To the Editor:
A full-term, male patient was born in the United Kingdom to parents of Hazars ethnic background, weighing 3.5 kg (50th percentile) and regaining his birth weight by Day 12. There was no significant family history, and he had two healthy siblings. He presented to hospital at 5 weeks of age with fever, respiratory distress, tachypnea, and a C-reactive protein level of 59 mg/L (reference range, <5 mg/L). During the next 6 months, he had a persistent cough and three further admissions. Chest radiographs showed persistent perihilar changes; he received multiple courses of antibiotics, although tests for bacterial and viral infections were consistently negative.
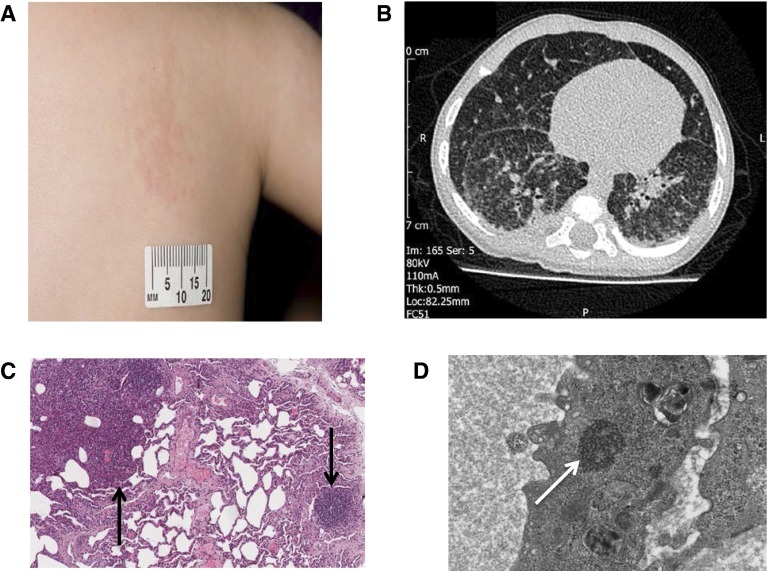
He was referred for specialist assessment at 7 months of age with ongoing respiratory symptoms, intermittent fever, and failure to thrive (weight, 5.59 kg; 0.4th percentile). He was tachypneic with moderate respiratory distress but had normal oxygen saturations in room air. He had clusters of raised maculopapular, erythematous lesions on his neck, back, arm, and legs first noted at 5 weeks of age (Figure 1A). Some of the features of this case have been previously reported in the form of an abstract (1).
Figure 1.
(A) Raised, papular, erythematous rash on the trunk. (B) Chest computed tomography scan showing widespread, slightly nodular interstitial opacification in both lungs. There are a number of small (<1 cm) peripheral cysts within the right lower and middle lobes. (C) Light microscopy microphotograph of lung biopsy (low magnification, hematoxylin and eosin stain), showing solid area composed of mixed infiltrate (left arrow) and chronic inflammation (right arrow). (D) Electron microscopy photograph of lung endothelium, showing tuboreticular inclusions (arrow).
Initial investigations found a C-reactive protein level of 10 mg/L, microcytic anemia with mean cell volume of 66.5 fl (reference range, 68–84 fl), and hemoglobin 100 g/L (reference range, 111–141 g/L). White blood cell count, renal function, liver function, thyroid function, and sweat chloride were normal. Chest radiograph showed increased interstitial and peribronchial markings throughout both lungs. Chest computed tomography showed features of interstitial lung disease (ILD; Figure 1B).
Immunological testing showed raised IgG (13.8 g/L; reference range, 3.0–9.0 g/L) and IgA (1.2 g/L; reference range, 0.2–0.7 g/L) levels, abnormal lymphocyte proliferation, highly positive antinuclear antibody screen with positive SS-A(Ro), and raised plasma viscosity (1.77 mPa ⋅ s; reference range, 1.50–1.72 mPa ⋅ s). Extensive investigations for bacteria, viruses, and fungi were performed on peripheral blood, skin biopsy, and bronchoalveolar lavage samples; all were negative.
A lung biopsy showed active inflammation with type 2 pneumocyte hyperplasia: epithelial hyperplasia of the bronchioles and surrounding lymphocytic infiltrate (Figure 1C). Ultrastructure examination with electron microscopy showed endothelial tuboreticular inclusions, which are suggestive of excess type 1 IFN, as a result of either exogenous treatment or endogenous overproduction (Figure 1D).
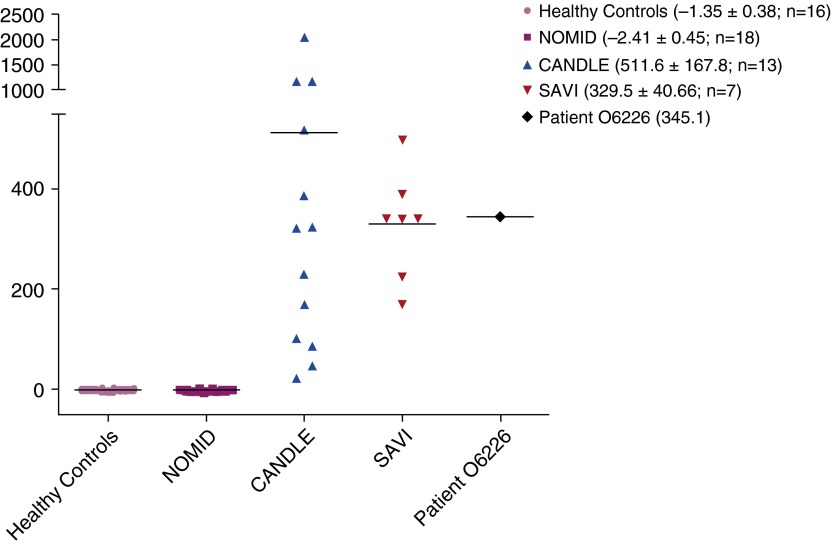
Given the constellation of ILD, systemic inflammation, and persistent rash dating from 5 weeks of age, a diagnosis of STING-associated vasculopathy with onset in infancy (SAVI) was considered. Genetic analysis by Sanger sequencing confirmed a heterozygous somatic mutation (c. 463 G>A, p.V155M) in exon 5 of TMEM173 (NM_198282.3), the gene encoding stimulator of interferon genes (STING). Genetic testing of the parents showed the mutation had occurred de novo. Whole-blood gene expression studies demonstrated a strong IFN signature, with IFN scores similar to those of other patients with SAVI (Figure 2).
Figure 2.
Gene expression of selected IFN response genes was determined by Nanostring (NanoString Technologies, Seattle, WA), and an IFN score was calculated for healthy controls, patients with varying autoinflammatory conditions, and our patient (patient O6226). Standardized IFN score (y-axis) is the sum of six Nanostring counts that were standardized by subtracting the mean of healthy controls and dividing by the SD of the healthy controls. IFN response genes assessed in this study were IFI27, IFI44, IFI44L, ISG15, RSAD2, and USP18. Mean and SD of the IFN score are depicted in parentheses for each group of individuals. CANDLE = chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome (mediated by excess IFN); NOMID = neonatal onset multisystem inflammatory disease (mediated by excess IL-1β); SAVI = STING-associated vasculopathy with onset in infancy (mediated by excess IFN).
Treatment with 3 days of intravenous methylprednisolone (10 mg/kg) yielded no clinical improvement. The patient gained weight with supplemental nasogastric feeding but had persistent tachypnea, and he subsequently became hypoxemic, requiring low-flow oxygen therapy. He then commenced monthly intravenous immunoglobulin at 2 g/kg; after 4 months of treatment, there has been some clinical improvement, with reduced tachypnea and a stable oxygen requirement.
The clinical syndrome of SAVI is characterized by early-onset (<8 wk of age) cutaneous vasculitis, fevers, ILD, and systemic inflammation. On laboratory testing, common features include positive autoantibodies (particularly a persistently raised antinuclear antibody) and raised IgG and IgA (2–5), all of which are features observed in our patient. Our patient developed a more central rather than peripheral (ears, nose, digits) rash exacerbated by cold exposure more commonly seen in patients with SAVI (6).
Of the previously described cases, 11 of 12 patients had clinically apparent lung disease (3, 4, 6, 7). Importantly, three of these patients have died in adolescence from pulmonary complications (3, 7). Lung toxicity from exogenous type 1 IFN treatment has been reported in patients with multiple sclerosis (8, 9) and chronic hepatitis C virus infection (10). Despite the documented link between chronic type 1 IFN exposure and lung pathology, it is perplexing that SAVI is currently the only known type 1 interferonopathy in which lung involvement is a common and major clinical feature.
Autosomal-dominant, gain-of-function mutations in the TMEM173 gene underlie the pathogenesis of SAVI. TMEM173 encodes STING protein, an adaptor molecule linking sensing of foreign (viral and bacterial) DNA to the production of type 1 IFNs as part of the innate immune response. These gain-of-function mutations lead to constitutive activation of STING and upregulated type 1 IFN production. STING is expressed in alveolar macrophages, bronchial epithelium, and alveolar type 2 pneumocytes (3), which presumably explains the specific lung pathology seen in SAVI.
In addition, in vitro studies show that STING also acts directly on endothelial cells, causing inflammation and initiating the coagulation cascade. Thus, these TMEM173 mutations are postulated to mediate chronic vessel endothelium inflammation, leading to the vasculitic rash and vasoocclusive processes seen in SAVI (3).
The predominance of early and significant respiratory symptoms, lack of characteristic peripheral rash in this patient, and variable phenotype described in family members with the same, inherited TMEM173 mutation (4) illustrates the variable phenotype of patients with SAVI; further research into the genotype–phenotype correlation and prognosis is warranted.
Treatment options in SAVI remain limited. Liu and colleagues reported a lack of response to glucocorticoids, disease-modifying antirheumatic drugs, and biological therapies (3). Isolated improvement in ILD has been documented in one patient after pulsed methylprednisolone and mycophenolate mofetil (6). Given the pathogenesis in SAVI, there is potential benefit from Janus kinase inhibitors to block type 1 IFN signaling, despite constitutively activated STING. The Janus kinase inhibitor baricitinib has shown benefit in adult patients with a range of inflammatory conditions, but there are limited pediatric data. An approved clinical protocol to assess the therapeutic benefit of baricitinib in patients with either SAVI or chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE) interferonopathy is ongoing and has recently published encouraging results in patients with CANDLE (ClinicalTrials.gov number NCT01724580) (11).
To conclude, a diagnosis of SAVI has significant implications for patients and families, not only from the high mortality risk but also from the significant morbidity. The respiratory component of the disease may predominate, and pulmonary complications have been the cause of death in previous reports. In the context of early age of onset, ILD, failure to thrive, fevers, and rash, we urge respiratory pediatricians to consider SAVI as a differential diagnosis and to request testing for TMEM173 mutations and IFN gene signature to confirm the diagnosis.
Supplementary Material
Footnotes
S.L.N.C.’s research at the National Institutes of Health was supported by the Above and Beyond Research Funding Committee and UK National Institute for Health Research Academic Clinical Fellowship program.
Author Contributions: All authors were involved in the care of the patient; S.L.N.C. and E.J.P. drafted and revised the manuscript; A.A.d.J. performed the diagnostic testing for STING-associated vasculopathy with onset in infancy; and A.A.d.J., R.G.-M., T.N.H., and A.V.R. revised the draft manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Pellowe EJ, Clarke SLN, Hiliard TN, Ramanana AV. Interstitial lung disease caused by STING-associated vasculopathy with onset in infancy (SAVI) [abstract] Thorax. 2015;70:A1–A254. doi: 10.1164/rccm.201510-2102LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–874. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, Goudin N, Frémond ML, Nitschke P, Molina TJ, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow YJ, Manel N. Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429–440. doi: 10.1038/nri3850. [DOI] [PubMed] [Google Scholar]
- 6.Munoz J, Rodière M, Jeremiah N, Rieux-Laucat F, Oojageer A, Rice GI, Rozenberg F, Crow YJ, Bessis D. Stimulator of interferon genes-associated vasculoapthy with onset in infancy: a mimic of childhood granulomatosis with polyangiitis. JAMA Dermatol. 2015;151:872–877. doi: 10.1001/jamadermatol.2015.0251. [DOI] [PubMed] [Google Scholar]
- 7.Omoyinmi E, Melo Gomes S, Nanthapisal S, Woo P, Standing A, Eleftheriou D, Klein N, Brogan PA. Stimulator of interferon genes-associated vasculitis of infancy. Arthritis Rheumatol. 2015;67:808. doi: 10.1002/art.38998. [DOI] [PubMed] [Google Scholar]
- 8.Petousi N, Thomas EC. Interferon-β-induced pulmonary sarcoidosis in a 30-year-old woman treated for multiple sclerosis: a case report. J Med Case Reports. 2012;6:344. doi: 10.1186/1752-1947-6-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty SD, Harris ME, Schreiner AM, Crow MK. Sarcoidosis triggered by interferon-Beta treatment of multiple sclerosis: a case report and focused literature review. Semin Arthritis Rheum. 2012;42:206–212. doi: 10.1016/j.semarthrit.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Slavenburg S, Heijdra YF, Drenth JP. Pneumonitis as a consequence of (peg)interferon-ribavirin combination therapy for hepatitis C: a review of the literature. Dig Dis Sci. 2010;55:579–585. doi: 10.1007/s10620-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteleagre G, Reinhart A, Brogan P, Berkun Y, Zlotgorski A, Brown D, Chira P, Gao L, Dare J, Schalm S, et al. Preliminary response to Janus kinase inhibition with baricitinib in chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE) Pediatr Rheumatol Online J. 2015;13:O31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.