Abstract
Rationale: Low maximally attained lung function increases the risk of chronic obstructive pulmonary disease irrespective of the subsequent rate of lung function decline.
Objectives: We aimed to determine if there were individuals with a distinct, persistently low lung function trajectory in the CRS (Tucson Children’s Respiratory Study).
Methods: The CRS, an ongoing birth cohort study, enrolled 1,246 participants between 1980 and 1984. Latent class linear mixed effects modeling of the ratio of FEV1 to FVC was used to identify distinct lung function trajectories among participants with two or more spirometry measurements between ages 11 and 32 years.
Measurements and Main Results: Among 599 participants with 2,142 observations, a model with two distinct trajectories (a low trajectory [n = 56; 9.3%] and a normal trajectory) fit the data significantly better than a model with only one trajectory (P = 0.0007). As compared with those with a normal trajectory, participants with a persistently low trajectory were more likely to have a history of maternal asthma (20.0% vs. 9.9%; P = 0.02); early life lower respiratory illness caused by respiratory syncytial virus (41.2% vs. 21.4%; P = 0.001); and physician-diagnosed active asthma at age 32 years (43.9% vs. 16.2%; P < 0.001). Individuals with a persistently low trajectory also demonstrated lower lung function as measured by average maximal expiratory flow at functional residual capacity during infancy and at age 6 years.
Conclusions: A distinct group of individuals in a nonselected population demonstrates a persistently low lung function trajectory that may be partly established at birth and predisposes them to chronic obstructive pulmonary disease later in life.
Keywords: respiratory function tests, chronic obstructive pulmonary disease, asthma, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
Recent work suggests low lung function trajectory in adults increases the risk for chronic obstructive pulmonary disease later in life regardless of the rate of lung function decline. We proposed to identify individuals with a persistently low lung function trajectory from childhood into adulthood using an unsupervised statistical approach and to determine early life exposures associated with a persistently low trajectory.
What This Study Adds to the Field
We examined lung function data from the Tucson Children’s Respiratory Study, a nonselected birth cohort that has been ongoing for almost four decades. Using latent class analysis, we found that nearly 1 in 10 participants demonstrated persistently low lung function from childhood into adulthood, and this trajectory was associated with maternal asthma, low lung function in infancy, early life respiratory syncytial virus lower respiratory infection, and later life asthma. Low lung function trajectory, which is likely to be an important pathway to chronic obstructive pulmonary disease, may be partly established at birth and influenced by early life exposures.
The natural history of lung function is characterized by dramatic periods of growth from birth through adolescence, after which most individuals attain their maximal lung function during the third decade of life (1). Beyond this plateau, lung function declines with aging, with rapid deterioration occurring in some individuals as a result of factors, such as smoking (2). Rapid lung function decline has been thought to be critical in the pathophysiology of chronic obstructive pulmonary disease (COPD), ever since Fletcher and Peto (3) first described this phenomenon in their London smoking cohort. The Fletcher-Peto model assumes that all individuals achieve normal maximal lung function before experiencing rapid decline as a result of smoking or other exposures. In contrast to this widely accepted paradigm, Burrows and colleagues (4) and advocates of the Dutch hypothesis (5, 6) proposed that early life factors may be important in COPD pathogenesis because of effects on lung growth and development. Therefore, with respect to lung function, there may be more than one pathway to COPD. This concept is strongly supported by recent analysis of spirometry data from three independent cohort studies published by Lange and colleagues (7), which shows that low lung function in early adulthood is an important contributor to the origins of COPD. However, this study was unable to determine if there was a distinct group of individuals who attained a lower lung function plateau in early adulthood, or if they were simply the tail of the normal distribution of lung function in the population. Moreover, information regarding early life exposures was not available for these cohorts to evaluate their impact on later life lung function trajectory.
To date, investigation of the potential childhood origins of COPD has been challenging because of the long time frame between early life exposures and later life COPD diagnosis and issues related to recall bias. However, the CRS (Tucson Children’s Respiratory Study) provides the unique opportunity to study lung function trajectories from early life into adulthood and to identify early life epidemiologic and physiologic characteristics associated with low lung function trajectory. We hypothesized that distinct lung function trajectories could be identified in the CRS cohort using unsupervised latent class analysis (8, 9), including (1) normal lung function trajectory, (2) persistently low lung function trajectory, and (3) trajectory of decline from normal to low lung function. Some of the results of these studies have been previously reported in the form of an abstract (10).
Methods
Study Design
The CRS is a longitudinal nonselected birth cohort study of 1,246 individuals who were born in Tucson, Arizona between 1980 and 1984. The overall study design has been previously described (11, 12). Informed consent was obtained from parents at the time of enrollment and then later from the participants. The University of Arizona institutional review board approved the study.
Data Collection
Information about demographics, parental asthma, and parental smoking was collected by questionnaire at the time of enrollment. Acute lower respiratory illnesses (LRI) during the first 3 years were ascertained by pediatrician assessment and confirmed by chest radiograph when pneumonia was suspected (13, 14). Nasopharyngeal swabs were collected at the time of LRI for respiratory syncytial virus (RSV) culture and/or immunofluorescence. An episode was considered to be positive for RSV if culture, immunofluorescence, or both were positive. Partial expiratory flow-volume curves were obtained for a subset of participants during infancy using the chest compression technique, and maximal expiratory flow at FRC (V′maxFRC) was recorded (15). V′maxFRC was also measured at age 6 years using voluntary expiratory maneuvers, as previously reported (15). Spirometry was performed at survey visits conducted at ages 11, 16, 22, 26, and 32 years. Spirometric measurements were obtained at age 11 with a custom-built pneumotachometer connected to a portable computer and at ages 16 and 22 with a portable Schiller Spirovit-SP1 (Schiller AG, Barr, Switzerland). Spirometry was measured at ages 26 and 32 with a Koko Legend spirometer (nSpire Health, Inc., Longmont, CO). Peak flow variability was assessed at age 11 using methods previously described (16). Active asthma, defined by episodes or attacks of asthma and/or active wheeze in the prior year and confirmed by physician diagnosis, was determined by questionnaires at each survey visit, which were completed by the parent before age 18 and the participant thereafter. Smoking status was determined by questionnaire and confirmed by salivary cotinine.
Statistical Analysis
We modeled longitudinal measurements of the ratio of FEV1 to FVC (FEV1/FVC) using a latent class linear mixed effects model, adjusting for sex (8, 9). Although the function of interest was the growth and plateau phase of FEV1, we initially used FEV1/FVC ratio as the measure to assess lung function between ages 11 and 32 years. It is well established that there is uneven growth in height and lung function as well as different progression through stages of pubescent maturation between individuals over the course of adolescence (17–19). In these circumstances, adjustment of an individual’s FEV1 for height relative to other individuals during this interval is inaccurate, and the best method to adjust FEV1 for size is to calculate the FEV1/FVC ratio. Our group and others have shown that the ratio more accurately detects the effects of factors influencing lung function during this period of rapid somatic and lung growth, permitting better comparison across groups than FEV1% predicted (20, 21).
We fit longitudinal models for FEV1/FVC ratio with subject specific random effects for slope and intercept and a fixed effect for sex. We evaluated latent mixture distributions for random slopes and intercepts for up to three latent classes. The clustering of random effects was unsupervised. In addition, uncorrelated errors and autoregressive (AR1) error structure were assessed. Model selection was performed using Akaike Information Criterion (AIC). When applied to longitudinal lung function measurements, this approach allows data-driven discovery of latent classes of lung function trajectory and establishment of individual subjects' probabilities of class membership. Participants were included in this analysis if they had prebronchodilator spirometry measured at least two times between the ages of 11 and 32 years. We also performed a sensitivity analysis in which we restricted analyses to those participants who contributed three or more spirometry measurements. The statistical programming language R (version 3.1.2) was used for data exploration, analyses, and graphics (R packages ggplot2 and lcmm). Additional details regarding these methods are available in the online supplement.
Lung function trajectory class group characteristics were compared using the chi-square test for categorical variables and Student’s t test for continuous variables (with 95% confidence intervals). Comparisons use a P value of 0.05 to determine significance. For comparison of physiologic measurements, results were adjusted for sex using multiple linear regression. V′maxFRC was natural logarithm-transformed, adjusted for infant length or child height, and standardized for average length or height of participants as previously reported (15). Comparison of lung function trajectory classes was performed using Stata version 12 (StataCorp, College Station, TX).
The predicted FEV1 and FVC, respectively, were computed for each participant’s spirometry measurements using their maximum observed height with sex- and race-specific Hankinson reference equations at age 18 for females and age 20 for males. We then calculated the percentage of maximum predicted FEV1 (pmpFEV1) and FVC (pmpFVC) achieved using each individual’s measured lung function divided by their maximum predicted lung function (22). Linear mixed effects models were used to compare pmpFEV1 and pmpFVC longitudinal profiles for the trajectory classes. Estimated means and 95% confidence intervals were computed for each class at each observation age.
Results
Among 1,246 individuals enrolled in the CRS, 599 participants had two or more spirometry measurements performed between age 11 and 32 years, for a total of 2,142 observations (119 with two observations, 166 with three observations, 164 with four observations, and 150 with five observations). Compared with these 599 included participants, excluded participants had slightly lower birth weight and their parents were slightly younger, had fewer years of education, and were more likely to smoke at the time of enrollment (see Table E1 in the online supplement).
Longitudinal modeling results indicated that three lung function trajectory classes were selected by AIC (see Table E2). However, only seven individuals were assigned to a class exhibiting normal initial FEV1/FVC values with a trajectory of rapid FEV1/FVC decline. This class thus remains speculative, and we elected to use the two-class model for further analyses, comparing characteristics of each class. A two-class model showed a better fit than the one class model, as assessed by the AIC. Model comparison using −2 log likelihood ratio also indicated that two latent classes were preferred to one class (χ23 = 17.1; P = 0.0007).
Among the 599 participants in the two-group latent class analysis, 56 (9.3%) were assigned to a persistently low lung function trajectory using probability greater than or equal to 0.5 to define class membership. Of the 56 participants assigned to the low trajectory class, most (n = 33; 58.9%) had a probability greater than or equal to 0.7 of belonging to their trajectory class (see Table E3). Similarly, among the 543 participants assigned to a normal trajectory class, most (n = 408; 75.1%) had a probability greater than or equal to 0.9 of belonging to the normal trajectory class (see Table E3). Compared with the normal trajectory class, the persistently low trajectory class demonstrated an average absolute difference in FEV1/FVC ratio of approximately 9% over the course of the 21-year follow-up (see Table E4).
Participants assigned to normal or persistently low trajectory class did not differ by sex, race/ethnicity, or birth weight (Table 1). Participants assigned to the low lung function trajectory class were more likely to have a history of maternal asthma (20.0% vs. 9.9%; P = 0.02), but there was no difference with respect to paternal asthma (Table 1). There were no differences in antenatal or childhood exposures, such as parental smoking or day care but the proportion of participants who experienced lower respiratory infection caused by RSV during the first 3 years of life was higher among the persistently low class compared with the normal class (41.2% vs. 21.4%; P = 0.001) (Table 2). There was also no difference in the proportion of active smokers during adolescence and young adulthood by lung function trajectory class (Table 2).
Table 1.
Enrollment Characteristics of CRS Participants and Their Parents by Lung Function Trajectory Class
| Characteristic | N | Low Trajectory | Normal Trajectory | P Value |
|---|---|---|---|---|
| Male sex, % (n/N) | 599 | 50.0 (28/56) | 48.8 (265/543) | 0.87 |
| Race/ethnicity, % (n/N) | 599 | |||
| White | 66.1 (37/56) | 63.4 (344/543) | 0.53 | |
| Mexican American | 26.8 (15/56) | 29.4 (160/543) | ||
| African American | 5.3 (3/56) | 2.8 (15/543) | ||
| Other | 1.8 (1/56) | 4.4 (24/543) | ||
| Birth weight, g, mean (SD) | 599 | 3,538 (506) | 3,482 (466) | 0.40 |
| Parental age, yr, mean (SD) | ||||
| Maternal | 599 | 27.7 (4.7) | 27.7 (4.5) | 0.92 |
| Paternal | 592 | 30.6 (8.0) | 30.0 (5.6) | 0.90 |
| Parental asthma,% (n/N)* | ||||
| Maternal | 589 | 20.0 (11/55) | 9.9 (53/534) | 0.02 |
| Paternal | 562 | 5.8 (3/52) | 12.4 (63/510) | 0.16 |
| In utero smoke exposure, % (n/N)† | ||||
| Maternal | 599 | 14.3 (8/56) | 14.7 (80/543) | 0.93 |
| Paternal | 592 | 25.0 (14/56) | 28.2 (151/536) | 0.61 |
Definition of abbreviation: CRS = Tucson Children’s Respiratory Study.
Ever physician diagnosis of asthma.
Active parental smoking at birth.
Table 2.
Childhood and Early Adult Life Exposures and Physiologic Characteristics of CRS Participants by Lung Function Trajectory Class
| N | Low Trajectory | Normal Trajectory | P Value | ||
|---|---|---|---|---|---|
| Exposure, % (n/N) | |||||
| Parental smoking* | 491 | 51.1 (24/47) | 55.9 (248/444) | 0.53 | |
| Early day care† | 578 | 7.6 (4/53) | 7.2 (38/525) | 0.93 | |
| Early life RSV-LRI‡ | 533 | 41.2 (21/51) | 21.4 (103/482) | 0.001 | |
| Active smoking§ | 596 | 35.7 (20/56) | 32.4 (175/540) | 0.62 | |
| Physiologic measure, mean (95% CI) | |||||
| V′maxFRC in infancy, ml/s | 107 | 65.6 (45.0–95.7) | 118.5 (108.0–129.9) | 0.003 | |
| V′maxFRC at 6 yr, ml/s | 444 | 872.6 (795.0–957.7) | 1,212.2 (1,180.1–1,245.1) | <0.001 | |
| Peak flow variability at 11 yr, amp%mean | 489 | 0.103 (0.086–0.124) | 0.074 (0.070–0.078) | 0.001 | |
| Maximally attained FEV1 | 599 | <0.001 | |||
| Absolute, L | 3.53 (3.34–3.72) | 3.86 (3.80–3.92) | |||
| Percent predicted, % | 85.6 (83.3–87.9) | 94.5 (93.7–95.4) | |||
| Maximally attained FVC | 599 | <0.001 | |||
| Absolute, L | 5.06 (4.78–5.34) | 4.75 (4.67–4.83) | |||
| Percent predicted, % | 105.4 (102.8–107.9) | 100.3 (99.1–100.9) |
Definition of abbreviations: CI = confidence interval; CRS = Tucson Children’s Respiratory Study; LRI = lower respiratory illness; RSV = respiratory syncytial virus; V′maxFRC = maximal expiratory flow at FRC.
Mother or father ever reported smoking between enrollment and age 10 years.
Entry into large day care in first 6 months of life.
RSV-LRI during first 3 years.
Any smoking between ages 16 and 26 years.
Among participants with available data, we examined physiologic characteristics by lung function trajectory class. For the subset with lung function measured in infancy, sex-adjusted V′maxFRC was significantly lower among participants assigned to the persistently low class compared with the normal class (Table 2). Similar results were obtained for sex-adjusted V′maxFRC measured at age 6 years. Participants assigned to the persistently low class also demonstrated greater peak flow variability at 11 years of age compared with those assigned to the normal class (Table 2).
With respect to clinical characteristics, individuals assigned to the persistently low class were more likely to have active asthma confirmed by physician diagnosis than those assigned to the normal class, and this finding was consistent across all CRS surveys conducted between the ages of 6 and 32 years (Table 3).
Table 3.
Physician-diagnosed Active Asthma among CRS Participants by Lung Function Trajectory Class
| Survey Age (yr) | N | Low Trajectory [% (n/N)] | Normal Trajectory [% (n/N)] | P Value |
|---|---|---|---|---|
| 6 | 589 | 26.4 (14/53) | 7.7 (41/536) | <0.001 |
| 11 | 585 | 39.6 (21/53) | 15.2 (81/532) | <0.001 |
| 16 | 540 | 30.8 (16/52) | 18.0 (88/488) | 0.027 |
| 22 | 542 | 41.8 (23/55) | 16.8 (82/487) | <0.001 |
| 26 | 499 | 40.4 (21/52) | 17.7 (79/447) | <0.001 |
| 32 | 429 | 43.9 (18/41) | 16.2 (63/388) | <0.001 |
Definition of Abbreviation: CRS = Tucson Children’s Respiratory Study.
Active asthma was ascertained by written questionnaire completed by the participant and/or their parent and was defined by a history of physician diagnosis of asthma and active wheeze during prior year.
In addition to our main analysis that used a model specifying two latent classes including participants with at least two lung function observations, we also performed a sensitivity analysis restricted to those individuals with at least three observations and found similar results (see Table E5). Furthermore, we performed our analysis using a model specifying two latent classes that included adjustment for physician-diagnosed active asthma as a time-dependent variable that demonstrated similar findings (see Table E6). We also explored the effects of including covariates for prenatal smoke exposure and participant smoking at each age. The AIC results and model parameter estimates regarding the number of latent classes and longitudinal profiles are nearly identical to our main model results.
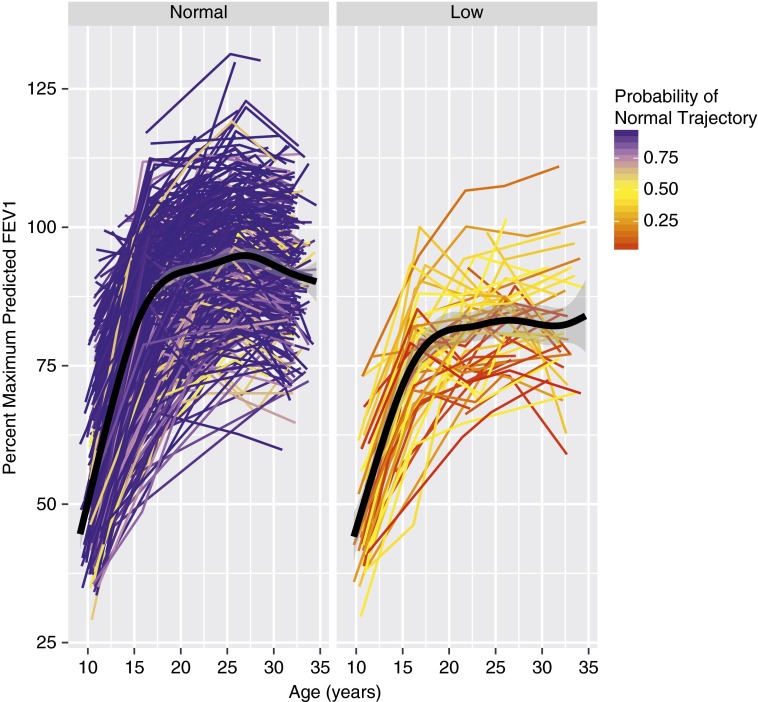
Figure 1 and Figure E1 respectively show individual and modeled pmpFEV1 for the two lung function trajectories obtained using FEV1/FVC ratio. The two discrete trajectories grew in parallel starting at age 11 and reached mean plateaus in lung function that differed by 11% of predicted maximal FEV1 by age 26. Likewise, we examined FVC trajectories and found that pmpFVC was higher on average among low trajectory individuals during adolescence but there was no substantial difference after maximal lung function was achieved by age 26 (mean difference, 2.6%; P = 0.13) (see Figure E2).
Figure 1.
Lung function growth over time expressed as percent of maximal predicted FEV1 for individuals assigned to low lung function trajectory versus normal trajectory from preadolescence into the fourth decade of life. Among 599 participants with at least two spirometry measurements, 543 were assigned to a normal trajectory and 56 to a low trajectory using latent class analysis.
Discussion
Using latent class analysis, we identified two major distinct lung function trajectories extending from preadolescence into the fourth decade of life in the CRS cohort: a persistently low lung function trajectory and a normal lung function trajectory. Participants assigned to the persistently low trajectory reached a maximum level of lung function that was approximately 10 percentage points lower (for both percent of predicted FEV1 values and FEV1/FVC ratio) than those in the normal trajectory.
Recent observations in three general population cohorts followed from early to middle adulthood into senescence have suggested that there may be two major patterns of normal lung function decline with aging, one associated with normal initial lung function and a second with low lung function attainment in early adult life (7). These patterns closely resemble the two distinct trajectories we described herein that are evident between preadolescence and early adult life. In the adult cohort studies, the deleterious effects of smoking were superimposed onto these different patterns of lung function attainment before decline, resulting in excessive decline in subjects with normal initial lung function. Both rapid decliners and those with lower initial lung function were more likely to attain the threshold level of lung function that is used to diagnose COPD compared with those with normal initial lung function and normal rate of decline (7).
Our findings thus indicate that the persistently low lung function trajectory associated with COPD in adult life has its origins during childhood. As such, our conceptual paradigm of the natural history of COPD should be expanded to include individuals whose low lung function trajectory may have its origins in fetal and early postnatal life. Our results suggest that these individuals are not just the tail end of the normal distribution of lung function in the population but constitute a separate, distinct subpopulation. Identifying the risk factors for belonging to this low lung function trajectory could be the basis for the development of novel strategies for the prevention of COPD.
We identified several characteristics that were associated with the low lung function trajectory. Not surprisingly, the low lung function trajectory was associated with lower lung function in infancy and in the early school years. Moreover, the low lung function trajectory was associated with increased peak flow variability and higher prevalence of active asthma than the normal trajectory. This is consistent with findings from the Childhood Asthma Management Program study that found low baseline lung function and airway hyperresponsiveness were associated with reduced lung function growth among children with asthma (23). Children with asthma have been shown to have lower levels of lung function at birth (24), but they also have demonstrated deficits in lung function growth, especially during the first 6 years of life (25), suggesting that both congenital and postnatal mechanisms are involved. Interestingly, members of the persistently low group were more likely to have mothers, but not fathers, with asthma. We have shown previously that children with maternal asthma, especially when breast-fed, have lower lung function than their peers (26). It is tempting to speculate that epigenetic mechanisms associated with maternal asthma may play a role in the development of chronic airflow limitation in children (27).
Participants in the low trajectory were twice as likely to have experienced early life RSV-LRI compared with those in the normal trajectory. We previously demonstrated that RSV-LRI was associated with diminished lung function at age 11 years in this same cohort (28), and the current results strongly suggest that such deficits persist up to the fourth decade of life. We surmise that a strategy of primary prevention of RSV infection in early life could decrease the incidence of COPD in adult life, although we cannot exclude the possibility that at least some of the observed deficits in lung function could have predated the episode of RSV-LRI.
Our study strengths include the use of longitudinal pulmonary function data from a well-characterized prospective birth cohort, which allowed us to examine the relationships between early life factors and lung function trajectory through early adulthood. We used an unsupervised modeling strategy that did not require us to identify arbitrary cutoffs to define “abnormal” lung function. In addition, our model was flexible in that it allowed the changes in lung function over time to vary between the different lung function trajectory classes. We also identified probabilities of class membership for each individual after model fitting. Weaknesses of this modeling strategy include that we selected an arbitrary probability cutoff of greater than or equal to 0.5 to define class membership, and we assumed sex differences to be stable over time. We also chose to include individuals in the analysis who had at least two measurements of lung function to maximize the generalizability of our results, but we confirmed our findings in an analysis restricted to participants with three or more measurements.
In conclusion, latent class analysis identified a distinct subpopulation with a persistently low lung function trajectory from preadolescence into adulthood representing almost 1 in 10 participants in the CRS. The persistently low group had lower lung function in early life, higher prevalence of maternal asthma, and higher incidence of early life RSV-LRI. The low lung function trajectory likely represents an important pathway to COPD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors are grateful to Lynn Taussig for starting the Tucson Children’s Respiratory Study; Bruce Saul for data management; and study nurses Marilyn Lindell, Lydia de la Ossa, and Nicole Pargas for data collection and participant follow-up.
Footnotes
The Tucson Children’s Respiratory Study was supported by a grant from the National Institutes of Health NHLBI (2R01HL056177). This project was also supported by a career development award from the Arizona Health Sciences Center (C.E.B.) and a grant from the American Lung Association (C.E.B.).
Author Contributions: C.E.B. and F.D.M. contributed to the design of the work, data analysis and interpretation, and drafting and revising of the manuscript. D.B., I.C.J., and Z.J.L. contributed to the design of the work, data analysis and interpretation, and drafting of the manuscript. D.A.S., W.J.M., and A.L.W. contributed to the data acquisition, analysis, and interpretation and critical revision of the manuscript. L.B.G., T.F.C., and S.G. contributed to the data analysis and interpretation and critical revision of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0753OC
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 2.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899–909. doi: 10.1016/S0140-6736(14)60446-3. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977;115:751–760. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- 5.Postma DS, Weiss ST, van den Berge M, Kerstjens HA, Koppelman GH. Revisiting the Dutch hypothesis. J Allergy Clin Immunol. 2015;136:521–529. doi: 10.1016/j.jaci.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, de Marco R, Norbäck D, Raherison C, Villani S, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 7.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 8.Verbeke G. E L. A linear mixed-effects model with heterogeneity in the random-effects population. J Am Stat Assoc. 1996;91:217–221. [Google Scholar]
- 9.Proust-Lima C, Phillips V, Diakite A, Liquet B.lcmm: extended mixed models using latent classes and latent processes [accessed 2015 Oct 24]. Available from: http://CRAN.R-project.org/package=lcmm
- 10.Berry CE, Jenkins IC, Billheimer DD, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Lung function trajectories in the Tucson Children’s Respiratory Study. Am J Respir Crit Care Med. 2015;191:A2314. [Google Scholar]
- 11.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675, quiz 676. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 12.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 13.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135:607–616. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voraphani N, Stern DA, Wright AL, Guerra S, Morgan WJ, Martinez FD. Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190:392–398. doi: 10.1164/rccm.201311-2095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 16.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, Martinez FD. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946–952. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal M, Bain SH, Cramer D, Helms P, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: I--Spirometry. Thorax. 1993;48:794–802. doi: 10.1136/thx.48.8.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borsboom GJ, van Pelt W, Quanjer PH. Interindividual variation in pubertal growth patterns of ventilatory function, standing height, and weight. Am J Respir Crit Care Med. 1996;153:1182–1186. doi: 10.1164/ajrccm.153.3.8630565. [DOI] [PubMed] [Google Scholar]
- 19.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol. 1996;21:383–397. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–1488. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, Wise RA, Szefler SJ, Sharma S, Kho AT, et al. CAMP Research Group. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 25.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilbert TW, Stern DA, Morgan WJ, Martinez FD, Wright AL. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am J Respir Crit Care Med. 2007;176:843–848. doi: 10.1164/rccm.200610-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVries A, Vercelli D. Early predictors of asthma and allergy in children: the role of epigenetics. Curr Opin Allergy Clin Immunol. 2015;15:435–439. doi: 10.1097/ACI.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.