Abstract
Objective
To assess whether medial tibiofemoral joint space width (JSW) on 3D standing CT (SCT) correlates more closely with MRI cartilage morphology (CM) and meniscal scores than does radiographic 2D JSW.
Methods
Participants in the Multicenter Osteoarthritis Study (MOST), who had standing fixed-flexion PA knee radiographs, were recruited. Medial tibiofemoral 3D JSW on SCT and 2D JSW on fixed flexion radiographs were compared with medial tibiofemoral cartilage and meniscal morphology using the WORMS system. Associations between the area of the articular surface with 3D JSW <2.5mm on SCT, radiographic minimal 2D JSW and the WORMS-CM and meniscal scores were assessed using Spearman’s rho.
Results
For the 19 participants included (33 knees), mean±SD age was 66.9±5.4, BMI was 29.5±4.4 kg/m2, 42.1% were female and KL grades were 0 (21.2%), 1 (36.4%), 2 (18.2%), and 3 (24.2%). The articular surface area with 3D JSW<2.5mm on SCT correlated with WORMS-CM scores for the central medial tibia (rs=0.84, p<0.001) and central medial femur (rs=0.60, p<0.007) and posterior medial meniscal tear (rs=0.39, p<0.026), as did other cut-points for 3D JSW. Correlations with radiographic minimal 2D JSW were −0.66, −0.52, and −0.40 respectively, differing from SCT only for tibial cartilage (p=0.001).
Conclusions
Greater surface area with a low JSW, measured by SCT, correlates more strongly with the severity of tibial cartilage lesions, while correlating with medial femoral cartilage and meniscal damage to a similar extent as radiographic minimal JSW. SCT may enable valid stratification of participants in clinical trials, through quickly and inexpensively characterizing OA features.
INTRODUCTION
Radiography is one of the most commonly performed imaging examinations for diagnosis and evaluation of knee osteoarthritis (OA). With the increasing prevalence of knee OA, due in part to a rising number of older adults and in the prevalence of obesity, the use of radiography is likely to increase in the coming years. MRI is the definitive imaging modality for detailed structural evaluation. However, the insensitivity of radiographs has been a critical barrier to progress in diagnosing and advancing understanding of this disease.
Joint space width (JSW) is used to indicate knee joint health and is most often measured as the distance between the projected femoral and tibial margins on standing radiographs. The alignment of the radiographic beam with bone surfaces of the tibia has a large impact on the apparent JSW, because plain radiographs of the knees capture a 3D structure in 2D projection. Variations in the beam angle thus cause imprecision in JSW measurements. To accurately and reliably assess JSW in this manner, superimposition of the posterior and anterior edges of the tibial plateau is required. Repeated x-ray exposures at a variety of beam angles, or use of fluoroscopy to guide positioning, can be necessary to optimally visualize the JSW. This process can be time-consuming, requires repeated patient irradiation, and suffers from incorrect coronal angulation or transverse rotation that cannot be corrected following acquisition.
Despite JSW being the only acceptable outcome measure for Phase III trials in the U.S.,(1) these factors contribute to radiographs failing to detect evidence of OA in the knee for years after OA begins.(2, 3) In addition, 70% of knees without radiographic OA have evidence of cartilage damage visualized by MRI,(4) and this lack of evidence of OA on plain radiographs, contributes to low sensitivity,(5) and responsiveness(6) of radiographic JSW measurements. These limitations restrict the ability of clinicians to detect features of knee OA until joint damage is advanced and hamper assessment of the efficacy of new therapies within the usual 1–2 year time frame of clinical trials.
Advances in upright cone-beam CT have enabled imaging of the knees while standing (i.e., standing CT, or SCT). SCT images are unencumbered by overlapping anatomy, potentially improving concurrent validity with MRI and obviating the need for separate radiographic acquisitions at multiple beam angles. SCT imaging has been shown to be more sensitive and accurate for detection of osteophytes and subchondral cysts than conventional fixed-flexion radiography.(7) Both cartilage and meniscal damage are important contributors to reduction in JSW.(8) If JSW measurements from SCT reflect cartilage and meniscal morphology more validly than radiographs, then the cost and duration of knee OA clinical trials could be reduced by enabling recruitment of people with more valid baseline disease status and more sensitive evaluation of morphological outcomes.
The purpose of this study was therefore to assess whether the association between cartilage and meniscal morphology visualized by MRI (WORMS)(9) and medial tibiofemoral JSW measured on low-dose SCT is greater than the association with JSW measured on fixed-flexed radiographs.
METHODS
Participants
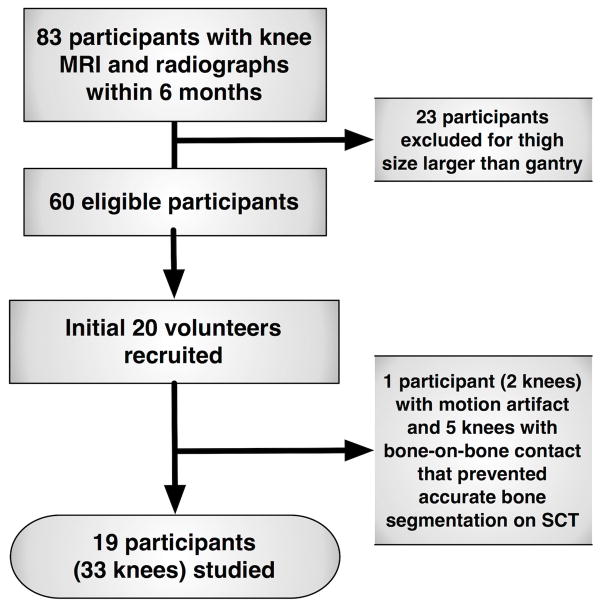
This investigation was conducted as an ancillary study following the 84-month visit of the Multicenter Osteoarthritis Study (MOST), an NIH-funded longitudinal observational study of 3026 community-dwelling men and women with knee osteoarthritis or known risk factors for knee osteoarthritis. Enrollment for MOST has been described previously.(10) Participants at the Iowa site were eligible if they had bilateral, standing fixed-flexion knee radiographs in the prior 6 months, knees discordant for Kellgren Lawrence(11) (KL) grade (to include a range of JSW), a KL grade <4 and a distal thigh width on PA radiographs that did not exceed the 38.1cm SCT gantry width. Out of 83 participants with knee MRI and radiographs within 6 months, the first 20 volunteers who met inclusion criteria were enrolled (Figure 1). All participants completed a University of Iowa institutional review board-approved informed consent process in compliance with the Helsinki Declaration.
FIGURE 1.
Flow Diagram of Participants Included
Radiographic JSW measurements
Bilateral, standing fixed-flexion posteroanterior (PA) radiographs of the tibiofemoral compartments were obtained at the 84-month MOST clinic visit(12) and were graded according to the KL grading system, as previously described(11, 12). Radiographic minimal JSW between the projected medial femoral and tibial bone margins was measured using a validated semi-automated software tool.(13, 14) This measurement of minimal JSW has previously been reported to have a root mean square standard deviation (RMSSD) of 0.16 mm when repositioning healthy knees and 0.18 mm in OA knees,(13) supporting its reproducibility.
SCT JSW measurements
A commercial scanner (PedCAT, Curvebeam LLC, Warrington, PA) was modified to enable imaging of both knees in a standing fixed-flexed configuration. A custom radiolucent positioning system was used to maintain foot external rotation and fixed knee flexion angles, with participants’ thighs and hands contacting the unit for stability and prevention of motion. The scanner produced pulsed cone-beam x-ray on a 30×30 cm amorphous silicon flat-panel detector over a 360° projection angle, with a total scan time of 32 seconds (effective dose equivalent 0.1 mSv, in comparison with ~0.04 mSv for acquisition of a set of knee radiographs). A 3D dataset with isotropic resolution of 0.37mm and FOV of 200×350mm was reconstructed from initial cone-beam projections. Reconstructed images were uploaded to a PACS as a standard DICOM CT image stack with image matrix of 768 × 768 pixels, and bones were segmented in a semi-automated manner. Medial tibiofemoral JSW was measured as the intra-articular closest distances between points on the tibiofemoral bony articular margins in the following manner.
Segmentation of tibial and femoral margins on the SCT images utilized custom MATLAB code to identify bone and non-bone portions, by applying thresholding algorithms based on the higher density of bone in comparison with the adjacent soft tissues. The segmentation masks for each sagittal slice were manually corrected to ensure the accuracy of the segmented bone models. The voxellated triangulated mesh surfaces of the tibia and femur from the raw segmentations were then lightly smoothed using Geomagic Studio software (Geomagic, Inc., Research Triangle Park, NC) to repair local errors invariably introduced during surface generation from the segmentations.
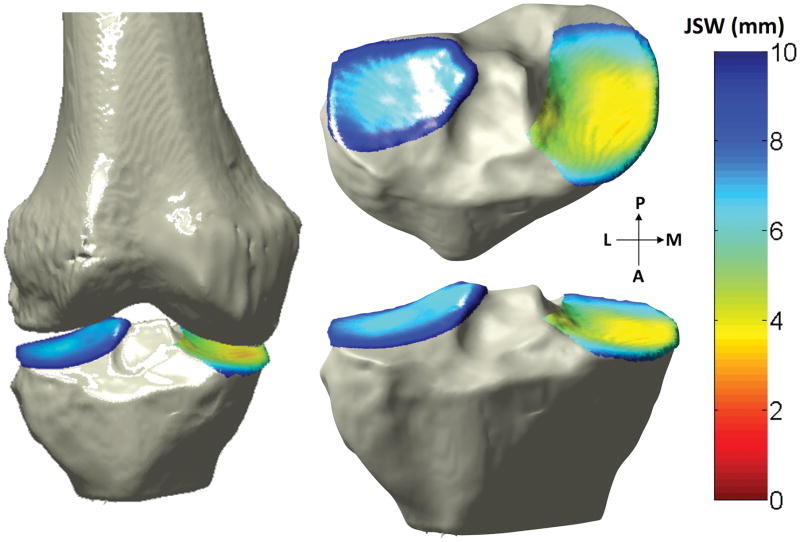
It was these smoothed tibial and femoral subchondral surfaces that were used to locate the nearest-neighboring element of the femur for each element on the tibia. The distance between each element on the tibial subchondral bone surface and its nearest neighbor on the femoral subchondral surface was defined as the 3D JSW. Each distance was assigned a color, and these colors were overlaid on the tibial articular surface, producing a color-coded map of the 3D JSW at every point on the subchondral surface (Figure 2).
FIGURE 2.
Illustration of 3D JSW measured by SCT (L=lateral, M=medial, P=posterior, A=anterior)
To facilitate comparisons of individual MRI based scorings of cartilage morphology (CM) and meniscal morphology with the approximately 18,000 measurements of 3D JSW from the SCT, a summary measure of the SCT JSW data was defined. The area of each element on the tibial surface was calculated and paired with the previously calculated distance to produce distance-surface area data. A proximity threshold of 10mm was selected in order to define the contacting regions of the joint, and the surface area of every element with a distance ≤10mm was summed to generate the total tibial subchondral area. The tibial subchondral areas of the medial and lateral compartments also were computed. Then, we calculated the percent of these areas with JSW <2.5mm, to indicate how much of the surface was in abnormally close proximity. The reproducibility of SCT measurements of medial tibiofemoral JSW measurements acquired 2 weeks apart, using these methods, had a test-retest reliability ICC of 0.97 (95%CI: 0.94–1.00), a root mean square error of 1.5% and a root mean square standard deviation of 2.7%.
MRI assessment of cartilage and meniscal morphology
Knee MRI was completed using a 1-Tesla ONI OrthOne extremity MRI scanner (GE Healthcare, Waukesha, WI). Musculoskeletal radiologists, each with greater than 10 years of experience reading knee MRI, assessed the morphology of the central medial tibiofemoral cartilage and posterior horn of the medial meniscus on axial and sagittal proton-density weighted fat-suppressed (PDFS) fast spin echo sequences and a coronal STIR sequence, using the Whole Organ MRI Scoring (WORMS) system (0–6 for cartilage morphology {CM} and 0–4 for meniscus).(9) This scoring system characterizes the presence and severity of cartilage and meniscal damage visualized by MRI. For example, for cartilage morphology, 0=normal thickness and signal; 1=normal thickness but increased T2 signal; 2=partial-thickness focal defect <1 cm in greatest width; 2.5=full-thickness focal defect <1 cm in greatest width; 3=mixture of partial-thickness defects and normal thickness; 4= partial-thickness loss ≥75% of the region; 5=multiple areas of full-thickness loss; 6=full-thickness loss ≥75% of the region. The central region of the cartilage and posterior horn of the meniscus were selected as these were the regions accounting for tibiofemoral inter-bone distances in the ~20° semi-flexed pose used for the SCT and radiographs.
Statistical Methods
We first compared 3D JSW data to WORMS-CM scores for the central medial tibia and central medial femur by generating scatter plots of the percent of 3D JSW <2.5mm vs. individual medial cartilage and meniscus WORMS scores The associations between the percent articular surface area with JSW <2.5mm and the ordinal WORMS scores were assessed using Spearman’s rho, including one randomly selected knee per participant to avoid intra-participant covariance between limbs. To assess for statistically significant differences between the correlation coefficients for (1) MRI vs. Radiographs and (2) MRI vs. SCT within the sample, Steiger’s Z-test was used and the signs for negative correlations were inverted to avoid spuriously finding significant differences.(15) Confirmatory analyses, using the percent areas with JSW <2.0mm and <3.0mm were conducted to assess for consistency of the relationship between 3D JSW and WORMS-CM. Finally, to compare ability to detect full-thickness cartilage lesions, receiver operator characteristic (ROC) curves were plotted for the sensitivity vs. (1 – specificity) for thresholds of SCT JSW (% area with JSW<2.0mm and 2.5mm) and radiographic minimal JSW for knees with both tibia and femur having full thickness cartilage lesions visualized on MRI. C-statistics for each of these prediction models were calculated. Statistical analyses were completed using SAS versions 9.2 and 9.3 (SAS Inc, Cary, NC) and an alpha level of <.05 was used for determination of statistical significance.
RESULTS
Out of the 40 knees imaged in the 20 participants who were recruited, 7 either could not be validly segmented from SCT due to motion (2 knees in 1 participant) or absence of a joint space (n = 5 knees). For the 19 participants included (33 knees), mean±SD age was 66.9±5.4 years, BMI was 29.5±4.4 kg/m2, 42.1% were female and KL grades were 0 (21.2%), 1 (36.4%), 2 (18.2%), and 3 (24.2%).
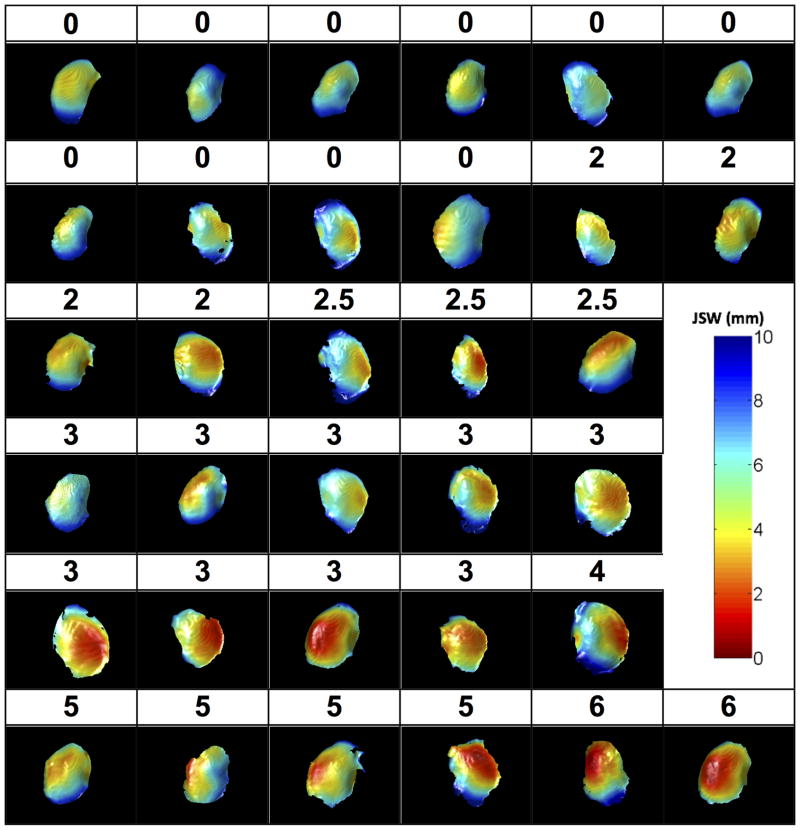
Plots of the JSW for the medial tibiofemoral compartment for each of the 33 knees along with the corresponding WORMS-CM scores for the central medial tibia are depicted in Figure 3. The percent area with JSW<2.5mm correlated very well with WORMS-CM scores for the central medial tibia (rs =.84, p <.001) and central medial femur (rs =.60, p=.007) (Table 1). Sensitivity analysis found similar correlations when using the % area with JSW<3.0mm. The correlation of minimal JSW from fixed-flexed radiographs with central medial tibial cartilage morphology (rs =−.66, p <.001) was significantly lower (p=.001; Table 1). While there was not a statistically significant association between the presence of medial meniscal extrusion diagnosed by MRI and JSW by either SCT or radiographs, there were significant associations with damage in the posterior horn of the medial meniscus. For meniscal findings by MRI, there were no significant differences between the correlations of JSW parameters by SCT vs. those for radiographs.
FIGURE 3.
Joint Space Width Distribution and Whole Organ MRI Score for Cartilage Morphology (WORMS-CM) scores for all included knees (Numbers refer to grades of cartilage morphology using the WORMS-CM grading system)
TABLE 1.
Spearman Correlations Between WORMS Cartilage Morphology and Meniscal Scores, and JSW Measurements on SCT and Radiographs
| WORMS Cartilage Morphology | Central Medial Femur | Central Medial Tibia | Medial Meniscal Extrusion | Medial Meniscal Tear, Posterior |
|---|---|---|---|---|
| % Joint Area with JSW<2.5mm on SCT | rs = .60 p=.007 |
rs = .84* p <.001 |
rs =.29 p=.097 |
rs =.39 p = .026 |
| % Joint Area with JSW<3.0mm on SCT | rs = .62 p=.005 |
rs = .82* p <.001 |
rs =.32 p=.068 |
rs =.40 p = .022 |
| Radiographic Minimum JSW (mJSW) | rs = −.52 p = .002 |
rs = −.66 p <.001 |
rs =.31 p=.076 |
rs = −.40 p = .018 |
p-values for comparison of rs for SCT vs. MR with the rs for radiographic mJSW vs. MR were ≤ .001. P-values for all other comparisons of rs were > .2.
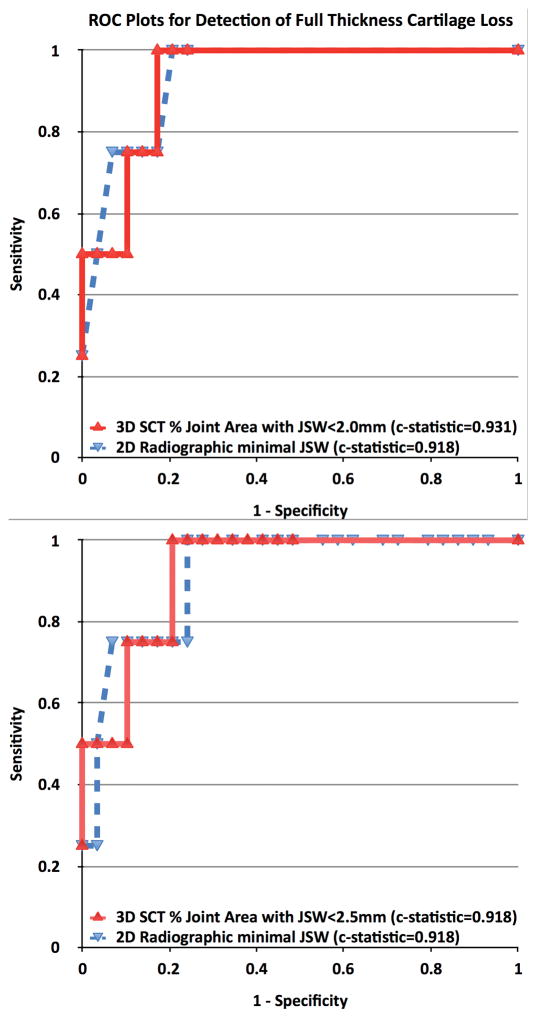
For detection of full thickness cartilage lesions, 7% of the joint area with JSW ≤2.5mm was 75% sensitive and 90% specific (c-statistic 0.918). In comparison, a radiographic minimum JSW of 2.6mm was 75% sensitive and 93% specific (c-statistic 0.918). Confirmatory analysis using 12% of the joint area with JSW ≤2.0mm also was 75% sensitive and 90% specific (c-statistic 0.931). Thus, the sensitivity and specificity for detection of full thickness cartilage lesions did not differ between SCT and radiographic measures of JSW. ROC curves are presented in Figure 4.
FIGURE 4.
ROC Plots of Sensitivity vs. 1-Specificity for Detection of Full Thickness Cartilage Loss on the Central Medial Femur and Tibia: % Joint Area with JSW<2.0mm and <2.5mm on SCT (top and bottom respectively) and radiographic minimum JSW
DISCUSSION
While relatively insensitive to disease status, knee radiography remains the most widely used diagnostic tool for detecting OA and patient stratification in clinical trials. Radiographs are used to visualize bony features characteristic of knee OA and to provide an indirect estimate of cartilage thickness and meniscal integrity through measurement of JSW. The current study compared the performance of SCT in measuring medial JSW with semiautomated assessment of radiographic minimum JSW, each vs. an MRI-based semi-quantitative measure of cartilage and meniscal morphology. While minimum JSW has been reported to be more reproducible and more sensitive to change than other radiographic measurements of JSW, SCT measures of JSW correlated better with MRI semi-quantitative assessments of central medial tibial cartilage morphology than did radiographic measurement of minimal JSW. These data support greater criterion validity of SCT assessment of JSW in assessing tibial cartilage health, although the correlations for femoral cartilage and meniscal damage did not significantly differ between SCT and radiographs.
Although MRI has the advantage of superior visualization of soft tissue, weight bearing imaging provides more functional assessments of the knee joint and can detect meniscal extrusions not detected when non-weight bearing.(16) Weight bearing imaging has also been found to be more sensitive to change in articular cartilage thickness than non-weight bearing quantitative MRI measurements in knees with KL3 OA.(2) The most likely explanation for this difference is that the cartilage and meniscus compress when standing in comparison with when in the unloaded position in which standard MRI is acquired. Recent data suggest this is especially true for cartilage.(17) However, the non-weight bearing condition during MRI may explain the lower correlations of MRI measures of meniscal pathology with weight-bearing JSW.
While there are some similarities in the techniques of standing radiography and SCT, there are also some differences that favor the SCT technique. One consideration that remains similar between SCT and fixed-flexed knee radiography is the importance of positioning of the joint for both cross-sectional assessment of JSW and longitudinal assessment of change in JSW. To visualize the central area of the tibiofemoral joint most frequently affected by cartilage lesions, knees must be positioned with appropriate degrees of flexion and external rotation.(18) However, while with radiographs, the beam angle must be appropriately selected for each knee to achieve parallel alignment of the anterior and posterior margins of the medial tibial plateau, with a maximum intermargin distance of 1–1.5 mm or less,(18) beam angle is not a consideration with 3D SCT. The absence of overlapping bony structures when imaged in 3D may explain the higher correlations between medial tibiofemoral JSW by SCT and cartilage morphology by MRI.
Recognizing that standing imaging has diagnostic advantages, evidence from the current study supports that radiographs may not be the optimal imaging modality for assessing knee OA. In addition to the higher correlation of JSW by SCT with central tibial cartilage morphology, some knees in our study that were graded as KL3 by radiographs were found to have areas of bone on bone contact by SCT imaging. Furthermore, SCT in this study required less than 2 minutes and approximately twice the effective radiation dose of plain radiographs of the knee to acquire 360° views of the joint, a much greater amount of information than is acquired with coronal, lateral and patellar plain radiographic views. Thus, SCT imaging may also be more time efficient than some radiographic protocols, in addition to having similar radiation dose to acquiring exposures at varying beam angles(19) and lower radiation dose than when using fluoroscopic positioning.
In prior work, SCT assessment was found to be more sensitive and accurate than radiographs for detecting marginal osteophytes and subchondral cysts, two of the primary bony features associated with OA.(7) A recent study, using a different tomographic technique, i.e. digital tomosynthesis, reported similar results, most likely due to the avoidance of superimposition with use of tomographic imaging in comparison with standard radiography.(19) The presence of a “definite” osteophyte is the most common criterion used for diagnosis of OA (KL grade 2) and the presence and degree of joint space narrowing is used to assess severity on both the KL and OARSI grading scales. Thus, together with the results of the current study, these findings suggest that the greater sensitivity and concurrent validity of SCT for diagnosis of bony features of knee OA in the prior study (7) and at least equivalent assessment of JSW in the current study may permit improved diagnosis and classification of knee OA disease severity.
One key advantage to more sensitive and valid office-based measures of knee OA is the potential to reduce size, costs and duration of clinical trials. The high cost of OA clinical trials has been a significant barrier to their conduct, and it is mostly due to the large numbers of subjects and the greater than 2 years of follow-up required to detect a clinical response. These same limitations hinder clinical care. In comparison with knee radiographs, imaging with SCT appears to provide higher sensitivity and accuracy for bony features (7) and equivalent sensitivity and specificity for full-thickness cartilage loss, without significantly increasing the radiation dose, time or cost of image acquisition. The modifications to a currently available low dose standing foot and ankle CT scanner have improved on each of these factors, while also providing the capability to consistently image the 3D position of the knee for JSW measurements. The better correlation with central tibial cartilage morphology than radiographs holds potential to reduce sample sizes needed for clinical trials, through including knees with known OA status or those most likely to progress and to detect progression. These factors could reduce the number of people exposed to test drugs and reduce the cost and duration of knee OA clinical trials, accelerating scientific progress and advancing clinical care.
Limitations of this study included the relatively small sample of knees included and the use of MRI, rather than direct visualization of the cartilage with arthroscopy. The sample size was sufficient to suggest differences in correlations between MRI assessment of tibial cartilage morphology and JSW by SCT in comparison with when measured on fixed-flexed radiographs. While direct visualization of cartilage was not performed, the WORMS system is a validated and widely used semi-quantitative assessment of cartilage morphology. The greater agreement between SCT measurement of JSW and MRI assessment of tibial cartilage morphology, as well as the equivalent correlations between SCT and radiographs for femoral cartilage damage, support the need to assess the extent to which the responsiveness of SCT measurement of JSW may improve longitudinal assessment of worsening beyond what is achieved with radiographs.
Another point worth further discussion is that the 2D JSW measure from radiographs in this carefully controlled context already had a very high sensitivity and specificity for detecting cartilage lesions, so much so that the summary measures of 3D JSW from SCT were near equivalent to the 2D measures. It is important to keep in mind that the MOST radiographic acquisition protocol is more rigorous than what commonly occurs clinically, and that the SCT dataset is insensitive to x-ray projection angle. Also, additional summary measures of 3D JSW, some of which might reasonably be expected to have superior sensitivity, remain to be developed. This stands in contrast to the 2D JSW measure, which has been developed over a period of many years.
In conclusion, in comparison with measurement of mJSW on radiographs, greater surface area with a low JSW, as determined by SCT, correlates more strongly with the presence and severity of partial and full thickness cartilage lesions on the central medial tibia, as determined by semi-quantitative MRI. No statistically significant differences in the correlations between measurements of JSW on SCT and radiographs and MRI were detected for femoral cartilage or meniscal damage. In the context of prior findings, the improved ability to detect tibial cartilage damage with SCT may enable valid stratification of participants in clinical trials at baseline and follow-up, through quickly and inexpensively characterizing OA features.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
Standing CT (SCT) imaging of knees provides joint space width (JSW) measurements that correlate more highly with MRI assessments of the presence and severity of medial tibial cartilage lesions than does minimum JSW by plain radiographs.
This advance may improve ability to validly stratify participants in clinical trials at baseline and follow-up, through quickly and inexpensively characterizing OA features.
Footnotes
DISCLOSURES: This study was supported by an Arthritis Foundation Innovative Research Grant and NIH grants to: The University of Iowa (AG18832); Boston University (AG18820); and the University of California San Francisco (AG19069). The study sponsors had no involvement in the study conception, design, data collection, analysis or interpretation. Dr. Guermazi is President of Boston Imaging Core Lab (BICL), and consultant to MerckSerono, Genzyme and TissueGene. Dr. Segal is one of the inventors of the knee positioning system used in the scanner, but has no equity interest and has not received licensing or royalty income.
Contributor Information
N.A. Segal, Email: segal-research@kumc.edu.
E. Frick, Email: eric-frick@uiowa.edu.
J. Duryea, Email: jduryea@bwh.harvard.edu.
A. Guermazi, Email: Ali.Guermazi@bmc.org.
M.C. Nevitt, Email: MNevitt@psg.ucsf.edu.
J.C. Torner, Email: james-torner@uiowa.edu.
D.T. Felson, Email: dfelson@bu.edu.
D.D. Anderson, Email: don-anderson@uiowa.edu.
References
- 1.FDA, editor. Federal Register. Aug 14, 2007. Clinical Development Programs for Human Drugs, Biological Products, and Medical Devices for the Treatment and Prevention of Osteoarthritis; p. 100. DOCKET NO. 1998D-0077 (FORMERLY 98D-0077) [Google Scholar]
- 2.Guermazi A, Roemer FW, Felson DT, Brandt KD. Motion for debate: osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum. 2013;65(11):2748–58. doi: 10.1002/art.38086. [DOI] [PubMed] [Google Scholar]
- 3.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52(10):3152–9. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 4.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kijowski R, Blankenbaker DG, Stanton PT, Fine JP, De Smet AA. Radiographic findings of osteoarthritis versus arthroscopic findings of articular cartilage degeneration in the tibiofemoral joint. Radiology. 2006;239(3):818–24. doi: 10.1148/radiol.2393050584. [DOI] [PubMed] [Google Scholar]
- 6.Cicuttini F, Hankin J, Jones G, Wluka A. Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthritis Cartilage. 2005;13(8):722–7. doi: 10.1016/j.joca.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Segal NA, Nevitt MC, Lynch JA, Niu J, Torner JC, Guermazi A. Diagnostic performance of 3D standing CT imaging for detection of knee osteoarthritis features. The Physician and sports medicine. 2015;2015:1–8. doi: 10.1080/00913847.2015.1074854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JG, McAlindon T, Dimasi M, Carey J, Eustace S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54(8):502–6. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 9.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM R. 2013;5(8):647–54. doi: 10.1016/j.pmrj.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35(10):2047–54. [PMC free article] [PubMed] [Google Scholar]
- 13.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27(3):580–91. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 14.Duryea J, Zaim S, Genant HK. New radiographic-based surrogate outcome measures for osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11(2):102–10. doi: 10.1053/joca.2002.0866. [DOI] [PubMed] [Google Scholar]
- 15.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–51. [Google Scholar]
- 16.Stehling C, Souza RB, Hellio Le Graverand MP, Wyman BT, Li X, Majumdar S, et al. Loading of the knee during 3. 0T MRI is associated with significantly increased medial meniscus extrusion in mild and moderate osteoarthritis. Eur J Radiol. 2012;81(8):1839–45. doi: 10.1016/j.ejrad.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh M, Souza RB, Wyman BT, Hellio Le Graverand MP, Subburaj K, Link TM, et al. Differences between X-ray and MRI-determined knee cartilage thickness in weight-bearing and non-weight-bearing conditions. Osteoarthritis Cartilage. 2013;21(12):1876–85. doi: 10.1016/j.joca.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Hellio Le Graverand MP, Mazzuca S, Duryea J, Brett A. Radiographic grading and measurement of joint space width in osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):485–502. doi: 10.1016/j.rdc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi D, Xu L, Roemer FW, Hunter DJ, Li L, Katur AM, et al. Detection of osteophytes and subchondral cysts in the knee with use of tomosynthesis. Radiology. 2012;263(1):206–15. doi: 10.1148/radiol.12111649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.