Abstract
Rationale: In heritable pulmonary arterial hypertension with germline mutation in the bone morphogenetic protein receptor type 2 (BMPR2) gene, right ventricle (RV) dysfunction is associated with RV lipotoxicity; however, the underlying mechanism for lipid accumulation is not known.
Objectives: We hypothesized that lipid accumulation in cardiomyocytes with BMPR2 mutation occurs owing to alterations in lipid transport and impaired fatty acid oxidation (FAO), which is exacerbated by a high-lipid (Western) diet (WD).
Methods: We used a transgenic mouse model of pulmonary arterial hypertension with mutant BMPR2 and generated a cardiomyocyte cell line with BMPR2 mutation. Electron microscopy and metabolomic analysis were performed on mouse RVs.
Measurements and Main Results: By metabolomics analysis, we found an increase in long-chain fatty acids in BMPR2 mutant mouse RVs compared with controls, which correlated with cardiac index. BMPR2-mutant cardiomyocytes had increased lipid compared with controls. Direct measurement of FAO in the WD-fed BMPR2-mutant RV showed impaired palmitate-linked oxygen consumption, and metabolomics analysis showed reduced indices of FAO. Using both mutant BMPR2 mouse RVs and cardiomyocytes, we found an increase in the uptake of 14C-palmitate and fatty acid transporter CD36 that was further exacerbated by WD.
Conclusions: Taken together, our data suggest that impaired FAO and increased expression of the lipid transporter CD36 are key mechanisms underlying lipid deposition in the BMPR2-mutant RV, which are exacerbated in the presence of dietary lipids. These findings suggest important features leading to RV lipotoxicity in pulmonary arterial hypertension and may point to novel areas of therapeutic intervention.
Keywords: pulmonary arterial hypertension, fatty acid oxidation, fatty acid transporter (CD36), right ventricular lipotoxicity, lipotoxic cardiomyopathy
At a Glance Commentary
Scientific Knowledge on the Subject
Lipid accumulation in the right ventricle (RV) with bone morphogenetic protein receptor type 2 (BMPR2) mutation is shown to be associated with failure of RV hypertrophy in both the animal model and the human heritable form of pulmonary arterial hypertension (PAH), but the mechanisms of lipid accumulation in the RV are as yet unknown.
What This Study Adds to the Field
The current study unravels the principal mechanisms leading to RV lipotoxicity in heritable PAH using a novel cultured cardiomyocyte cell line with mutant BMPR2 expression and a transgenic mouse model of PAH with the same BMPR2 mutation. Our studies demonstrate that impaired fatty acid oxidation is the key mechanism underlying lipid accumulation in the BMPR2-mutant RV, and increased expression of the lipid transporter molecule CD36 also contributes to lipid accumulation. This study further demonstrates that these alterations are exacerbated in the presence of elevated dietary lipids. These findings will help develop novel areas of therapeutic intervention.
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by progressive obliteration of the pulmonary vasculature, which causes right ventricle (RV) dysfunction leading to right heart failure and ultimately death (1). Patients with heritable PAH (HPAH) with germline mutations in the bone morphogenetic protein receptor type 2 (BMPR2) gene have been reported to present approximately 10 years earlier and with more severe disease and compromised hemodynamics compared with patients with idiopathic PAH (2–5), suggesting that in PAH, mutations in BMPR2 are associated with distinct RV disease phenotypes. Transforming growth factor-β/BMP superfamily signaling has been linked to the development of maladaptive left ventricular hypertrophy (6), although little is known about this pathway in the RV. In humans with HPAH and in HPAH animal models we have published that expression of mutant BMPR2 in RV is associated with failure of RV hypertrophy, increase in transforming growth factor-β signaling pathway genes, abnormalities in fatty acid metabolic genes, and increased lipid deposition in RV cardiomyocytes (7), implying a lipotoxic cardiomyopathy (8, 9), but the mechanism of RV lipid accumulation in PAH is currently unknown.
In cardiomyocytes, lipid accumulation could be due to increased uptake of free lipids via fatty acid transporter molecules, impaired fatty acid oxidation (FAO), or increased lipid synthesis within the cell. In cardiomyocytes, fatty acids are the predominant energy source. However, cardiomyocytes are capable of metabolic plasticity and thus can adapt to changes in the environment by switching to other substrates (10). Although mitochondrial shift from fatty acids to glucose may be a critical mediator of RV dysfunction in PAH, growing evidence suggests that impairment of fatty acid metabolism and mitochondrial dysfunction can possibly promote lipid deposition and RV failure (7, 11–13). In addition, a high-lipid (Western) diet (WD) may also induce cardiomyocyte lipid accumulation and promote RV dysfunction (14). We therefore hypothesized that lipid accumulation in cardiomyocytes with BMPR2 mutation occurs due to impaired FAO, which is exacerbated by WD. We tested this hypothesis using a transgenic mouse model of PAH with ubiquitous expression of mutant BMPR2 and also in vitro using a novel cardiomyocyte cell line with BMPR2 mutation. Some of the results of these studies have been previously reported in the form of an abstract (15, 16).
Methods
See Methods in the online supplement for details.
Results
Long-Chain Fatty Acids Accumulate in the Mouse RV with BMPR2 Mutation
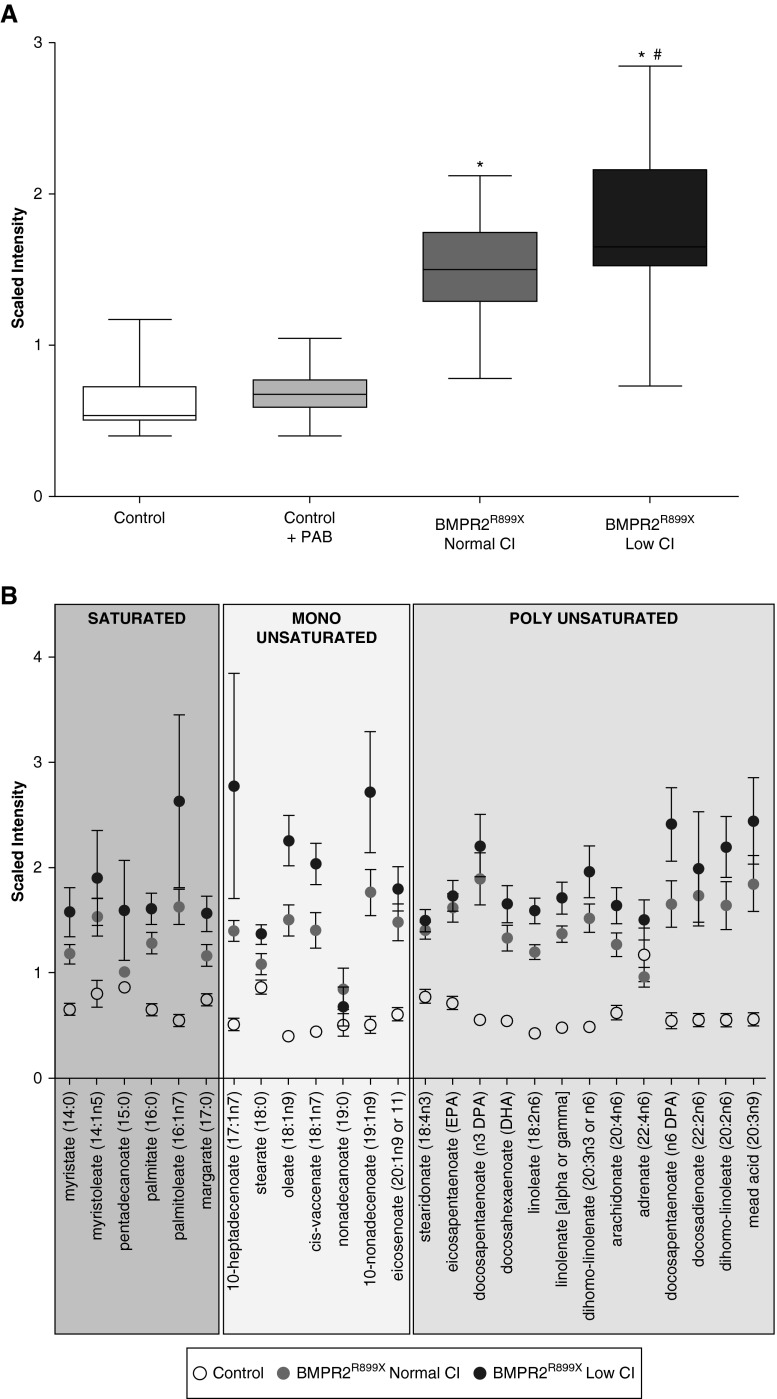
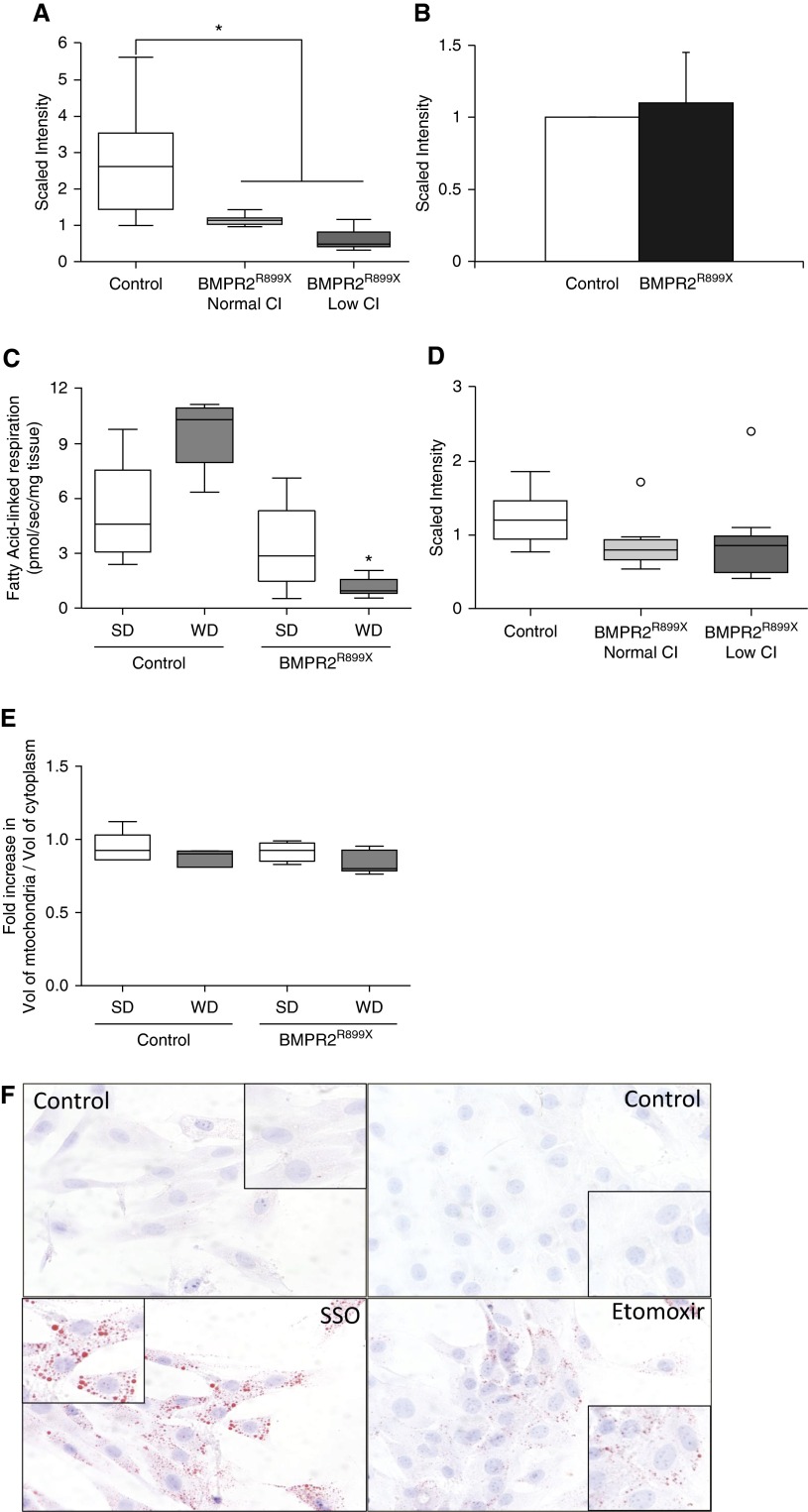
We have previously identified elevated triglycerides in the RV of our transgenic rodent model (7), but a more detailed speciation of lipid intermediaries accumulating as a function of BMPR2 mutation has not been performed. Using a metabolomics approach, we compared the lipid distribution in RV tissue from BMPR2 mutant mice to control littermates with or without pulmonary arterial banding as a model of adaptive hypertrophy, all fed standard diet (SD). We found a significant increase in long-chain fatty acids (LCFAs) in BMPR2 mutant mice RV compared with control mice (Figure 1). The LCFAs present in the BMPR2 mutant RV were saturated or monounsaturated and polyunsaturated LCFAs (Figure 1B). There was no change in the distribution of LCFAs in mice with pulmonary arterial banding compared with control mice. We also explored whether reduced cardiac function in our transgenic rodent model was associated with higher levels of lipid deposition. As there is a range of cardiac index (CI) in pulmonary hypertension, we stratified the mutant mice by CI (with lower or with preserved CI; see Table E1 in the online supplement) and linked to RV function. We found that in the BMPR2 mutant mouse RV, lower CI was associated with increased accumulation of LCFAs compared with mutant mice with normal CI (Figure 1A). These findings indicate that in a rodent model of BMPR2 mutation, RV dysfunction is associated with accumulation of LCFA that is not present in a model of isolated pressure overload. Moreover, higher LCFA levels were associated with worse RV function, suggesting that degree of lipid deposition correlates inversely with RV function.
Figure 1.
Long-chain fatty acids (LCFAs) are increased in mouse right ventricle (RV) in the presence of bone morphogenetic protein receptor type 2 (BMPR2) mutation. (A) Total amount of LCFAs in control (white box; n = 7), control with pulmonary arterial banding (PAB; light gray box; n = 8), BMPR2R899X with normal cardiac index (CI; dark gray box; n = 7), and BMPR2R899X with low CI (black box; n = 8). *P < 0.0001. LCFAs are significantly increased in the mouse RV with BMPR2 mutation compared with control littermates; #P < 0.009. In the mouse RV with BMPR2 mutation, LCFAs are increased further in the group with the lower CI. (B) Distribution of saturated and unsaturated LCFAs in mouse RV; control (open circles), BMPR2R899X with normal CI (gray circles), and BMPR2R899X with low CI (black circles).
Characterization of H9c2 Cells
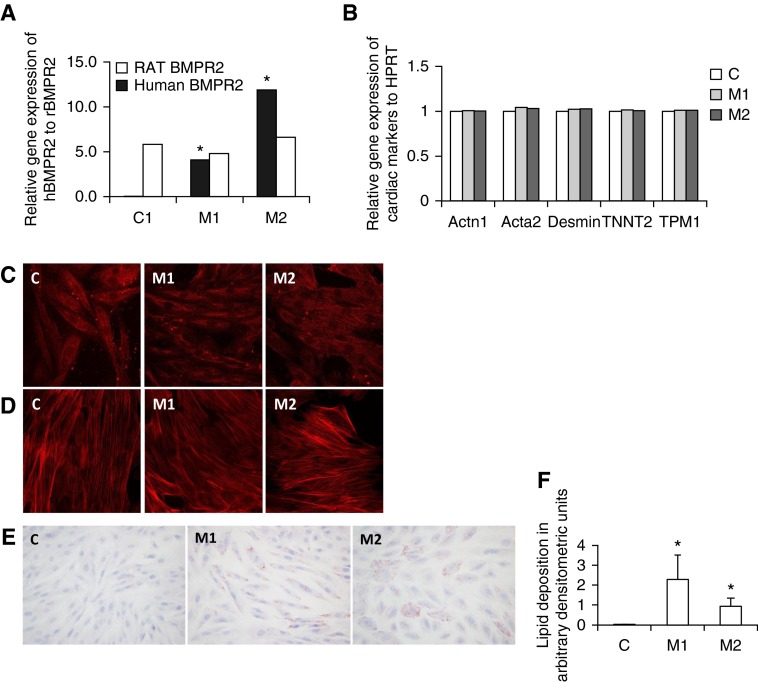
To show that RV lipid accumulation in the mutant BMPR2 phenotype occurs independent of pulmonary vascular disease, we next performed cell culture experiments in the H9c2 cell line (17–19). We generated H9c2 clones stably expressing either empty vector (C) or two forms of human BMPR2 gene: mutation in cytoplasmic (M1) or kinase domain (M2) (Figure 2A). All the H9c2 clones expressed cardiac-specific markers (Acta1; Acta2; Desmin; troponin T2, cardiac type; tropomyosin 1α) (Figure 2B), and demonstrated immunoreactivity for cardiac troponin T (Figure 2C) and F-actin (Figure 2D), markers of cardiomyocytes. To determine if lipid accumulation is present in these cell lines and thus independent of load stress, we performed Oil Red O stain on control and mutant H9c2 cells (Figure 2E) and quantified the accumulated lipids using Image J software. We found lipid accumulation was exclusively present in the cardiomyocytes that expressed mutant BMPR2 gene (Figure 2F). Our results show that lipid accumulation in BMPR2 mutant H9c2 cells is a consequence of BMPR2 mutation and does not require load stress.
Figure 2.
Characterization of H9c2 cells. (A) Rat bone morphogenetic protein receptor type 2 (BMPR2) gene (open bars) is expressed in control (C), mutant1 (M1), and mutant2 (M2) cells. Human mutant BMPR2 gene (solid bars) is expressed in M1 and M2 but not in C cells. *P < 0.05. (B) Cardiac markers Actn1, Acta2, Desmin, TNNT2, and TPM1 are equally expressed in C (white bars), M1 (light gray bars), and M2 (dark gray bars). (C) Cardiac TNNT2 and (D) F-actin are present in C, M1, and M2 cells. (E) Lipid deposition is present in in M1 and M2 cells compared with C as seen by Oil Red O staining. (F) Bar graph represents lipid deposition. HPRT = hypoxanthine phosphoribosyltransferase; TNNT2 = troponin T2, cardiac type; TPM1 = tropomyosin 1α.
Cardiomyocyte Expression and Localization of the Fatty Acid Transporter CD36 Is Altered by Both BMPR2 Mutation and Dietary Lipids
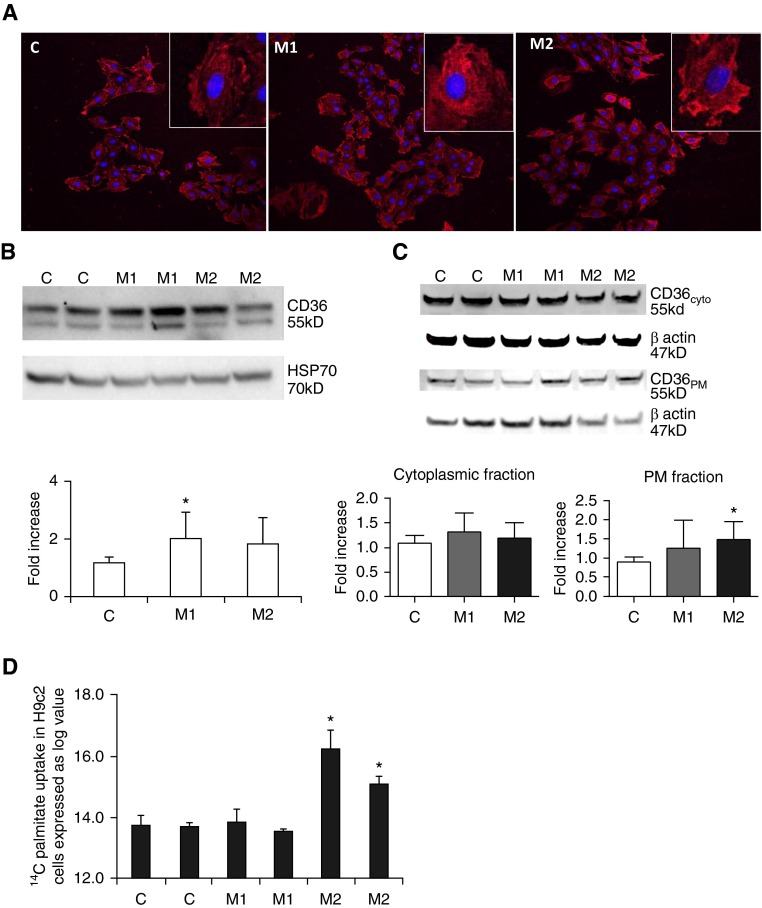
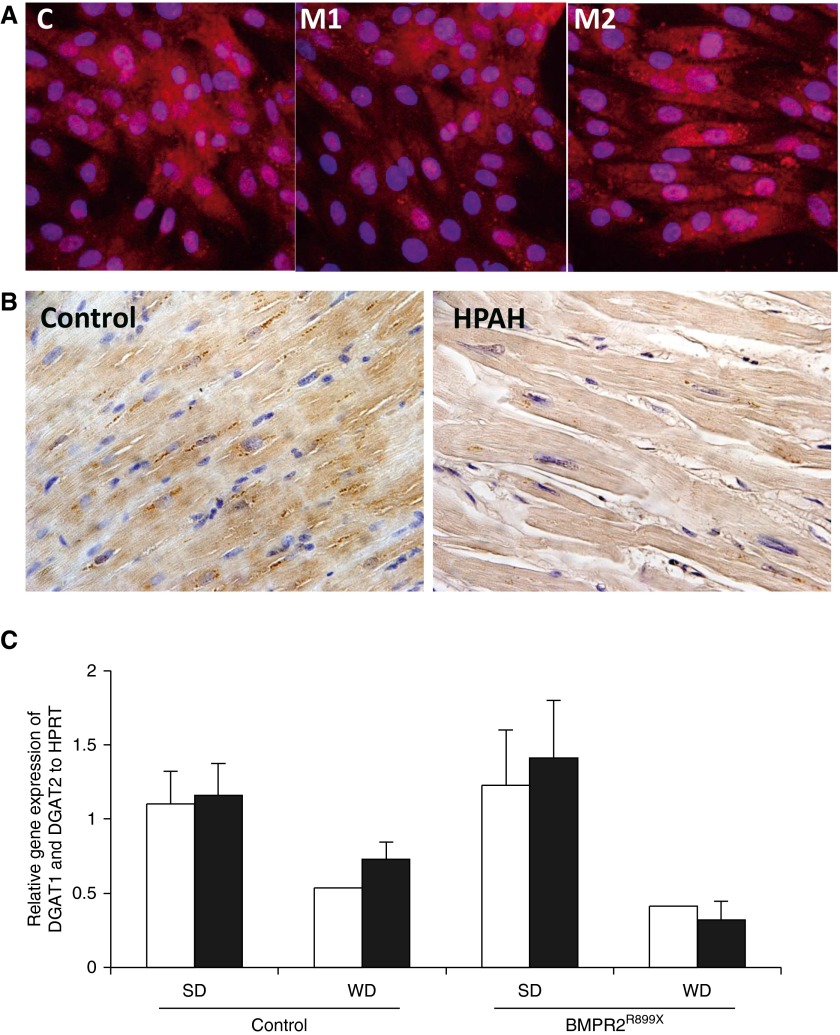
The observed lipid deposition in BMPR2 mutant cardiomyocytes could be a result of increased uptake of fatty acids, dysregulation of fatty acid metabolism, or increased synthesis of lipids. We investigated each of these features in our mouse model and cultured cardiomyocytes with BMPR2 mutation. CD36, the main fatty acid transporter molecule, facilitates fatty acid uptake by being recruited to the sarcolemma from the intracellular compartment (20–23). This molecule plays a role in the pathogenesis of various cardiac metabolic diseases, including alterations in cellular lipid metabolism (cardiac lipotoxicity) leading to cardiac contractile dysfunction (24–26). We tested the presence of CD36 in lipid accumulation in BMPR2 mutant H9c2 cells. We found an increase in CD36 protein in mutants, which was significant (P = 0.02) in M1 and trends toward significance (P = 0.06) in M2 cells compared with controls (C) (Figure 3B). In control cells, CD36 was predominantly localized in cytoplasm, whereas in M1 and M2 cells the localization of CD36 was in both the cytoplasm and the plasma membrane (Figure 3A). To confirm the immunohistochemical findings and determine if there was increased plasma membrane localization in both mutant lines, we performed subcellular fractionation and enriched plasma membrane and cytoplasmic fractions from H9c2 cells. In the plasma membrane fraction, there was a significant increase in CD36 protein in the mutant M2 cells, but not the Cy fraction, compared with control cells (Figure 3C). To demonstrate a mechanistic role for CD36 in mutant BMPR2 cells, we performed radioactive palmitate uptake assay in C, M1, and M2 cells using 14C palmitate. We found a significant uptake of radioactive palmitate in M2 mutant cells compared with control but not in M1 mutant cells (Figure 3D). These findings indicate that in cardiomyocytes with BMPR2 mutation, particularly the M2 cell line, increased CD36 protein expression, and its redistribution may contribute to the observed lipid accumulation.
Figure 3.
CD36 protein is increased in H9c2 cells with bone morphogenetic protein receptor type 2 mutation. (A) CD36 protein (red) is localized in cell membrane and cytoplasm of control (C), mutant1 (M1), and mutant2 (M2) cells with greater staining in both mutant lines. Nucleus is stained blue. (B) Total CD36 protein is increased in M1 (n = 4; *P < 0.05) but not M2 (n = 6) compared with C cells (n = 6). Bar graph represents total protein. (C) In plasma membrane (PM) fraction, CD36 is increased in M2 cells (n = 4; *P < 0.05) but not in M1 cells (n = 4) compared with C cells (n = 4); in cytoplasmic fraction, CD36 was similar between C (n = 4), M1 (n = 4), and M2 (n = 4) cells. (D) 14C palmitate uptake is observed in M2 cells (n = 3; P < 0.05) but not M1 (n = 3) and C (n = 3) cells. HSP70 = heat shock protein 70.
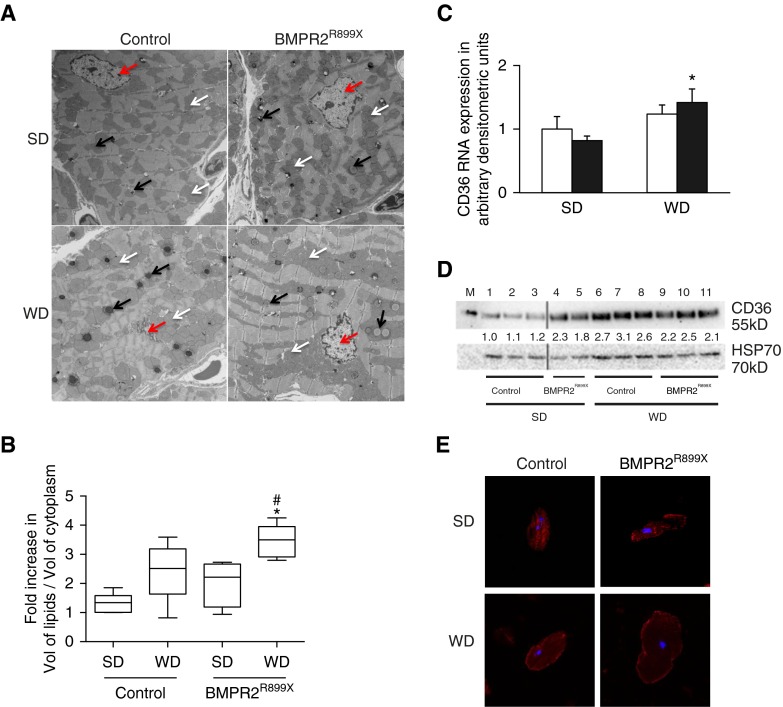
We have previously found that in the BMPR2 mutant mouse model of PAH, WD resulted in increased right ventricular systolic pressure and penetrance as well as weight gain despite the consumption of the same amount of chow compared with control littermates (7, 27), but the effects of WD on RV lipid content has not been studied. We used electron microscopy to quantify the volume of RV tissue lipid droplets in RV from control and BMPR2 mutant mice fed either SD or WD diet. We found that when on SD there was a trend toward increase in the volume of lipids (P = 0.06) in BMPR2 mutant mouse RV compared with control mouse RV (Figures 4A and 4B). With WD, the volume of lipids was significantly higher in BMPR2 mutant mouse RV than in BMPR2 mutant littermates (P < 0.03) and control littermates (P < 0.001) on SD (Figures 4A and 4B). In RV tissue from all the groups, the lipid droplets were clearly visible close to mitochondria, suggesting that the lipids may be underused for mitochondrial FAO. These data show that WD alone is associated with RV lipid deposition, and this is further augmented in the context of BMPR2 mutation.
Figure 4.
In mouse right ventricle (RV), CD36 protein is increased and associated with lipid accumulation as a result of bone morphogenetic protein receptor type 2 (BMPR2) mutation or Western diet (WD). (A) Electron microscopic images of lipid bodies (black arrows), mitochondria (white arrows), and nucleus (red arrows) in RV tissue from control or BMPR2R899X mice fed standard diet (SD) or WD. (B) Bar graph representing quantitative analysis of fold increase of volume of lipids to volume of cytoplasm in RV tissue from control or BMPR2R899X mice fed SD or WD. Volume of lipids is increased in RV cardiomyocytes from BMPR2R899X mice fed WD compared with BMPR2 mutant littermates (#P < 0.05) and control littermates (*P < 0.001) fed SD. (C) CD36 gene expression is unchanged in BMPR2R899X (solid bars) compared with control mice (open bars). CD36 gene expression is increased in RV from BMPR2R899X and control mice fed WD compared with SD (*P < 0.05). (D) CD36 protein trended toward an increase in RV from BMPR2R899X (n = 2) fed SD and was significantly higher in RV from BMPR2R899X (n = 3; P < 0.005) and control mice (n = 3; P < 0.005) fed WD compared with control mice (n = 3) fed SD. (E) In primary cultures of mouse RV cardiomyocytes, in control mice fed SD, CD36 (red) is localized in cytoplasm, whereas in BMPR2R899X fed SD as well as in RV cardiomyocytes from BMPR2R899X and control mice fed WD, CD36 is distinctly localized in cytoplasm and plasma membrane. Nucleus is blue in color. HSP70 = heat shock protein 70.
To determine if dietary lipids could trigger increase or redistribution of CD36, we measured Cd36 expression in control and BMPR2 mutant mice fed SD or WD. We found that Cd36 gene expression was similar in RV from control and BMPR2 mutant mice fed either SD or WD (Figure 4C). However, with WD, Cd36 gene expression trended toward an increase in control RV and was significantly higher in BMPR2 mutant RV compared with respective groups on SD. At the protein level, with SD there was a twofold increase in CD36 protein in BMPR2 mutant RV compared with control mouse RV (Figure 4D). With WD there was also a twofold increase in CD36 protein in control and BMPR2 mutant RV, which was significant compared with control RV from mice fed SD. We then sought to demonstrate the cellular localization of CD36 using primary cultures of mouse RV cardiomyocytes. In the RV cardiomyocytes from control mice fed SD, CD36 is predominantly localized in the cytoplasm, but in RV cardiomyocytes from BMPR2 mutant mice, CD36 is localized to the plasma membrane and in the cytoplasm. Similarly, in RV cardiomyocytes from mice fed WD, CD36 was localized in the cytoplasm and to the plasma membrane both in control and BMPR2 mutant RV cardiomyocytes (Figure 4E), similar to our earlier findings in the H9c2 cell line. Taken together, these findings suggest that as a consequence of BMPR2 mutation, expression of CD36 fatty acid transporter protein is increased in cardiomyocytes. Furthermore, an increased availability of dietary lipids results in further increase in CD36 and redistribution in RV cardiomyocytes.
The RV with Mutant BMPR2 Expression Demonstrates Suppression of FAO
In cardiomyocytes, ATP is generated via fatty acid β-oxidation. In the classical pathway of FAO, fatty acids are bound to Coenzyme A after entering the cell and subsequently form fatty-acyl carnitines to facilitate their entry into the mitochondria, where they undergo β-oxidation. Although other animal models of RV failure have been found to have suppressed FAO, whether this is present as a consequence of BMPR2 mutation is unknown (28–32). We first investigated the intermediaries of the FAO pathway using our metabolomics data in BMPR2 mutant RV cardiomyocytes. There was a significant decrease in long-chain acylcarnitines in BMPR2 mutant mouse RV compared with control mouse RV (Figure 5A, Figure E1). And, when BMPR2 mutant mouse RVs were stratified based on cardiac function, the levels of these acylcarnitines, although not significantly decreased, show a trend with lower CI (Figure 5A). We found that the levels of metabolites in carnitine synthesis were similar in control and BMPR2 mutant mice RV (Figure 5B), suggesting that although carnitines are present in the cytoplasm, acylcarnitines are not formed in BMPR2 mutant mouse RV to undergo mitochondrial β-oxidation.
Figure 5.
Fatty acid β-oxidation is impaired in right ventricle (RV) cardiomyocytes with mutant bone morphogenetic protein receptor type 2 (BMPR2). (A) Acylcarnitines (medium- and long-chain acylcarnitines; see Figure E1) are significantly decreased in mouse RV with BMPR2 mutation and decrease in cardiac index (CI; *P < 0.001). White, control (n = 7); light gray, BMPR2R899X with normal CI (n = 7); dark gray, BMPR2R899X with low CI (n = 8). (B) Metabolites of carnitine metabolism are unaffected by mutant BMPR2 in mouse RV (P not significant). (C) Mutant BMPR2 shows a trend to decrease in mitochondrial Δ oxygen flux in response to palmitoylcarnitine, which is significant when fed Western diet (WD; *P < 0.002). (D) There is a trend toward decrease in β-hydroxybutyrate with mutant BMPR2 and lower CI in mouse RV (open circles indicate outliers). (E) In RV cardiomyocytes, volume of mitochondria is unchanged with BMPR2 mutation and WD. (F) β-Oxidation pathway inhibitors sulfosuccinimidyl oleate (SSO) and etomoxir cause lipid accumulation (red color) in control H9c2 cells. SD = standard diet.
We further directly tested mitochondrial β-oxidation in the context of BMPR2 mutation. Using high-resolution respirometry, we determined the incremental change in oxygen (O2) consumption supported by an acylcarnitine substrate (palmitoylcarnitine) in permeabilized mouse RV tissue from control and BMPR2 mutant mice fed either SD or WD. With SD, we found a trend toward decrease in FAO-attributable O2 consumption in BMPR2 mutant mouse RV compared with control mouse RV (Figure 5C). With WD, FAO-attributable O2 consumption was nearly obliterated in BMPR2 mutant mouse RV, whereas control RV, although not significant, show an increase in FAO-supported O2 consumption in the context of a WD (Figure 5C). Supporting this evidence of reduced fat metabolism, our metabolomics analysis also demonstrated a trend toward a decrease in β-hydroxybutyrate, a metabolic product of fatty acid breakdown and ketogenesis, in BMPR2 mutant mouse RV compared with control mouse RV (Figure 5D). The baseline respiration as represented by Complex I–linked respiration (in the presence of glutamate and malate) was not different between the diet conditions or genotypes (Figure E2).
We further investigated mitochondrial volume in RV cardiomyocytes by electron microscopy to determine if the observed decrease in β-oxidation was due to reduced mitochondrial volume. We found that the volume of mitochondria in BMPR2 mutant mouse RV cardiomyocytes was similar to RV cardiomyocytes from control mice fed either SD or WD, suggesting decrease in mitochondrial FAO-attributable O2 consumption is independent of size or number mitochondria (Figure 5E, Figure E3). Taken together, these findings indicate that the FAO pathway in the BMPR2 mutant RV is impaired at multiple points, including transport into the mitochondria and β-oxidation itself.
We next sought to test the hypothesis that impaired FAO by itself is sufficient to cause lipid accumulation in control cardiomyocytes. We treated control H9c2 cells with two inhibitors of FAO pathway: sulfosuccinimidyl oleate (SSO) and etomoxir. SSO is not only an established inhibitor of CD36 but it also effectively inhibits mitochondrial metabolism at several sites, with inhibition of palmitoylcarnitine-supported respiration being the most potent inhibitory effect observed (half maximal inhibitory concentration around 5 μM) (33). Etomoxir is known to inhibit mitochondrial fatty acid β-oxidation by irreversibly binding to carnitine pamitoyltransferase-1 and preventing entry of LCFAs into the mitochondrial matrix (33, 34). We found that in the presence of either SSO or etomoxir there was significant lipid accumulation in control H9c2 cells (Figure 5F). Indeed, significant lipid accumulation for SSO was observed in spite of its inhibitory effects on CD36, suggesting that the inhibition of FAO is an overriding driving force for lipid accumulation. Together, these findings suggest that in cardiomyocytes lipid accumulation can be recapitulated through impairment of mitochondrial FAO, suggesting this is a key mechanism underlying lipid accumulation in the BMPR2 mutant RV, which is exacerbated in the presence of dietary lipids.
De Novo Lipid Synthesis in RV Cardiomyocytes Is Unaffected by BMPR2 Mutation
DGAT (acyl CoA: diacylglycerol acyltransferase) is a microsomal enzyme that catalyzes the conversion of diacylglycerol (DAG) to triglycerides (35). As our previous findings showed increased accumulation of triglycerides in human RV with BMPR2 mutation (7), we sought to determine if the expression of DGAT1 is altered in cardiomyocytes in the presence of BMPR2 mutation. In H9c2 cells we found that DGAT1 protein was localized in the cytoplasm in mutant and control cells (Figure 6A). There was no difference in the DGAT1 localization in mutant cells compared with controls. We confirmed this finding in human RV from autopsy specimens of PAH (n = 3; Table E2) and specimens from healthy control subjects (n = 3) who died free of cardiovascular disease. In human RV tissue, DGAT1 protein was localization in the cytoplasm and as expected was similar in HPAH compared with controls (Figure 6B). We next sought to determine the mRNA expression of the two DGAT isoforms (Dgat1 and Dgat2) in BMPR2 mutant RV tissue and compare with control RV tissue from mice fed either SD or WD. We found that with either SD or WD, there was no difference in the Dgat1 and Dgat2 gene expression in BMPR2 mutant mouse RV compared with control mouse RV. However, there was a trend toward a decrease in DGAT genes (1 and 2) in control RV and a significant decrease in BMPR2 mutant RV when fed WD compared with SD (Figure 6C). These data demonstrate the lipid accumulation in BMPR2 mutant RVs is not due to increased synthesis.
Figure 6.
In cardiomyocytes, bone morphogenetic protein receptor type 2 (BMPR2) mutation does not up-regulate acyl CoA: diacylglycerol acyltransferase (DGAT). (A) In control (C), mutant1 (M1), and mutant2 (M2) cells, DGAT1 localized in cytoplasm and nucleus. (B) In right ventricle (RV) cardiomyocytes from control specimens (n = 3) and specimens from subjects with heritable pulmonary arterial hypertension (HPAH; n = 3), DGAT1 localized mainly in the cytoplasm (brown color). Nucleus is blue. (C) In mouse RV, Dgat1 (open bars) and Dgat2 (solid bars) gene expression was similar in control and BMPR2R899X when fed standard diet (SD) and trended toward a decrease in control RV and significantly decreased (P < 0.05) in BMPR2 mutant RV with Western diet (WD). HPRT = hypoxanthine phosphoribosyltransferase.
Discussion
In this series of experiments, we explored the mechanisms through which BMPR2 mutation in cardiomyocytes results in lipid accumulation, We used both cell culture and animal models as well as human tissue and direct measurements of respirometry to demonstrate altered transport of fats into cardiomyocytes with BMPR2 mutation and, moreover, demonstrated a key role for suppression of FAO in promotion of lipid accumulation. In addition, our findings suggest that high-fat diet could also increase lipid deposition in RV cardiomyocytes through increased transport, which is further exaggerated in the presence of mutated BMPR2. Our findings provide mechanistic insight into lipid accumulation in RV failure in PAH, which has previously been demonstrated to be lipotoxic (7).
A major challenge to studying mechanisms of lipid deposition was the lack of a cell culture model of BMPR2 mutation. To address this problem, we developed a novel cardiomyocyte cell line, H9c2 stably transfected with mutant BMPR2. The two mutant constructs used to transfect H9c2 cells are present in families with HPAH (7, 36, 37). The M1 mutant is described as a BMPR2 gene with a 2579-2580delT resulting in a frameshift at amino acid 859 resulting in 10 missense amino acids and a stop leads to deletion of cytoplasmic tail. M2 is the BMPR2 gene with a C993T mutation resulting in R332X in kinase domain deletion. With the exception of expression of the BMPR2 mutation, the BMPR2 mutant H9c2 cells were genetically similar to cells expressing empty vector and the stably transfected H9c2 cells expressed cardiomyocyte markers (38), suggesting that the presence of mutant BMPR2 did not affect cellular characterization. In addition, these mutant cell lines also expressed the cardiac markers, troponin T and F-actin, shown to be expressed by H9c2 cells in culture (38). The advantages of these cell lines were (1) to demonstrate that lipid accumulation in cardiomyocytes can occur in the absence of load stress, which is only possible in an ex vivo model; and (2) to mechanistically study the effects of BMPR2 mutation on CD36 trafficking and mitochondrial function. A key finding in our data was that lipid accumulation occurs in the cultured cardiomyocytes with BMPR2 mutation, thus confirming that the lipid effects of BMPR2 mutation do not require pulmonary vascular disease and are dependent on altered BMPR2 signaling within the cardiomyocyte.
When RV is in its basal metabolic state, LCFAs are the predominant source of energy production (60–90%), and carbohydrates (e.g., glucose) are secondary source (39, 40). In PAH, changes in fatty acid metabolism can promote RV dysfunction and may underlie the poor prognosis in patients with pulmonary hypertension (7, 11–13). Thus, alterations in lipid transport, metabolism, and storage can potentially contribute to RV dysfunction in PAH. CD36, a major fatty acid transporter molecule, accounts for the majority of fatty acid uptake in cardiomyocytes. It is stored in intracellular compartments and recruited to sarcolemma to facilitate fatty acid uptake. External stimuli such as insulin, diet, caffeine, etc., can trigger relocalization of CD36 to the sarcolemma (25, 41, 42). Similarly, deficiency of CD36 in hearts can influence fatty acid uptake and oxidation, as reported in compromised recovery from ischemic injury (43, 44). Our combined data in both RV tissue from transgenic mice and BMPR2 mutant cardiomyocytes demonstrate that BMPR2 mutation can independently up-regulate CD36 at the protein level, not at the mRNA transcription level, and trigger relocalization of CD36 in cultured cardiomyocytes. Furthermore, our results also indicate that exogenous palmitate uptake is dependent on BMPR2 mutation type (7, 36). BMPR2 mutation in kinase domain (M2, required for SMAD [suppressor of mothers against decapentaplegic] signaling) but not the deletion of cytoplasmic tail (M1, SMAD signaling is intact) results in increased uptake of fatty acids, which may lead to increased lipid accumulation. Thus, our data support the evidence that relocalization of CD36 likely contributes to chronic elevation in the uptake of LCFAs into the heart followed by myocardial lipid accumulation (30, 39) and also support further studies of the mechanism of lipid accumulation patterns dependent on BMPR2 mutation type.
In cardiomyocytes, FAO is the major source of ATP production and oxygen consumption (60–90%), whereas glucose metabolism is considered a secondary source, (10–40%) of energy production (45). In context to RV specifically, most of the literature is focused on glucose metabolism, and very limited information is available on alteration of FAO in PAH. Others and we have demonstrated indirect evidence for impaired fatty acid metabolism in RVs from PAH and suppression of FAO genes in rodent models of RV failure (7, 11, 12, 46, 47). Our metabolomics study indicated that BMPR2 mutation is associated with decrease in long- and medium-chain acylcarnitines, an important intermediate of the FAO pathway, that are transported from the cytoplasm into the mitochondria. In addition, our high-resolution respirometry data directly show reduced mitochondrial O2 consumption with fat substrate, confirming the inability of mitochondria from BMPR2 mutant RVs to augment β-oxidation in the presence of fat substrate. However, this is not the only metabolic derangement present in Bmpr2 mutants (48), and it is possible that other metabolic pathways are exerting a modifier effect. Furthermore, electron microscopic studies indicated increase in the lipid droplets close to mitochondria in mouse RV with BMPR2 mutation, similar to that shown in Zucker rat myocardial cells, where lipid deposits were clearly visible close to mitochondria (49). This could suggest that reduced mitochondrial use of lipids by RV cardiomyocytes with BMPR2 mutation could result in increased accumulation of lipid intermediates in the cytoplasm, which can lead to a “lipotoxic cardiomyopathy” (50). Our data strongly support impairment in FAO in BMPR2 mutant cardiomyocytes, and they directly measure respirometry in addition to indirect markers. When combined with our prior demonstration of lipotoxicity associated with BMPR2 mutation in the RV, our findings suggest that impaired FAO is contributing to impaired RV compensation in HPAH (51).
It is known that increases in availability of LCFA as a result of a high-fat diet leads to increases in fatty acid uptake by CD36 (52). In accordance with the literature, our findings indicate an increase in CD36 protein with WD, which can potentially contribute to a lipotoxic cardiomyopathy (41, 53, 54). Furthermore, our high-resolution respirometry results indicate that in the mouse RV, dietary lipids can increase FAO-attributable mitochondrial O2 consumption, suggesting a distinctly different mechanism of lipid accumulation than mutant BMPR2 phenotype. However, in contrast, FAO-attributable mitochondrial β-oxidation is strikingly impaired by dietary lipids in the mutant BMPR2 mouse RV. These data suggest that WD alone may potentially have important effects on the RV and further suggest that WD may exacerbate, but is not required for, the BMPR2 mutant RV phenotype.
Our study does have some limitations. First, our studies do not demonstrate how BMPR2 leads to altered CD36 protein expression and localization. Prior work has suggested altered cytoskeletal function may underlie these changes (55), but we have not directly confirmed this, and further studies are warranted. Moreover, our studies focused on the metabolism of fat, but we have not studied the interaction of glucose and lipid in BMPR2 mutant RVs or cardiomyocytes, which is an important area of future study. Finally, although a cardiomyocyte line, the H9c2 cells are not specifically RV, though their findings are recapitulated in the isolated RVs from transgenic rodents. We are presently addressing this limitation using mutant BMPR2 embryonic stem cell–derived cardiomyocytes.
In summary, in HPAH with BMPR2 mutation the key mediators of RV lipid accumulation are impaired mitochondrial FAO as well as increased protein synthesis, and altered localization of CD36 can lead to an increase uptake of free fatty acids. Dietary lipids further exacerbate these alterations. A deeper understanding of the complex mechanisms of metabolic remodeling of the RV will aid in the development of targeted treatments of RV failure in PAH.
Supplementary Material
Footnotes
Supported by grants P01 HL 108800-01A1 (J.H.N. and A.R.H.), 1 R01 HL122417-01A1 (A.R.H.), and K08 HL121174 (J.P.F.), and a Parker B. Francis Foundation Fellowship (J.P.F.). Microscopy experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource, supported by National Institutes of Health grants CA68485, DK20593, DK58404, DK59637, and EY08126.
Author Contributions: Conception and design of the work: M.H.T., E.L.B., J.P.F., N.P., J.A., M. Funke, C.G., W.G.J., M. Freeman, J.H.N., J.W., and A.R.H. Acquisition of data and analysis and interpretation of data for the work: M.H.T., J.P.F., N.P., M. Funke, C.G., W.G.J., J.W., and A.R.H. Initial drafting of the manuscript for important intellectual content: M.H.T., E.L.B., and A.R.H. All authors provided critical revisions of the manuscript and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201507-1444OC on March 8, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tonelli AR, Zein J, Ioannidis JP. Geometry of the randomized evidence for treatments of pulmonary hypertension. Cardiovasc Ther. 2013;31:e138–e146. doi: 10.1111/1755-5922.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girerd B, Montani D, Eyries M, Yaici A, Sztrymf B, Coulet F, Sitbon O, Simonneau G, Soubrier F, Humbert M. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 2010;11:73. doi: 10.1186/1465-9921-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Larkin EK, Austin ED, Hemnes AR. Shorter survival in familial versus idiopathic pulmonary arterial hypertension is associated with hemodynamic markers of impaired right ventricular function. Pulm Circ. 2013;3:589–598. doi: 10.1086/674326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, Montani D, Souza R, Simonneau G, Soubrier F, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig EB, Morse JH, Knowles JA, Chada KK, Khan AM, Roberts KE, McElroy JJ, Juskiw NK, Mallory NC, Rich S, et al. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27:668–674. doi: 10.1016/j.healun.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte TGF-β signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranowski M, Górski J. Heart sphingolipids in health and disease. Adv Exp Med Biol. 2011;721:41–56. doi: 10.1007/978-1-4614-0650-1_3. [DOI] [PubMed] [Google Scholar]
- 9.Ussher JR, Folmes CD, Keung W, Fillmore N, Jaswal JS, Cadete VJ, Beker DL, Lam VH, Zhang L, Lopaschuk GD. Inhibition of serine palmitoyl transferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. Plos One. 2012;7:e37703. doi: 10.1371/journal.pone.0037703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem. 1994;55:1–11. doi: 10.1002/jcb.240550002. [DOI] [PubMed] [Google Scholar]
- 11.Takeyama D, Kagaya Y, Yamane Y, Shiba N, Chida M, Takahashi T, Ido T, Ishide N, Takishima T. Effects of chronic right ventricular pressure overload on myocardial glucose and free fatty acid metabolism in the conscious rat. Cardiovasc Res. 1995;29:763–767. [PubMed] [Google Scholar]
- 12.Kim Y, Goto H, Kobayashi K, Sawada Y, Miyake Y, Fujiwara G, Chiba H, Okada T, Nishimura T. Detection of impaired fatty acid metabolism in right ventricular hypertrophy: assessment by I-123 β-methyl iodophenyl pentadecanoic acid (BMIPP) myocardial single-photon emission computed tomography. Ann Nucl Med. 1997;11:207–212. doi: 10.1007/BF03164765. [DOI] [PubMed] [Google Scholar]
- 13.Nagaya N, Goto Y, Satoh T, Uematsu M, Hamada S, Kuribayashi S, Okano Y, Kyotani S, Shimotsu Y, Fukuchi K, et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ventricle. J Nucl Med. 1998;39:1676–1680. [PubMed] [Google Scholar]
- 14.Aurich AC, Niemann B, Pan R, Gruenler S, Issa H, Silber RE, Rohrbach S. Age-dependent effects of high fat-diet on murine left ventricles: role of palmitate. Basic Res Cardiol. 2013;108:369. doi: 10.1007/s00395-013-0369-6. [DOI] [PubMed] [Google Scholar]
- 15.Talati M, Fessel JP, Penner N, Funke M, Jerome WG, West JD, Hemnes AR. Lipid deposition in cardiomyocytes with BMPR2 mutation is associated with increased lipid import and decreased fatty acid oxidation [abstract] Am J Respir Crit Care Med. 2015;191:A5139. [Google Scholar]
- 16.Talati M, Penner N, Funke M, Trammell A, Fessel J, West J, Hemnes A. BMPR2 mutation and western diet are associated with altered lipid transport and lipotoxicity in the right ventricle [abstract] Am J Respir Crit Care Med. 2014;189:A2338. [Google Scholar]
- 17.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 18.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 19.Sipido KR, Marban E. L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circ Res. 1991;69:1487–1499. doi: 10.1161/01.res.69.6.1487. [DOI] [PubMed] [Google Scholar]
- 20.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355:204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res. 2001;42:751–759. [PubMed] [Google Scholar]
- 22.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 23.van Oort MM, van Doorn JM, Bonen A, Glatz JF, van der Horst DJ, Rodenburg KW, Luiken JJ. Insulin-induced translocation of CD36 to the plasma membrane is reversible and shows similarity to that of GLUT4. Biochim Biophys Acta. 2008;1781:61–71. doi: 10.1016/j.bbalip.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Ouwens DM, Diamant M, Fodor M, Habets DD, Pelsers MM, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–1948. doi: 10.1007/s00125-007-0735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coort SL, Luiken JJ, van der Vusse GJ, Bonen A, Glatz JF. Increased FAT (fatty acid translocase)/CD36-mediated long-chain fatty acid uptake in cardiac myocytes from obese Zucker rats. Biochem Soc Trans. 2004;32:83–85. doi: 10.1042/bst0320083. [DOI] [PubMed] [Google Scholar]
- 26.Glatz JF, Angin Y, Steinbusch LK, Schwenk RW, Luiken JJ. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2013;88:71–77. doi: 10.1016/j.plefa.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 27.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41:861–871. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heresi GA, Aytekin M, Newman J, DiDonato J, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:661–668. doi: 10.1164/rccm.201001-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–911. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-γ activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 33.Drahota Z, Vrbacký M, Nůsková H, Kazdová L, Zídek V, Landa V, Pravenec M, Houstek J. Succinimidyl oleate, established inhibitor of CD36/FAT translocase inhibits complex III of mitochondrial respiratory chain. Biochem Biophys Res Commun. 2010;391:1348–1351. doi: 10.1016/j.bbrc.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 34.Wolf HP. Possible new therapeutic approach in diabetes mellitus by inhibition of carnitine palmitoyltransferase 1 (CPT1) Horm Metab Res Suppl. 1992;26:62–67. [PubMed] [Google Scholar]
- 35.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol. 2008;295:L744–L755. doi: 10.1152/ajplung.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talati M, West J, Zaynagetdinov R, Hong CC, Han W, Blackwell T, Robinson L, Blackwell TS, Lane K. BMP pathway regulation of and by macrophages. Plos One. 2014;9:e94119. doi: 10.1371/journal.pone.0094119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47:125–131. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 39.van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- 40.Bing RJ, Siegel A, Vitale A, Balboni F, Sparks E, Taeschler M, Klapper M, Edwards S. Metabolic studies on the human heart in vivo: I. Studies on carbohydrate metabolism of the human heart. Am J Med. 1953;15:284–296. doi: 10.1016/0002-9343(53)90082-5. [DOI] [PubMed] [Google Scholar]
- 41.Sung MM, Koonen DP, Soltys CL, Jacobs RL, Febbraio M, Dyck JR. Increased CD36 expression in middle-aged mice contributes to obesity-related cardiac hypertrophy in the absence of cardiac dysfunction. J Mol Med (Berl) 2011;89:459–469. doi: 10.1007/s00109-010-0720-4. [DOI] [PubMed] [Google Scholar]
- 42.Lally JS, Jain SS, Han XX, Snook LA, Glatz JF, Luiken JJ, McFarlan J, Holloway GP, Bonen A. Caffeine-stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol (Oxf) 2012;205:71–81. doi: 10.1111/j.1748-1716.2012.02396.x. [DOI] [PubMed] [Google Scholar]
- 43.Irie H, Krukenkamp IB, Brinkmann JF, Gaudette GR, Saltman AE, Jou W, Glatz JF, Abumrad NA, Ibrahimi A. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci USA. 2003;100:6819–6824. doi: 10.1073/pnas.1132094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, Dyck JR. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J Mol Cell Cardiol. 2013;63:180–188. doi: 10.1016/j.yjmcc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 46.Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med (Berl) 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6:136–144. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 50.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brittain EL, Goyal SK, Sample MA, Leacche M, Absi TS, Papa F, Churchwell KB, Ball S, Byrne JG, Maltais S, et al. Minimally invasive fibrillating mitral valve replacement for patients with advanced cardiomyopathy: a safe and effective approach to treat a complex problem. J Thorac Cardiovasc Surg. 2014;148:2045–2051.e1. doi: 10.1016/j.jtcvs.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han XX, Glatz JF, Luiken JJ, Lally J, Dyck DJ, et al. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. Am J Physiol Endocrinol Metab. 2009;296:E738–E747. doi: 10.1152/ajpendo.90896.2008. [DOI] [PubMed] [Google Scholar]
- 53.Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-α transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-α activators. Circulation. 2010;121:426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 55.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–L484. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.