Abstract
Backgound: This study evaluated whether the hydration status affected health-related quality of life (HRQOL) during 12 months in peritoneal dialysis (PD) patients.
Methods: The hydration status and the HRQOL were examined at baseline and after 12 months using a bioimpedance spectroscopy and Kidney Disease Quality of Life-Short Form, respectively in PD patients. Four hundred eighty-one patients were included and divided according to the baseline overhydration (OH) value; normohydration group (NH group, -2L≤ OH ≤+2L, n=266) and overhydration group (OH group, OH >+2L, n=215). Baseline HRQOL scores were compared between the two groups. The subjects were re-stratified into quartiles according to the OH difference (OH value at baseline - OH value at 12 months; <-1, -1 - -0.1, -0.1 - +1, and ≥+1L). The relations of OH difference with HRQOL scores at 12 months and the association of OH difference with the HRQOL score difference (HRQOL score at baseline - HRQOL score at 12 months) were assessed.
Results: The OH group showed significantly lower baseline physical and mental health scores (PCS and MCS), and kidney disease component scores (KDCS) compared with the NH group (all, P<0.01). At 12 months, the adjusted PCS, MCS, and KDCS significantly increased as the OH difference quartiles increased (P<0.001, P=0.002, P<0.001, respectively). In multivariate analysis, the OH difference was independently associated with higher PCS (β = 2.04, P< .001), MCS (β=1.02, P=0.002), and KDCS (β=1.06, P<0.001) at 12 months. The OH difference was independently associated with the PCS difference (β = -1.81, P<0.001), MCS difference (β=-0.92, P=0.01), and KDCS difference (β=-0.90, P=0.001).
Conclusion: The hydration status was associated with HRQOL and increased hydration status negatively affected HRQOL after 12 months in PD patients.
Keywords: bioimpedance, fluid overload, overhydration, peritoneal dialysis, quality of life.
Introduction
Euvolemia is a predictor of outcome in peritoneal dialysis (PD) patients 1, 2. It is because volume overload is related with cardiac dysfunction 3, 4, arterial stiffness 5 and inflammation 6. Although achievement of euvolemia is crucial in dialysis patients, assessment of volume status is relatively crude in clinical practice. Bioimpedance spectroscopy (BIS) measures conductance and reactance at different frequencies by measuring the flow of electrical current through the body, and allows accurate measurement of fluid status 7. Different indices of hydration status are provided by the BIS, including extracellular water (ECW), intracellular water (ICW), total body water (TBW), and overhydration (OH). The ECW/TBW is most widely accepted as a hydration index, however it can be confounded by obesity 8, and it does not give the degree of tissue hydration. By contrast, the OH data provides an estimate of hydration in liters allowing the clinician to easily set a target weight for the patient without calculating an index 1. Recently it was reported that the OH value was an independent predictor of death in PD patients 1.
Health-related quality of life (HRQOL) is a predictor of mortality in end-stage renal disease (ESRD) patients 9, 10. Multiple factors are known to affect HRQOL in ESRD patients, including underlying disease, nutrition, inflammation, adverse effects of treatment modality, social support and rapport with care providers 10-14. Recent literature showed that body composition is associated with HRQOL in hemodialysis patients 15. It was also reported that hydration status is related with HRQOL in elderly dialysis patients, which included a relatively small number of patients 16. However, whether the hydration status affects HRQOL has not been evaluated in a large number of dialysis patients in a prospective manner.
The Quality of Life of Dialysis (QOLD) study was designed to analyze the change in HRQOL, depressive symptoms, and body composition of dialysis patients in Korea. In this prospective, observational multi-center study, 708 PD patients were recruited from 24 centers in Korea. In the current analyses, we analyzed 481 PD patients who were eligible for both the hydration status and HRQOL data at baseline and after 12 months to examine the hypothesis that hypervolemia is associated with worse HRQOL in PD patients.
Methods
Study population
We studied PD patients who participated in the QOLD study. The QOLD study is a prospective, observational multi-center study to analyze the change in HRQOL, depressive symptoms, and body composition of dialysis patients in Korea. Inclusion criteria were age ≥18 years and incident or prevalent dialysis patients. Exclusion criteria were those who had psychiatric disease, current malignancy or liver cirrhosis, who were bed-ridden, or who cannot undergo bioimpedance analysis because of defibrillators, artificial joints, pins or limb amputations. The study visits were conducted at each center at baseline and 12 months by study coordinators. At each visit (baseline and after 12 months), HRQOL, depressive symptoms, and body composition were assessed. Seven hundred eight PD patients were recruited from 24 centers in Korea.
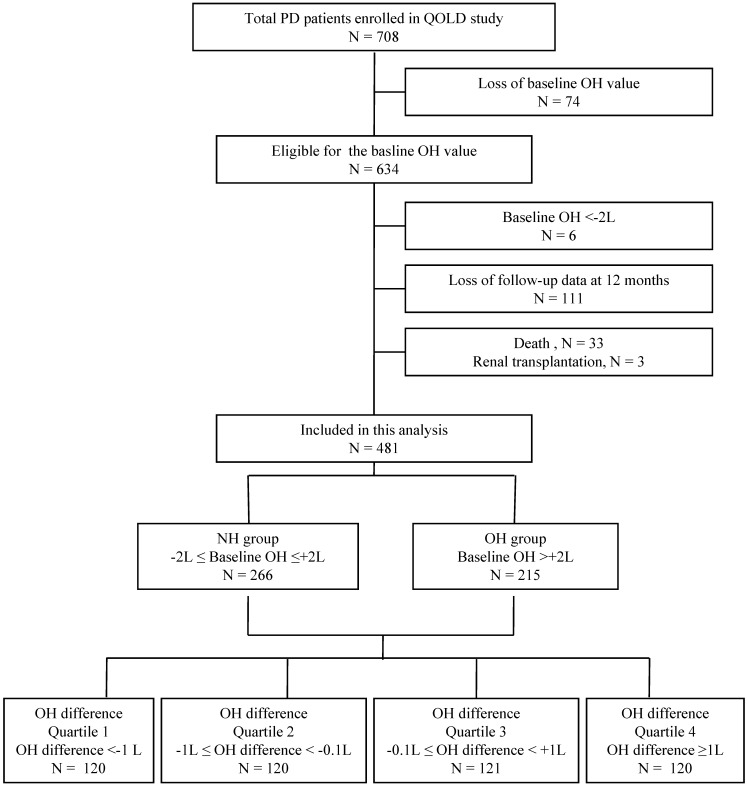
In the current analyses, 481 PD patients, who were eligible for both the hydration status and HRQOL data at baseline and after 12 months, were included. As shown in Figure 1, 634 patients were eligible for the baseline OH value. We used the baseline OH value to classify the hydration status of the patients. The overhydration group (OH group) was defined according to a previous study which showed that 2.0 liters was a reasonable cutoff value for OH in PD patients (OH >+2L) 17. Normohydration group (NH group) was defined as patients with baseline OH value between ±2L (-2L≤ OH ≤+2L). Six patients who were in an underhydration status (OH <-2L) were excluded from the current analysis as this study was to compare the OH group and NH group. Additionally, 111 patients who were lost for follow-up data, 33 patients who died during the study period and 3 patients who received renal transplantation were excluded. Among 481 patients included in this study, 266 patients were in the NH group and 215 patients were in the OH group at baseline.
Figure 1.
Patient population included in this study.
The subjects were additionally stratified into quartiles according to the change in the OH value during 12 months. The change in the OH value was defined as the OH difference, which is the difference between the baseline OH value and that at 12 months (OH difference = OH value at baseline - OH value at 12 months). The OH difference quartiles were; quartile 1 (OH difference <-1L, n = 120), quartile 2 (-1L≤ OH difference <-0.1L, n = 120), quartile 3 (-0.1L≤ OH difference <+1L, n = 121), and quartile 4 (OH difference ≥+1L, n = 120).
Instruments
HRQOL was examined using the Korean version of Kidney Disease Quality of Life-Short Form (KDQOL-SF) 18 at baseline and at 12 months. The KDQOL-SF includes 36 items derived from a generic, validated instrument (SF-36) as well as 43 kidney disease-targeted items and one overall health-rating item. This instrument has been validated in the ESRD population 19. The SF-36 domain includes subscales of physical functioning, role-physical, bodily pain, general health, emotional well-being, role-emotional, social function, and vitality. The kidney disease-targeted items include subscales of symptom/problem list, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, dialysis staff encouragement, and patient satisfaction to staff. Responses to the KDQOL-SF were used to determine the physical health component scores (PCS), mental health component scores (MCS), and kidney disease component scores (KDCS). The change in each component score of KDQOL-SF was defined as the HRQOL score difference, which is the difference between the baseline score and score at 12 months (PCS difference = PCS at baseline - PCS at 12 months; MCS difference = MCS at baseline - MCS at 12 months; KDCS difference = KDCS at baseline - KDCS at 12 months).
Measurements of hydration status and body composition
The hydration status and body composition were assessed at baseline and after 12 months. The BIS device (Body Composition Monitor, Fresenius Medical Care, Germany) was used to measure bioimpedance at 50 frequencies between 5 and 1000 kHz. The measurement was performed by placing electrodes on one hand and one foot in the BIS device and entering current height and weight data into the machine. BIS measurements were performed with the peritoneal dialysate in situ, and were performed by one reference PD physician or nurse in each center. ECW, ICW, TBW, and OH were determined from the measured impedance data. The OH/ECW was calculated as the percentage of OH to ECW. The lean tissue index (LTI) was calculated as the quotient of lean tissue mass/height2 (kg/m2). The adipose tissue index (ATI) was calculated as the quotient of adipose tissue mass/height2 (kg/m2).
Other variables
Patients' comorbid status was quantified using the modified Charlson Comorbidity Index (CCI) 20. Blood pressure was recorded as the mean of two consecutive measurements with 5 minutes' interval, using one single calibrated device in each center. Height and weight were measured using one single calibrated device in each center. Body mass index (BMI) was calculated as the quotient of weight/height2 (kg/m2). Peritoneal membrane characteristics were determined based on the results of the peritoneal equilibration test (PET) at the time of body composition measurements. Dialysis adequacy (total KT/Vurea per week), mean of renal urea and creatinine clearance (renal CrCl), 24-h urine volume, ratio of dialysate to serum creatinine at 4-h PET (D/P Cr), and laboratory values were collected. Dietary protein intake was estimated from the normalized protein equivalent of nitrogen appearance (nPNA) following the equation: PNA=15.1 + 0.1945 urea appearance (mM/24 h) + protein losses (g/24 h) 21.
Statistical analysis
Continuous data are expressed as the mean ± standard deviation (SD) or the median (range). Categorical variables are expressed as percentage of total. The normality of the distribution was assessed by the Shapiro-Wilk test. Differences between the NH group and the OH group were determined using Student's t-test for variables with normal distribution or Wilcoxon rank-sum test for variables with non-normal distribution. Categorical variables were compared using a chi-square test or Fisher's exact test. Pearson's correlation analysis was used to determine the correlation between the OH difference and the HRQOL scores at 12 months. Analysis of covariance was used to compare differences in the HRQOL scores at 12 months between the OH difference quartiles. Linear regression test was used to determine the association of OH difference with the HRQOL scores at 12 months and the HRQOL score difference. Multivariate models included the significantly associated parameters according to their weight on univariate testing and clinically fundamental parameters. A P value of < 0.05 was considered to indicate a statistically significant difference and statistical analysis was performed using SAS.
Ethics statement and trial registration
All participants gave written informed consent, and the study protocol was approved by the following institutional review boards of the centers participated in the study: Korea University Guro Hospital, Catholic University of Korea Bucheon St. Mary's Hospital, Incheon St. Mary's Hospital, St. Vincent's Hospital, St. Paul's Hospital, Uijeongbu St. Mary's Hospital, Daejeon St. Mary's Hospital and Seoul St. Mary's Hospital, Soonchunhyang University Hospital, Hallym University Medical Center, Hanyang University Medical Center, KyungHee University Medical Center, Veterans Health Service Medical Center, Daejeon Sun Hospital, Eulji University Hospital, Dankook University Hospital, Konyang University Hospital, Chungnam National University Hospital, Inje University Haeundae Paik Hospital, Wonju Severance Christian Hospital, Chosun University Hospital, Presbyterian Medical Center, St. Carollo Hospital, and Keimyung University Dongsan Medical Center. The study was conducted from August 2010 to May 2014.
The study was registered at clinicaltrials.gov (NCT01668628), and was conducted in adherence to the Declaration of Helsinki. The authors confirm that all onging and related trials for this intervention have been registered. There was a delay in registering this study because centers were additionally recruited to participate in this study.
Results
Baseline characteristics
Table 1 shows the baseline characteristics and laboratory and bioimpedance measurements of the total patients and the comparison between the NH group and OH group. More male, diabetes, and continuous ambulatory PD patients were in the OH group than the NH group. The OH group showed higher CCI, total drained dialysate volume, and systolic blood pressure compared to the NH group. The OH group had higher D/P Cr and more patients with high average or high membrane transporter types than the NH group. The OH group consisted of less patients using 1.5% glucose bags only and more patients using 2.5% glucose bag at least once a day. The nPNA values were significantly lower in the OH group compared to the NH group.
Table 1.
Baseline characteristics and laboratory and bioimpedance measurements.
| Total patients in the current analysis | NH group | OH group | P | |
|---|---|---|---|---|
| n = 481 | n = 266 | n = 215 | ||
| Age | 51.3 ± 11.1 | 50.9 ± 11.6 | 51.8 ± 10.4 | 0.38 |
| Male (%) | 256 (53.2) | 117 (44) | 139 (64.7) | <0.001 |
| Dialysis vintage (mo) | 23.6 ± 34.1 | 25.1 ± 34.7 | 21.6 ± 33.3 | 0.27 |
| Diabetes (%) | 208 (43.2) | 75 (28.2) | 133 (61.9) | <0.001 |
| Cause of ESRD (%) | <0.001 | |||
| Diabetes | 198 (41.2) | 67 (25.2) | 131 (60.9) | |
| Hypertension | 179 (37.2) | 124 (46.6) | 55 (25.6) | |
| Glomerulonephritis | 52 (10.8) | 37 (13.9) | 15 (7) | |
| Others | 52 (10.8) | 38 (14.3) | 14 (6.5) | |
| CCI | 2.6 ± 1.0 | 2.4 ± 0.9 | 2.8 ± 1.1 | <0.001 |
| BMI (kg/m2) | 24.2 ± 3.5 | 24.2 ± 3.7 | 24.2 ± 3.3 | 0.96 |
| Systolic blood pressure (mmHg) | 132.2 ± 21.8 | 128.8 ± 19.1 | 136.4 ± 24.2 | <0.001 |
| Diastolic blood pressure (mmHg) | 80.7 ± 12.7 | 79.7 ± 12.5 | 81.9 ± 12.9 | 0.05 |
| Peritoneal dialysis modality (%) | <0.001 | |||
| CAPD | 424 (88.1) | 222 (83.5) | 202 (94) | |
| APD | 57 (11.9) | 44 (16.5) | 13 (6.1) | |
| D/P Cr at 4-h PET | 0.60 ± 0.11 | 0.59 ± 0.12 | 0.62 ± 0.11 | 0.005 |
| Type of membrane transport (%) | 0.007 | |||
| Low | 82 (17.0) | 59 (22.2) | 23 (10.7) | |
| Low average | 232 (48.2) | 125 (47) | 107 (49.8) | |
| High average | 148 (30.8) | 72 (27.1) | 76 (35.4) | |
| High | 19 (4.0) | 10 (3.8) | 9 (4.2) | |
| Total drained dialysate volume (mL/day) | 8469.3 ± 1328.0 | 8336.0 ± 1370.4 | 8637.4 ± 1255.6 | 0.01 |
| Dialysate usage (%) | ||||
| 1.5% glucose bags only | 205 (42.6) | 126 (47.4) | 79 (36.7) | 0.02 |
| 2.5% glucose bag at least once | 253 (52.6) | 127 (47.7) | 126 (58.6) | 0.02 |
| 4.25% glucose bag at least once | 33 (6.9) | 15 (5.6) | 18 (8.4) | 0.24 |
| Icodextrin bag usage | 18 (3.7) | 9 (3.4) | 9 (4.2) | 0.64 |
| 24-h urine volume (mL/day) | 763.9 ± 555.5 | 741.3 ± 540.6 | 793.6 ± 574.8 | 0.35 |
| Renal CrCl (mL/min/1.73m2) | 3.55 (0, 163.4) | 3.6 (0, 163.4) | 3.4(0, 74.3) | 0.67 |
| Total KT/Vurea per week | 2.4 ± 1.1 | 2.5 ± 1.1 | 2.3 ± 1.0 | 0.06 |
| nPNA (g/kg/day) | 0.58 ± 0.26 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.003 |
| Laboratory measurements | ||||
| Haemoglobin (g/dL) | 10.7 ± 1.4 | 10.8 ± 1.3 | 10.5 ± 1.4 | 0.003 |
| Creatinine (mg/dL) | 9.6 ± 3.7 | 9.8 ± 3.7 | 9.4 ± 3.7 | 0.24 |
| Sodium (mEq/L) | 138.8 ± 3.4 | 139 ± 3.4 | 138.6 ± 3.4 | 0.22 |
| Potassium (mEq/L) | 4.5 ± 1.6 | 4.4 ±0.8 | 4.5 ± 2.2 | 0.53 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.8 ± 0.4 | 3.4 ± 0.5 | <0.001 |
| Total cholesterol (g/dL) | 176.3 ± 42.6 | 179.6 ± 42.0 | 172.1 ± 43.0 | 0.06 |
| C-reactive protein (g/dL) | 0.2 (0, 61.2) | 0.2 (0, 61.2) | 0.2 (0, 18.8) | 0.06 |
| Bioimpedance measurements | ||||
| TBW (L) | 34.5 ± 7.1 | 32.7 ± 6.8 | 36.7 ± 6.8 | <0.001 |
| ECW (L) | 16.3 ± 3.5 | 14.8 ± 2.8 | 18.2 ± 3.2 | <0.001 |
| ICW (L) | 18.2 ± 4.1 | 17.9 ± 4.1 | 18.5 ± 4 | 0.13 |
| OH (L) | 2.2 ± 2.1 | 0.8 (-1.8, 2) | 3.5 (2.1, 9.6) | <0.001 |
| OH/ECW (%) | 12.2 ± 10.7 | 4.5 ± 6.2 | 21.7 ± 6.9 | <0.001 |
| LTI (kg/m2) | 14.4 ± 3.1 | 14.3 ± 3.1 | 14.4 ± 3.1 | 0.86 |
| ATI (kg/m2) | 8.8 ± 4.1 | 9.4 ± 4.3 | 8.1 ± 3.7 | <0.001 |
Values expressed with a plus/minus sign are the mean ± SD. Values expressed with a parentheses are the median (range).
NH group, normohydration group; OH group, overhydration group; ESRD, end-stage renal disease; CCI, Charlson comorbidity index; BMI, body mass index; CAPD, continuous ambulatory peritoneal dialysis; APD, automated peritoneal dialysis; D/P Cr at 4-h PET, the ratio of dialysate creatinine to plasma creatinine at 4-h peritoneal equilibration test; CrCl, creatinine clearance; nPNA, normalized protein equilvalent of nitrogen appearance; TBW, total body water; ECW, extracellular water; ICW, intracellular water; OH, overhydration; OH/ECW, the ratio of overhydration to extracellular water; LTI, lean tissue index; ATI, adipose tissue index.
At baseline, the OH group showed lower haemoglobin and albumin levels than the NH group. As expected, the OH group showed higher TBW, ECW, ICW, OH, OH/ECW values than the NH group. The ATI was significantly lower in the OH group compared to the NH group, but there was no difference in the LTI.
HRQOL scores at baseline
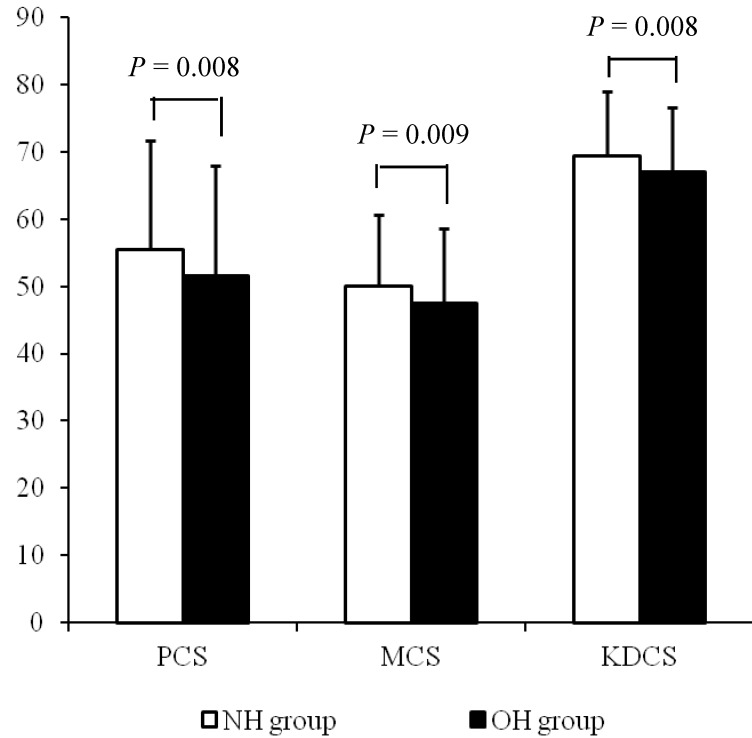
Each component score of KDQOL-SF at baseline was compared between the two groups. The average of PCS, MCS, and KDCS at baseline were significantly lower in the OH group compared with the NH group (NH vs. OH; PCS, 55.5 ± 16.2 vs. 51.5 ± 16.5, P = 0.008; MCS, 50.1 ± 10.6 vs. 47.5 ± 11.1, P = 0.009; KDCS, 69.3 ± 9.6 vs. 67.0 ± 9.6, P = 0.008; Fig 2).
Figure 2.
The HRQOL scores at baseline according to the hydration status. The average scores of PCS, MCS, and KDCS at baseline were significantly lower in the OH group compared with the NH group.
The subscales of the KDQOL-SF at baseline were compared (Table 2). Among the SF-36 domains, the OH group showed significantly lower scores in physical functioning, bodily pain, general health, and social function. Among the kidney disease-specific domains, the OH group showed significantly lower scores in effects of kidney disease, burden of kidney disease, and cognitive function.
Table 2.
Baseline HRQOL scores.
| Total patients (n = 481) | NH group (n = 266) | OH group (n = 215) | P | |
|---|---|---|---|---|
| SF-36 domains | ||||
| Physical functioning | 73.1 ± 22.4 | 75.3 ± 21.5 | 70.4±23.2 | 0.02 |
| Role-physical | 50 (0, 100) | 50 (0, 100) | 50(0,100) | 0.33 |
| Bodily pain | 76.5 ± 22.9 | 79 ± 20.5 | 73.5±25.3 | 0.01 |
| General health | 39.9 ± 21.7 | 42.8 ± 21.1 | 36.4±22.1 | 0.001 |
| Emotional well-being | 30.2 ± 14.8 | 29.7 ± 13.8 | 30.9±16 | 0.39 |
| Role-emotional | 74.5 ± 24.5 | 75.7 ± 22.5 | 73±26.8 | 0.25 |
| Social function | 69.6 ± 25.0 | 72.1 ± 24.5 | 66.5±25.2 | 0.01 |
| Vitality | 30.3 ± 14.3 | 30.2 ± 14.2 | 30.5±14.5 | 0.78 |
| Kidney disease-specific domains | ||||
| Symptom problem list | 79.2 ± 15.6 | 80.3 ± 14.7 | 77.8 ± 16.7 | 0.08 |
| Effect of kidney disease | 75.6 ± 16.2 | 77.3 ± 16.1 | 73.5 ± 16.2 | 0.01 |
| Burden of kidney disease | 36.4 ± 25.3 | 38.7 ± 25.9 | 33.5 ± 24.2 | 0.03 |
| Work status | 47.9 ± 25.3 | 47.6 ± 25 | 48.4 ± 25.8 | 0.73 |
| Cognitive function | 83.5 ± 15.9 | 85.5 ± 14.6 | 81.1 ± 17.1 | 0.004 |
| Quality of social interaction | 70.6 ± 14.4 | 71.0 ± 15.2 | 70 ± 13.3 | 0.45 |
| Sexual function | 65.0 ± 32.5 | 68.0 ± 32.4 | 61.2 ± 32.3 | 0.13 |
| Sleep | 69.3 ± 15.5 | 70.3 ± 15.6 | 68 ± 15.3 | 0.10 |
| Social support | 65.9 ± 23.6 | 66.7 ± 22.3 | 64.9 ± 25.2 | 0.41 |
| Dialysis staff encouragement | 100 (0, 100) | 100 (0, 100) | 100 (0, 100) | 0.81 |
| Patient satisfaction | 66.7 ± 17.1 | 67.4 ± 21.8 | 65.8 ± 22.3 | 0.43 |
Values expressed with a plus/minus sign are the mean ± SD. Values expressed with a parentheses are the median (range).
NH group, normohydration group; OH group, overhydration group.
Correlations between the OH difference and the HRQOL scores at 12 months
Table 3 shows the correlation coefficients between the OH difference and subscales of the KDQOL-SF at 12 months. In unadjusted analysis, the OH difference showed positive correlations with scores of bodily pain and patient satisfaction. After adjustment for the baseline OH value, the OH difference showed significant positive correlations with scores of physical functioning, role-physical, bodily pain, general health, role-emotional, and social function in the SF-36 domains. In the kidney disease-specific domains, the OH difference showed significant positive correlations with scores of symptom problem list, effect of kidney disease, burden of kidney disease, cognitive function, sleep, social support and patient satisfaction after adjustment for the baseline OH value.
Table 3.
Correlations of the OH difference with the HRQOL scores at 12 months.
| Unadjusted r | P | Adjusted ra | P | |
|---|---|---|---|---|
| SF-36 domains | ||||
| Physical functioning | 0.08 | 0.08 | 0.22 | <0.001 |
| Role-physical | 0.05 | 0.29 | 0.16 | <0.001 |
| Bodily pain | 0.10 | 0.02 | 0.18 | <0.001 |
| General health | 0.06 | 0.18 | 0.13 | 0.005 |
| Emotional well-being | 0.08 | 0.10 | 0.02 | 0.61 |
| Role-emotional | 0.08 | 0.08 | 0.13 | 0.006 |
| Social function | 0.03 | 0.46 | 0.12 | 0.01 |
| Vitality | 0.02 | 0.62 | 0.009 | 0.84 |
| Kidney disease-specific domains | ||||
| Symptom problem list | 0.01 | 0.78 | 0.09 | 0.046 |
| Effect of kidney disease | 0.05 | 0.30 | 0.15 | 0.001 |
| Burden of kidney disease | 0.07 | 0.15 | 0.13 | 0.004 |
| Work status | 0.03 | 0.58 | 0.02 | 0.71 |
| Cognitive function | 0.07 | 0.14 | 0.13 | 0.003 |
| Quality of social interaction0 | 0.008 | 0.85 | 0.03 | 0.51 |
| Sexual function | -0.11 | 0.13 | 0.10 | 0.17 |
| Sleep | 0.07 | 0.15 | 0.12 | 0.007 |
| Social support | 0.08 | 0.08 | 0.12 | 0.009 |
| Dialysis staff encouragement | 0.05 | 0.27 | 0.03 | 0.50 |
| Patient satisfaction | 0.12 | 0.01 | 0.11 | 0.01 |
aAdjusted for the baseline OH value.
Impact of the OH difference on the HRQOL scores at 12 months
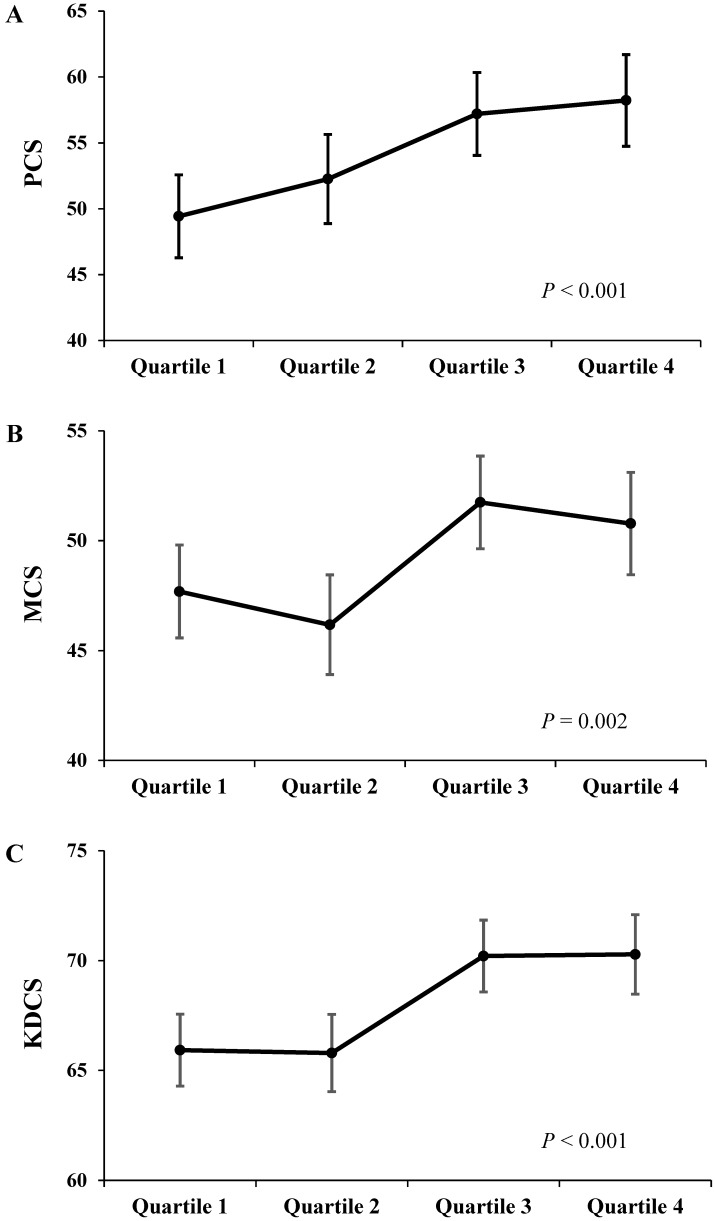
Each component score of KDQOL-SF at 12 months was compared between the OH difference quartiles (Fig 3). As the OH difference quartile increased, there was a significant trend toward an increase in adjusted PCS, MCS, and KDCS at 12 months after adjustments for age, sex, dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea per week, renal CrCl, nPNA, 24-h urine volume, the baseline OH value, and each baseline component score (PCS, P < 0.001; MCS, P = 0.002; KDCS, P < 0.001).
Figure 3.
The Adjusted HRQOL scores at 12 months according to the OH difference quartiles. As the OH difference quartile increased, there was a significant trend toward an increase in scores of PCS (A), MCS (B), and KDCS (C) at 12 months. Adjustments were made for age, sex, dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea, renal CrCl, nPNA, 24-h urine volume, the baseline OH value, and each component score at baseline (baseline PCS, MCS, and KDCS, respectively).
To evaluate whether the change in hydration status was associated with the HRQOL scores after 12 months, linear regression analysis was performed (Table 4). After adjustments for age and sex (Model 1), the OH difference showed significant positive associations with PCS (β = 2.18, 95% confidence interval [CI] 1.27 - 3.09, P < 0.001), MCS (β = 1.06, 95% CI 0.46 - 1.65, P < 0.001), and KDCS (β = 1.05, 95% CI 0.54 - 1.56, P < 0.001) at 12 months. These associations remained robust after adjustments for dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea per week, renal CrCl, nPNA, 24-h urine volume and the baseline OH value (Model 2; PCS, β = 2.25, 95% CI 1.23 - 3.28, P < 0.001; MCS, β = 1.08, 95% CI 0.40 - 1.77, P = 0.002; KDCS, β = 1.29, 95% CI 0.71 - 1.88, P < 0.001). Moreover, these associations were significant after adjustments for baseline PCS, MCS, and KDCS, respectively (Model 3; PCS, β = 2.04, 95% CI 1.01 - 2.97, P < 0.001; MCS, β = 1.02, 95% CI 0.38 - 1.65, P = 0.002; KDCS, β = 1.06, 95% CI 0.57 - 1.55, P < 0.001).
Table 4.
Regression coefficients of the OH difference for the HRQOL scores at 12 months.
| βa | 95% CI | P | |
|---|---|---|---|
| PCS at 12 months | |||
| Model 1 | 2.18 | 1.27, 3.09 | <0.001 |
| Model 2 | 2.25 | 1.23, 3.28 | <0.001 |
| Model 3 | 2.04 | 1.01, 2.97 | <0.001 |
| MCS at 12 months | |||
| Model 1 | 1.06 | 0.46, 1.65 | <0.001 |
| Model 2 | 1.08 | 0.40, 1.77 | 0.002 |
| Model 3 | 1.02 | 0.38, 1.65 | 0.002 |
| KDCS at 12 months | |||
| Model 1 | 1.05 | 0.54, 1.56 | <0.001 |
| Model 2 | 1.29 | 0.71, 1.88 | <0.001 |
| Model 3 | 1.06 | 0.57, 1.55 | <0.001 |
aRegression coefficient
Model 1: Adjusted for age and sex.
Model 2: Adjusted for Model 1 plus dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea per week, renal CrCl, nPNA, 24-h urine volume and the baseline OH value.
Model 3: Adjusted for Model 2 plus baseline scores of PCS, MCS, and KDCS, respectively.
Impact of the OH difference on the HRQOL score difference
To evaluate whether the change in hydration status was associated with the change in HRQOL scores, linear regression analysis was performed (Table 5). After adjustments for age and sex (Model 1), the OH difference was significantly negatively associated with the PCS difference (β = -1.64, 95% CI -2.47- -0.80, P < 0.001), and the KDCS difference (β = -0.48, 95% CI -0.95 - -0.01, P = 0.04). These associations remained robust after adjustments for dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea per week, renal CrCl, nPNA, 24-h urine volume and the baseline OH value (Model 2; PCS difference, β = -1.81, 95% CI -2.84 - -0.78, P < 0.001; MCS difference, β = -0.92, 95% CI -1.65 - -0.20, P = 0.01; KDCS difference, β = -0.90, 95% CI -1.44 - -0.36, P = 0.001).
Table 5.
Regression coefficients of the OH difference for the HRQOL score difference.
| βa | 95% CI | P | |
|---|---|---|---|
| PCS difference (PCS at baseline - PCS at 12 months) | |||
| Model 1 | -1.64 | -2.47, -0.80 | <0.001 |
| Model 2 | -1.81 | -2.84, -0.78 | <0.001 |
| MCS difference (MCS at baseline - MCS at 12 months) | |||
| Model 1 | -0.59 | -1.19, 0.006 | 0.052 |
| Model 2 | -0.92 | -1.65, -0.20 | 0.01 |
| KDCS difference (KDCS at baseline - KDCS at 12 months) | |||
| Model 1 | -0.48 | -0.95, -0.01 | 0.04 |
| Model 2 | -0.90 | -1.44, -0.36 | 0.001 |
aRegression coefficient
Model 1: Adjusted for age and sex.
Model 2: Adjusted for Model 1 plus dialysis vintage, diabetes, haemoglobin, albumin, CCI, total KT/Vurea per week, renal CrCl, nPNA, 24-h urine volume and the baseline OH value.
Discussion
The QOLD study is the first multi-center study of change in HRQOL, depressive symptoms, and body composition in PD patients. The results of the present study show that the baseline hydration status was associated with the baseline HRQOL scores and that the change in hydration status was related with the HRQOL scores after 12 months and the change in HRQOL scores in PD patients. The associations were significant after adjusting multiple factors including nutrition, anemia, residual renal function, dialysis adequacy, as well as the baseline hydration status and baseline HRQOL scores. These findings implicate that interventions to achieve euvolemia may potentially improve the HRQOL in PD patients.
HRQOL is a powerful predictor of mortality in ESRD patients 9, 10. Euvolemia is also a predictor of mortality in PD patients 1, 2. However, monitoring of HRQOL is not routinely done and accurate assessment of volume status is relatively crude in clinical practice. The novelty of this study is that we demonstrated that the hydration status was associated with HRQOL, not only at baseline, but also after 12 months. At baseline, the OH group showed better baseline PCS, MCS, and KDCS compared to the NH group. We speculate several reasons for this association. First, the OH group was more anemic than the NH group. A previous systematic review demonstrated that hematocrit level showed a consistent relationship with HRQOL in ESRD patients 22. Second, the OH group was more hypoalbuminemic than the NH group. Nutritional biomarkers including albumin are well known predictors of both generic and disease-specific HRQOL in ESRD patients 22. Third, the OH group was more diabetic and had multimorbidity, both of which were shown to be negatively associated with HRQOL in ESRD patients 23-25. After 12 months, the OH difference showed positive correlations with most of the subscales of the KDQOL-SF, after adjustment for the baseline OH value. This suggested that the decrease in hydration status (positive OH difference) was associated better HRQOL scores after 12 months. To strengthen the statistical power, we stratified the patients into quartiles according to the OH difference. The adjusted PCS, MCS, and KDCS after 12 months significantly increased as the OH difference quartiles increased. In regression analysis, the decrease in hydration status (positive OH difference) was independently associated with better PCS, MCS, KDCS after adjustments for multiple variables including the baseline OH value and baseline component score of KDQOL-SF. Moreover, the decrease in hydration status (positive OH difference) was independently associated with improvement in PCS, MCS, KDCS (negative PCS, MCS, and KDCS difference). These findings suggest that decrease in hydration status is associated with improvement in HRQOL score after 12 months. Interestingly, the OH difference was more strongly associated with PCS difference than it did with MCS difference or KDCS difference. As physical function is closely related with muscle mass and cardiac function, several mechanisms can be postulated. First, the increase in hydration status may reflect progressive muscle loss and malnutrition 26. Second, the increase in hydration status may be related to cardiac injury. It was reported that there is a longitudinal correlation between ECW and brain natriuretic peptide 27, which is strongly related with cardiac abnormalities in PD patients 28.
Hypervolemia was a frequent finding in our patients. Among 481 patients included in this study, 44.7% of patients were overhydrated. This finding is similar to that from a multi-center European study of 639 PD patients, which showed that 53.4% of patients were overhydrated and 24% had OH values equivalent to >2L 29. In this study, all hydration indices were higher in the OH group than the NH group. The total drained dialysate volume was also higher and more patients used 2.5% glucose dialysates at least once in the OH group. However, the use of 4.25% glucose dialysate or icodextrin was not different. Although the OH group showed higher peritoneal transport characteristics, the proportion of automated PD was lower and the use of icodextrin was not different compared with the NH group. The reason for this is unclear, since it was a multi-center study. However, these findings suggest that the PD prescription or fluid and salt restriction failed to achieve euvolemia in our study population.
In this study, the OH group was more likely to be male, diabetic, and hypoalbuminemic and to have multiple comorbidities and higher blood pressures, which is consistent with previous reports 19. The reason for the male predominance in the OH group is not clear, but a similar finding was shown in a study of non-dialysis dependent chronic kidney disease patients 30. The relationship between hydration status and hypoalbuminemia was also reported previously 29, 31. There may be several reasons. First, the low dietary protein intake may be involved, as the OH group demonstrated lower nPNA levels than the NH group. However, the LTI or the serum creatinine was not different between the two groups, suggesting that muscle mass was not different. Second, hypoalbuminemia per se determines tissue hydration. Radio labeled albumin used to determine plasma volume demonstrated that the excess fluid associated with hypoalbuminemia is due to extravascular rather than intravascular volume expansion, which results from reduced oncotic pressure 31. Third, the hydration status can also be affected by peritoneal membrane transport characteristics 32. Rapid peritoneal solute transport is associated with increased peritoneal protein losses, contributing to hypoalbuminemia 33. Our study population showed higher D/P Cr and more high and high average transporters in the OH group, which supports the association between hydration status and hypoalbuminemia.
There are limitations to this study. First, the effects of medications which may affect the hydration status or anemia were not analyzed. Second, data of dietary fluid and salt intake were lacking. Whether the overhydrated patients had excessive sodium and water intake or the prescription of PD was inadequate cannot be determined in this study. Third, the direct effect of hydration status on HRQOL could not be proven due to the observational manner of this study. Fourth, subjects with loss of data were excluded from the analysis.
In conclusion, this study demonstrates that the hydration status negatively affects the HRQOL in PD patients. Interventions to control volume overload may improve the HRQOL in PD patients with better outcomes.
Acknowledgments
The authors want to thank Kyungdo Han (Department of Biostatistics, College of Medicine, The Catholic University of Korea) for his statistical analysis.
References
- 1.O'Lone EL, Visser A, Finney H, Fan SL. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant. 2014;29:1430–7. doi: 10.1093/ndt/gfu049. [DOI] [PubMed] [Google Scholar]
- 2.Paniagua R, Ventura MD, Avila-Diaz M. et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant. 2010;25:551–7. doi: 10.1093/ndt/gfp395. [DOI] [PubMed] [Google Scholar]
- 3.Enia G, Mallamaci F, Benedetto FA. et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001;16:1459–64. doi: 10.1093/ndt/16.7.1459. [DOI] [PubMed] [Google Scholar]
- 4.Konings CJ, Kooman JP, Schonck M. et al. Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int. 2002;22:477–87. [PubMed] [Google Scholar]
- 5.Hogas S, Ardeleanu S, Segall L. et al. Changes in arterial stiffness following dialysis in relation to overhydration and to endothelial function. Int Urol Nephrol. 2012;44:897–905. doi: 10.1007/s11255-011-9933-x. [DOI] [PubMed] [Google Scholar]
- 6.Demirci MS, Demirci C, Ozdogan O. et al. Relations between malnutrition-inflammation-atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant. 2011;26:1708–16. doi: 10.1093/ndt/gfq588. [DOI] [PubMed] [Google Scholar]
- 7.Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: a cross-sectional study. Nephrol Dial Transplant. 2010;25:545–51. doi: 10.1093/ndt/gfp517. [DOI] [PubMed] [Google Scholar]
- 8.Craven JL, Rodin GM, Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–74. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SD, Norris L, Acquaviva K, Peterson RA, Kimmel PL. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2:1332–42. doi: 10.2215/CJN.03951106. [DOI] [PubMed] [Google Scholar]
- 10.Simic Ogrizovic S, Jovanovic D, Dopsaj V. et al. Could depression be a new branch of MIA syndrome? Clin Nephrol. 2009;71:164–72. doi: 10.5414/cnp71164. [DOI] [PubMed] [Google Scholar]
- 11.Chilcot J, Davenport A, Wellsted D, Firth J, Farrington K. An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dial Transplant. 2011;26:1628–34. doi: 10.1093/ndt/gfq611. [DOI] [PubMed] [Google Scholar]
- 12.Kimmel PL, Phillips TM, Simmens SJ. et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–44. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmel PL, Peterson RA, Weihs KL. et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57:2093–8. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 14.Plantinga LC, Fink NE, Harrington-Levey R. et al. Association of social support with outcomes in incident dialysis patients. Clin J Am Soc Nephrol. 2010;5:1480–8. doi: 10.2215/CJN.01240210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinson M, Ikizler TA, Morrell G. et al. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1082–90. doi: 10.2215/CJN.09200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laegreid IK, Bye A, Aasarod K, Jordhoy M. Nutritional problems, overhydration and the association with quality of life in elderly dialysis patients. Int Urol Nephrol. 2012;44:1885–92. doi: 10.1007/s11255-012-0280-3. [DOI] [PubMed] [Google Scholar]
- 17.Luo YJ, Wang T. What is the upper limitation of volume in Chinese peritoneal dialysis patients? Blood Purif. 2011;31:289–95. doi: 10.1159/000322615. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Kim S, Yong JS. et al. Reliability and validity of the Korean version of Kidney Disease Quality of Life instrument (KDQOL-SF) Tohoku J Exp Med. 2007;211:321–9. doi: 10.1620/tjem.211.321. [DOI] [PubMed] [Google Scholar]
- 19.Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT. Validation of the KDQOL-SF: a dialysis-targeted health measure. Qual Life Res. 2002;11:437–47. doi: 10.1023/a:1015631411960. [DOI] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–32. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom J, Heimburger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int. 1998;18:467–73. [PubMed] [Google Scholar]
- 22.Spiegel BM, Melmed G, Robbins S, Esrailian E. Biomarkers and health-related quality of life in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2008;3:1759–68. doi: 10.2215/CJN.00820208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Md Yusop NB, Yoke Mun C, Shariff ZM, Beng Huat C. Factors associated with quality of life among hemodialysis patients in Malaysia. PLoS One. 2013;8:e84152. doi: 10.1371/journal.pone.0084152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen VR, Mathiesen ER, Watt T, Bjorner JB, Andersen MV, Feldt-Rasmussen B. Diabetic patients treated with dialysis: complications and quality of life. Diabetologia. 2007;50:2254–62. doi: 10.1007/s00125-007-0810-1. [DOI] [PubMed] [Google Scholar]
- 25.Park HC, Yoon HB, Son MJ. et al. Depression and health-related quality of life in maintenance hemodialysis patients. Clin Nephrol. 2010;73:374–80. doi: 10.5414/cnp73374. [DOI] [PubMed] [Google Scholar]
- 26.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int; 2014. [DOI] [PubMed] [Google Scholar]
- 27.Davenport A. Changes in N-terminal pro-brain natriuretic peptide correlate with fluid volume changes assessed by bioimpedance in peritoneal dialysis patients. Am J Nephrol. 2012;36:371–6. doi: 10.1159/000343286. [DOI] [PubMed] [Google Scholar]
- 28.Wang AY, Lam CW, Wang M. et al. Diagnostic potential of serum biomarkers for left ventricular abnormalities in chronic peritoneal dialysis patients. Nephrol Dial Transplant. 2009;24:1962–9. doi: 10.1093/ndt/gfp067. [DOI] [PubMed] [Google Scholar]
- 29.Van Biesen W, Williams JD, Covic AC. et al. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One. 2011;6:e17148. doi: 10.1371/journal.pone.0017148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung SC, Kuo KL, Peng CH. et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–9. doi: 10.1038/ki.2013.336. [DOI] [PubMed] [Google Scholar]
- 31.John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ. Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1463–70. doi: 10.2215/CJN.09411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konings CJ, Kooman JP, Schonck M. et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003;18:797–803. doi: 10.1093/ndt/gfg147. [DOI] [PubMed] [Google Scholar]
- 33.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998;9:1285–92. doi: 10.1681/ASN.V971285. [DOI] [PubMed] [Google Scholar]