of experts in HCT outcomes designed a core set of case report forms for collection of acute and chronic graft-versus-host disease, post-transplant toxicities, engraftment, relapse, infection and immune reconstitution. This core set of case report forms are still in use today, constituting a consistent and streamlined data collection process. Reporting adverse events in the HCT setting has the potential to overwhelm the pharmacovigilance system but the Network developed an efficient model where only unexpected grades 3–5 adverse events are reported in an expedited manner while all expected events are reported on calendar-driven case report forms. This lean adverse event reporting process allows the Network to separate the background noise from the plethora of expected events after HCT while not compromising safety of study participants. The use of independent Medical Monitors (typically transplant physicians or disease matter experts) allows unbiased review of unexpected (or more frequent than usually expected) events and was instrumental in early detection of events that led to closing the umbilical cord blood cohort of an unrelated donor transplant trial for sickle cell disease and exclusion of busulfan-conditioning regimens from a trial evaluating sirolimus for GVHD prophylaxis after treatment of only eight and ten subjects, respectively (42, 25).
Center Performance
Successfully completing trials requires not only an efficient infrastructure but, more importantly, a cadre of high-performing centers committed to rapid activation and efficient accrual to trials that address important issues. Performance of BMT CTN centers is formally assessed annually in a report that evaluates scientific contributions, activation times, data quality and timeliness, protocol and laboratory compliance and accrual. Accrual rates are judged not only by the absolute number enrolled on a study, with a minimum number required from each center, but on how closely centers meet their individual projections. The latter has led to more careful consideration of accrual potentials by centers, which leads to more realistic accrual projections by the DCC and more rational decisions for protocol implementation by the Network. Center Performance Reports are shared openly at the annual February Steering Committee meeting; no names are changed to protect the innocent (or guilty). Centers failing to meet metrics in any area must submit a corrective action plan. Consistent failure to meet metrics can result in expulsion from the Network. In addition to ensuring timely accrual, the Network has, through this process, improved its performance on data quality and protocol and laboratory compliance, with all of the 20 Core Centers meeting standards in these areas in the 2015 assessment. Similar assessments are done for Affiliate Centers; past performance impacts the decision to allow participation in future protocols.
Adequate financial support for patient enrollment and good financial stewardship
Enrolling and monitoring patients on clinical trials requires substantial investment of time and resources. This is especially true for HCT trials, which require extensive data collection for the many adverse events expected in the early post-transplant time period. Most BMT CTN trials also require multiple laboratory assessments both to monitor toxicity and to better understand the molecular and immunologic determinants of outcomes. A formal assessment of the costs of enrolling patients and of meeting protocol requirements is done for each trial and per patient reimbursement rates are set by that assessment. Two studies of the actual time spent by center staff on trial activities have been done during the life of the Network to refine these estimates. The goal is to have participation in BMT CTN trials be cost-neutral, removing negative (or positive) financial incentives for patient enrollment so that decisions to activate are made on scientific grounds and compliance with protocol requirements is facilitated. This is important in an era when health care institutions face increasing financial constraints and pursuing activities that lose money becomes more and more untenable.
Together with a commitment to provide adequate reimbursement for trial-related costs, the Network has a commitment to provide careful oversight of limited NIH funds. The total amount of dollars available for trials for each funding period is known at the outset and the DCC has developed a budgeting model that allows the costs of each study to be forecast. Trial costs are reviewed quarterly, since changes in speed of accrual and protocol modifications can alter projected costs. The amount of money committed to existing or developing protocols and the amount available for new protocols are reviewed at each in person Steering Committee meeting. This transparency leads to accountability. The entire Steering Committee understands the Network’s financial limitations and takes responsibility for planning studies within a well-defined budget and within foci related to the distinct mission of each sponsor. This understanding also provides strong incentive for pursuing public-private partnerships that extend the power of that well-defined budget to address issues that would be otherwise impossible to support given the total NIH allocation. Such partnerships have increased the Network’s funding by >$360,000,000.
Lessons Learned and Areas for Improvement
Although we are proud of our accomplishments, it should be noted that this Network evolved over time and many of the processes described above grew out of painful experience. It took two years for the Network to launch its first trial in 2003! A few lessons from that and subsequent experiences were:
Do as many steps as possible in the protocol development process in parallel rather than sequentially;
-
Resistance to accrual can be triggered by seemingly trivial protocol requirements (remember, one person’s “trivial” is another person’s “critical”), so
Have supportive care practices not critical to the primary question implemented “per institutional protocol” as much as possible;
Try to understand the work flow implications of pre- and posttransplant evaluations, including the need for visits that might not otherwise occur;
Only perform the tests and collect data that are essential to the goals of the trial – “nice to have” is not a good rationale for a test or a data point;
Most HCT trials will need many centers to accrue successfully so this should be planned for from the beginning;
Never underestimate how long it will take to execute contracts with contributors and start early;
Stay aware of potentially competing trials – which means communicating with the community often and effectively; find ways to collaborate rather than compete whenever possible;
Encourage planning for ancillary studies and secondary analyses early, rather than waiting until the trial is complete.
Still problematic for the Network is efficient activation of trials at the individual center level, where protocols are subject to institution-specific processes and timelines for review and approval. Although the Network implemented some approaches to speed this process, such as early identification of a protocol champion at each center, frequent communication and close monitoring of each center’s progress, success has been variable. The Network is now piloting use of a single Institutional Review Board of record for some trials to try to speed this. Even the areas in which we think have effective processes require continuous monitoring and diligence to avoid falling into back into habits that delay development, activation and accrual.
The Future
HCT is a complex therapy and conducting effective multicenter trials must address complex issues. The Network has proven its ability to do this. It is now using this expertise to study alternative donor approaches able to extend HCT to populations with non-malignant diseases and restricted donor sources, e.g., patients with hemoglobinopathies and with marrow failure, as well as to patients with blood cancers. Two trials in sickle cell disease, one evaluating the effectiveness of HLA-matched related or unrelated donor transplantation as compared to standard of care in young adults with severe disease and the other the feasibility of HCT using HLA-mismatched related donors, are planned for 2016. Another trial will evaluate use of umbilical cord blood grafts and use of HLA-mismatched bone marrow grafts for patients with aplastic anemia. Trials in these rare blood disorders would not be feasible without the Network’s infrastructure and expertise. As novel approaches to cellular therapy of both malignant and non-malignant disease are developed, many of which focus on the same diseases as HCT, the Network is well-positioned to evaluate these approaches in the multicenter setting. It is already beginning to do so. In 2016, the Network will launch a study to evaluate the efficacy of a dendritic cell vaccine in improving response after autotransplantation for multiple myeloma. It is also planning trials using CAR-T cells and NK cells in the pre- and post-transplant settings as well as trials combining HCT with targeted anti-cancer therapies, based on emerging data that the activity of many novel therapies is augmented in the setting of a non-tolerant immune system as provided by an allograft. An example is the planned BMT CTN 1506 study which is the first trial testing, in a prospective randomized fashion, pilot data from several centers on the activity of FLT3 tyrosine kinase inhibition as maintenance therapy after allogeneic HCT for FLT3 ITD AML. Continued federal support and continued collaboration with the scientific community will allow us to leverage our infrastructure to facilitate translation of a growing understanding of molecular genetics and immunobiology into advances in clinical care and outcomes.
Highlights.
The BMT CTN was established in 2001 to conduct multicenter trials addressing important BMT issues.
It has opened 37 trials and published 57 papers addressing diverse issues in malignant and non-malignant disorders.
The scientific agenda is developed with broad input from the BMT community.
Participation is wide with >120 centers enrolling >8,000 patients over the Network’s history.
Use of the CIBMTR’s large observational database for trial planning contributes to success.
Acknowledgments
This work was supported by U10HL069294, U10HL069256, U10HL069249, U10HL069254, U10HL069274, U10HL069278, U10HL069286, U10HL069290, U10HL069291, U10HL069301, U10HL069310, U10HL069315, U10HL069330, U10HL069334, U10HL069348, U10HL108945, U10HL108987, U10HL109137, U10HL109322, U10HL109526, U10HL069233, U01HL069273 from the National Heart, Lung, and Blood Institute and the National Cancer Institute; U24-CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases; contract HHSH250201200016C with Health Resources and Services Administration. The BMT CTN Steering Committee includes the following individuals: Amin M. Alousi, MD Anderson Cancer Center, Houston; Claudio Anasetti, Moffitt Cancer Center, Tampa; Joseph H. Antin, Dana Farber Cancer Institute, Boston; Frederick R. Appelbaum (Immediate Past-Chair), Fred Hutchinson Cancer Research Center, Seattle; Asad Bashey, The Blood and Marrow Transplant Program at Northside Hospital, Atlanta; Dennis L. Confer, National Marrow Donor Program, Minneapolis; Steven M. Devine (Chair), Ohio State University Medical Center, Columbus; Nancy L. DiFronzo, National Institutes of Health-National Heart, Lung, and Blood Institute, Bethesda; Sergio A. Giralt, Memorial Sloan Kettering Cancer Center, New York; Stephan N. Grupp, Children’s Hospital of Philadelphia, Philadelphia; Parameswaran N. Hari, Medical College of Wisconsin, Milwaukee; Helen E. Heslop, Baylor College of Medicine, Houston; Mary M. Horowitz, Medical College of Wisconsin, Milwaukee; Richard J. Jones (Vice-Chair), The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore; Joanne Kurtzberg, Duke University Medical Center, Durham; Hillard M. Lazarus, Case Western Reserve University, Cleveland; Robert Lowsky, Stanford Hospital and Clinics, Stanford; Adam M. Mendizabal, The Emmes Corporation; Rockville; William Merritt, National Institutes of Health-National Cancer Institute, Bethesda; Ryotaro Nakamura, City of Hope National Medical Center, Duarte; Michael A. Pulsipher, Children’s Hospital of Los Angeles, Los Angeles; Voravit Ratanatharathorn, Karmanos Cancer Institute, Detroit; Edward A. Stadtmauer, University of Pennsylvania, Philadelphia; Patrick J. Stiff, Loyola University Medical Center, Maywood; Julie M. Vose, University of Nebraska Medical Center, Omaha; Daniel J. Weisdorf, University of Minnesota Medical Center, Minneapolis; Peter Westervelt, Washington University, St. Louis; John R. Wingard, University of Florida, Gainesville; Gregory A. Yanik, University of Michigan, Ann Arbor.
The Steering Committee would also like to acknowledge the following individuals who have greatly contributed to the success of the Network: Shelly Carter, Mary Eapen, Nancy Geller, Iris Gersten, Eric Leifer, Brent Logan, Marcelo Pasquini, Elizabeth Wagner and Roy S. Wu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Reilly RJ. Clinical Trials of Hematopoietic Cell Transplantations: Current Needs and Future Strategies. Biol Blood Marrow Transplant. 2000;6(2):79–89. doi: 10.1016/s1083-8791(00)70070-x. [DOI] [PubMed] [Google Scholar]
- 2.Weisdorf D, Carter S, Confer D, et al. Blood and Marrow Transplant Clinical Trials Network (BMT CTN): Addressing unanswered questions. Biol Blood Marrow Transplant. 2007;13(3):257–262. doi: 10.1016/j.bbmt.2006.11.017. discussion 255–256. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation: results of parallel Phase II trials using HLA-mismatched related bone marrow or unrelated umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devine SM, Owzar K, Blum W, et al. A Phase II Study of Allogeneic Transplantation for Older Patients with AML in First Complete Remission Using a Reduced Intensity Conditioning Regimen: Results from CALGB 100103 (Alliance)/BMT CTN 0502. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.7273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy PL, Owzar K, Hofmeister CC, et al. Phase III study of lenalidomide versus placebo after HCT for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavletic SZ, Carter SL, Kernan NA, et al. Influence of T-cell depletion on chronic graft-versus-host disease: results of a multicenter randomized trial in unrelated marrow donor transplantation. Blood. 2005;106(9):3308–3313. doi: 10.1182/blood-2005-04-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtzberg J, Prasad VK, Carter SL, et al. COBLT Steering Committee. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112(10):4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17(7):1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: Enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: Blood and Marrow Transplant Clinical Trials Network Protocol 0403. Biol Blood Marrow Transplant. 2014;20(6):858–864. doi: 10.1016/j.bbmt.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anasetti C, Logan BR, Lee SJ, et al. Blood and Marrow Transplant Clinical Trials Network. Peripheral blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell depleted peripheral blood stem cell transplantation for acute myeloid leukemia in first remission: results of the BMT CTN protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute GVHD treatment from the BMT CTN. Biol Blood Marrow Transplant. 2010;16(3):421–429. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(®) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-BMT CTN Protocol 0303. Biol Blood Marrow Transplant. 2012;18(5):690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine J, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a BMT CTN study. Blood. 2012;119(16):3854–3860. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189(10):5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolanos-Meade J, Wu J, Logan BR, et al. Lymphocyte phenotype during therapy for acute graft versus host disease: a brief report from BMT-CTN 0302. Biol Blood Marrow Transplant. 2013;19(3):481–485. doi: 10.1016/j.bbmt.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waller EK, Logan BR, Harris WA, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncology. 2014;32(22):2365–2372. doi: 10.1200/JCO.2013.54.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine JE, Braun TM, Harris AC, et al. A Prognostic Score for Acute Graft-Versus-Host Disease Based on Biomarkers: A Multicentre Study. Lancet Haematology. 2015;2(1):21–29. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtan SG, Verneris MR, Schultz KR, et al. Circulating Angiogenic Factors Associated with Response and Survival in Patients with AcuteGraft-Versus-Host Disease: Results from BMT CTN 0302 and 0802. Biol Blood Marrow Transplant. 2015;21(6):1029–1036. doi: 10.1016/j.bbmt.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen PB, Le-Rademacher J, Heather J, et al. Exercise and Stress Management Training Prior to Hematopoietic Cell Transplantation: Blood and Marrow Transplant Clinical Trial Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530–1536. doi: 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alousi A, Weisdorf D, Logan B, et al. Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute GVHD: A randomized Phase II trial from the BMT CTN. Blood. 2009;114(3):511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingard JR, Carter SL, Walsh TJ, et al. Randomized double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a Phase 3 biological assignment trial. Lancet Oncology. 2011;12(13):1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vose JM, Carter S, Burns LJ, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, melphalan (BEAM) compared with 131-Iodine tositumomab/BEAM with autologous stem cell transplantation for relapsed diffuse large B-cell lymphoma: results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0401 trial. J Clin Oncol. 2013;31(13):1662–1668. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/Sirolimus versus Tacrolimus/Methotrexate as GVHD Prophylaxis after Matched, Related Donor Allogeneic Hematopoietic Cell Transplantation. Blood. 2014;124(8):1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolaños-Meade J, Logan BR, Alousi AM, et al. Phase III Clinical Trial Steroids/Mycophenolate Mofetil vs Steroids/Placebo as Therapy for Acute Graft-versus-Host Disease: BMT CTN 0802. Blood. 2014;124(22):3221–3227. doi: 10.1182/blood-2014-06-577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner JE, Jr, Eapen M, Carter S, et al. One- versus Two-Unit Cord Blood Transplant for Leukemia. N Engl J Med. 2014;371(18):1685–1694. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderlini P, Wu J, Gersten I, et al. Cyclophosphamide conditioning in patients with severe aplastic anaemia given unrelated marrow transplantation: a phase 1–2 dose de-escalation study. Lancet Haematology. 2015;2(9):367–375. doi: 10.1016/S2352-3026(15)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wingard JR. Design issues in a prospective randomized double-blinded trial of prophylaxis with fluconazole versus voriconazole after allogeneic hematopoietic cell transplantation. Clin Infect Dis. 39:S176–180. doi: 10.1086/421953. 200. [DOI] [PubMed] [Google Scholar]
- 30.Logan BR. Optimal two-stage randomized Phase II clinical trials. Clinical Trials. 2005;2(1):5–12. doi: 10.1191/1740774505cn061oa. [DOI] [PubMed] [Google Scholar]
- 31.Ho VT, Cutler C, Carter S, et al. Toxicity Committee consensus summary, thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Keating K. Editorial: Prospective Clinical Trials in BMT Come of Age in the US: The Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2007;13(3):255–256. [Google Scholar]
- 33.Ferrara J, Anasetti C, Stadtmauer E, et al. BMT CTN State of the Science Symposium 2007. Biol Blood Marrow Transplant. 2007;13:1268–1285. doi: 10.1016/j.bbmt.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Logan B, Leifer E, Bredeson C, et al. Use of biological assignment in hematopoietic stem cell transplantation clinical trials. Clinical Trials. 2008;5(6):607–616. doi: 10.1177/1740774508098326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giralt S, Vesole DH, Somlo G, et al. Re: Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: A systematic review and meta-analysis. J Natl Cancer Inst. 2009;101(13):964. doi: 10.1093/jnci/djp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine JE, Logan B, Wu J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulsipher MA, Young NS, Tolar J, et al. Optimization of therapy for severe aplastic anemia based on clinical, biological and treatment response parameters: conclusions of an international working group on severe aplastic anemia convened by the BMT CTN, March 2010. Biol Blood Marrow Transplant. 2011;17(3):291–299. doi: 10.1016/j.bbmt.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz EM, Horowitz MM, DiFronzo NL, et al. Guidance for developing Phase II cell therapy trial proposals for consideration by the BMT CTN. Biol Blood Marrow Transplant. 2011;17(2):192–196. doi: 10.1016/j.bbmt.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohn DB, Dotti G, Brentjens R, et al. CARs on track in the clinic: workshop of the BMT CTN subcommittee on cell and gene therapy. Molecular Therapy. 2011;19(3):432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denzen EM, Burton Santibáñez ME, Moore H, et al. Easy-to-read informed consent forms for hematopoietic cell transplantation clinical trials. Biol Blood Marrow Transplant. 18(2):183–189. doi: 10.1016/j.bbmt.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamani NR, Walters MC, Carter S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the Phase II study from the BMT CTN. Biol Blood Marrow Transplant. 2012;18(8):1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolar J, Deeg HJ, Arai S, et al. Fludarabine-based conditioning for marrow transplantation from unrelated donors in severe aplastic anemia: early results of a cyclophosphamide dose deescalation study show life-threatening adverse events at predefined cyclophosphamide dose levels. Biol Blood Marrow Transplant. 2012;18(7):1007–1011. doi: 10.1016/j.bbmt.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasquini M, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logan BR, Zhang MJ. The use of group sequential designs with common competing risks tests. Statistics in Medicine. 2013;32(6):899–913. doi: 10.1002/sim.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giralt S, McCarthy PL, Anderson KC, et al. Anatomy of a successful practice-changing study: a Blood and Marrow Transplant Clinical Trials Network-National Cancer Institute Cooperative Group Collaboration. Biol Blood Marrow Transplant. 2013;19(6):858–859. doi: 10.1016/j.bbmt.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauskopf J, Chirila C, Graham J, et al. Cost-effectiveness analysis of voriconazole compared With fluconazole for prevention of invasive fungal infection in patients receiving allogeneic hematopoietic cell transplants. Am J Health Syst Pharm. 2013;70(17):1518–1527. doi: 10.2146/ajhp120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Switzer GE, Bruce JG, Harrington D, et al. Health-related QoL of bone marrow versus PBSC donors: a pre-specified subgroup analysis from a phase III RCT BMT CTN protocol 0201. Biol Blood Marrow Transplant. 2014;20(1):118–127. doi: 10.1016/j.bbmt.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara J. Blood and Marrow Transplant Clinical Trials Network: Progress since the State of the Science Symposium 2007. Biol Blood Marrow Transplant. 2014;20(2):149–153. doi: 10.1016/j.bbmt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth JA, Bensink ME, O’Donnell PV, et al. Design of a cost-effectiveness analysis alongside a randomized trial of transplantation using umbilical cord blood versus HLA-haploidentical related bone marrow in advanced hematologic cancer. J Comp Eff Res. 2014;3(2):135–144. doi: 10.2217/cer.13.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eapen M, O’Donnell P, Brunstein C, et al. Mismatched related and unrelated donors for allogeneic hematopoietic cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2014;20(10):1485–1492. doi: 10.1016/j.bbmt.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saber W, Le-Rademacher J, Sekeres M, et al. Multicenter Biologic Assignment Trial Comparing Reduced-Intensity Allogeneic Hematopoietic Cell Transplant to Hypomethylating Therapy or Best Supportive Care in Patients Aged 50 to 75 with Intermediate-2 and High-Risk Myelodysplastic Syndrome: Blood and Marrow Transplant Clinical Trials Network #1102 Study Rationale, Design, and Methods. Biol Blood Marrow Transplant. 2014;20(10):1566–1572. doi: 10.1016/j.bbmt.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atallah E, Bylow K, Troy J, et al. Treatment of older patients with high-risk myelodysplastic syndromes (MDS): The emerging role of allogeneic hematopoietic stem cell transplantation (Allo HSCT) Curr Hematol Malig Rep. 2014;9(1):57–65. doi: 10.1007/s11899-013-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard A, Fernandez-Vina MA, Appelbaum FR, et al. Recommendations for Donor HLA Assessment and Matching for Allogeneic Stem Cell Transplantation: Consensus Opinion of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2015;21(1):4–7. doi: 10.1016/j.bbmt.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Appelbaum F, Anasetti C, Antin J, et al. Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium 2014. Biol Blood Marrow Transplant. 2015;21(2):202–224. doi: 10.1016/j.bbmt.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacMillan ML, Robin M, Harris AC, et al. A Refined Risk Score for Acute Graft-versus-Host Disease that Predicts Response to Initial Therapy, Survival, and Transplant-Related Mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. doi: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khera N, Majhail NS, Brazauskas R, et al. Comparison of Characteristics and Outcomes of Trial Participants and Nonparticipants: Example of Blood and Marrow Transplant Clinical Trials Network 0201 Trial. Biology of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21(10):1815–1822. doi: 10.1016/j.bbmt.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplant, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant. 2015;21(12):2039–2051. doi: 10.1016/j.bbmt.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902) Cancer. doi: 10.1002/cncr.29717. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young JH, Logan BR, Wu J, et al. Infections following Transplantation of Bone Marrow or Peripheral-Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2015.09.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Institute of Medicine. A National Cancer Clinical Trials System for the 21st century: reinvigorating the NCI Cooperative Group Program. Washington (DC): The National Academies Press; 2010. [PubMed] [Google Scholar]
- 62.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24(28):4545–4552. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 63.Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: the Case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24(28):4553–4557. doi: 10.1200/JCO.2006.06.7819. [DOI] [PubMed] [Google Scholar]
- 64.Dilts DM, Sandler A, Cheng S, et al. Development of clinical trials in a cooperative group setting: the eastern cooperative oncology group. Clin Cancer Res. 2008;14(11):3427–3433. doi: 10.1158/1078-0432.CCR-07-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dilts DM, Cheng SK, Crites JS, et al. Phase III clinical trial development: A process of chutes and ladders. Clin Cancer Res. 2010;16(22):5381–5389. doi: 10.1158/1078-0432.CCR-10-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon DJ, Lauer MS. Publication of trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2014;370(8):782. doi: 10.1056/NEJMc1315653. [DOI] [PubMed] [Google Scholar]
- 67.Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637. doi: 10.1136/bmj.i637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laport GG, Wu J, Logan B, et al. Reduced-intensity conditioning with fludarabine, cyclophophamide, and high-dose rituximab for allogeneic heamtopoietic cell transplantation for follicular lymphoma: A phase two miulticenter trial from the Blood and Marrow Transplant Clinical Trials Network. BBMT. 2016 doi: 10.1016/j.bbmt.2016.04.014. 2016.04.014 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]