Abstract
Rationale: Sleep-disordered breathing (SDB) in children is associated with cognitive challenges. However, potential associations between SDB severity and neurocognitive function, as well as the presence of an SDB cutoff, have not been fully explored.
Objectives: To determine whether SDB-associated adverse changes in neurocognitive functioning are severity dependent.
Methods: A total of 1,010 snoring and nonsnoring children ages 5–7 years were prospectively recruited from public schools and underwent polysomnography and neurocognitive assessments of intellectual, attention, memory, language, and executive function development. The children were subdivided into four severity groups on the basis of apnea–hypopnea index (AHI), followed by comparisons of cognitive function, with a particular focus on standardized subtests of intellectual, language, attention, memory, and executive function.
Measurement and Main Results: Differential Ability Scales Verbal (P < 0.001) and Nonverbal (P = 0.002) performance, as well as global conceptual ability (IQ) (P < 0.001) scores, differed significantly across the groups, with individuals with higher AHI showing worse performance. Additionally, specific NEPSY (a Developmental Neuropsychological Assessment) subscores focused on attention and executive skills differed across the groups, indicating differences in levels of engagement and problem solving. Children with higher AHI (>5 per hour of total sleep time) were significantly more impaired than all three lower AHI groups, indicating a dose–response impact of SDB.
Conclusions: This large community-based sample of children highlights the significant deleterious impact of SDB, particularly in children with moderate to severe obstructive sleep apnea, and also that even snoring alone affects neurocognitive function. By affecting developing capabilities, as illustrated by cognitive measures in a severity-graded manner, SDB could adversely impact children’s capacity to attain academic and adaptive goals, ultimately hampering their ability to reach independence. Our findings support the need for increased awareness of SDB, with particular emphasis on children with more severe obstructive sleep apnea.
Keywords: children, cognitive function, obesity, obstructive sleep apnea, snoring
At a Glance Commentary
Scientific Knowledge on the Subject
Sleep-disordered breathing (SDB) is associated with an increased risk for cognitive dysfunction in children. However, it is unclear whether a severity-dependent association exists and whether a cutoff for SDB severity can be identified.
What This Study Adds to the Field
SDB is associated with cognitive dysfunction in a dose-dependent fashion in children, and the effect appears to be particularly marked in those with moderate to severe SDB.
Sleep-disordered breathing (SDB) is characterized by increased upper airway resistance, alveolar hypoventilation, and recurrent upper airway obstruction during sleep. Recurrent narrowing or collapse of the upper airway develops with sleep onset and is associated with both episodic hypoxemia and arousal. Following such arousal, the child typically drifts back to sleep, with the cycles then repeating throughout the night and resulting in sleep fragmentation and nonrestorative sleep (1). It is now recognized that pediatric SDB is an extremely frequent health problem. Contingent on the diagnostic criteria used, the prevalence may range between 1% and 5%, peaking at 2–8 years of age (2–5).
In children, the major morbidities thus far associated with SDB involve the occurrence of cognitive and behavioral deficits as well as reduced academic performance (6–15). However, not all children with SDB manifest cognitive impairments, even though the likelihood of such morbidity likely increases with the severity of SDB (16, 17) or in the presence of concurrent obesity (18, 19). Furthermore, several, but not all, studies have suggested that treatment of SDB leads to significant improvements in, or even to normalization of, cognitive performance if timely intervention is implemented (20–23). Across these studies, it has been implied that, for children with SDB, disruption in attention and executive function skills, as well as challenges with aspects of behavioral regulation, are associated with explicit challenges with consolidating learning, both in the classroom and at home (24). As such, cognitive dysfunction situates as a particular neurodevelopmental concern, given its impact on successful achievement of learning goals, as well as the development of adaptive skills that support independence over time. Yet, questions remain regarding both the level of impact and its relationship with degrees of SDB. Because in the vast majority of the published studies researchers have examined small cohorts and have not focused on a restricted and narrow range of school-age children, the potential associations between SDB severity and cognitive function have not been extensively explored. As a consequence, there is difficulty in establishing an important clinical cutoff that allows for cogent intervention decision making as well as a means for supporting a better understanding of these challenges within the academic and social worlds of these children.
To address these concerns in the present study, we hypothesized that prospective assessments of cognitive function in a large population of community-dwelling snoring and nonsnoring children in their early elementary school years, when the initial potential impact of SDB might occur, would enable delineation of a putative SDB severity cutoff beyond which the risk of cognitive deficits would be markedly increased. We believe that by examining the patterns of SDB symptoms present, as well as their potential relationships with cognitive function, and by articulating a potential dose–response effect of SDB, better determinations can be made about who is at most significant risk for challenges with cognitive development. In this way, we think that efforts can be guided toward more effectively understanding the impact of SDB over time.
Methods
The present pediatric cohort included children between the ages of 5 and 7 years residing in Louisville, Kentucky (recruited from October 2006 until November 2008), or in Chicago, Illinois (recruited from May 2009 until October 2014). This study was approved by the University of Louisville (protocol 474.99) and University of Chicago (protocol 09-115-B) human research ethics committees. Informed consent was obtained from the children’s parents or legal caregivers, and age-appropriate assent was also obtained from the children.
Children were recruited from the two sites using a consistent and identical protocol across settings. The use of children from these two cities was based on where two of the authors (D.G. and L.K.-G.) were situated professionally. Children from Louisville were recruited through a collaboration with the public school system and reflect the socioeconomic status and racial background of this urban setting. Similarly, the sample from Chicago is reflective of the community where the University of Chicago is located and that serves as its patient and school population base. This community is predominantly African American and of middle to lower socioeconomic status. Children from Chicago were recruited through community announcements and distribution of flyers across the medical center’s catchment area. All participation occurred as a result of parent agreement, and results derived from the study measures were shared with parents. Differences and similarities across the samples, integrated with this study, are addressed below.
Questionnaires
Parents of all children 5–7 years of age were invited to complete a detailed and validated questionnaire about their children’s sleeping habits, as previously described (25–27). The information gathered through the questionnaire included sex, age, ethnic background, whether the child had difficulty breathing during sleep, mouth breathing, witnessed apnea, and/or daytime sleepiness. Snoring and the severity of snoring were also included. For the question on snoring, the responses were graded as “never,” “rarely” (once per week), “occasionally” (twice per week), “frequently” (three times per week), and “almost always–always” (more than four times per week). Returned questionnaires were scanned into a computerized database and were subdivided according to snoring patterns into those of nonsnoring children (responses of never or rarely on snore and not applicable on loudness of snore in questionnaire) or those of habitually snoring children (responses of frequently or almost always–always [≥3 nights/wk] for snoring frequency and medium loud to loud on loudness of snoring).
Overnight Polysomnographic Studies
Height and weight were routinely obtained during the sleep study visit, and body mass index (BMI) Z scores were calculated using CDC guidelines (http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm). All children underwent standard nocturnal polysomnography (NPSG) evaluation with assessment of eight standard electroencephalography channels, bilateral electrooculography, electromyography, two-lead electrocardiography, oronasal airflow measurement using a thermistor, nasal pressure transducer, and end-tidal carbon dioxide, chest, and abdominal movement by respiratory inductance plethysmography, as well as with pulse oximetry, including pulse waveform using a commercially available data acquisition system (Polysmith; Nihon Kohden America, Inc., Irvine, CA), as previously described (28). All of the NPSG studies were scored as per the 2012 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events. Scores obtained before 2012 were recoded (29). Furthermore, arousals were classified as either spontaneous or respiratory, and corresponding indices (spontaneous arousal index and respiratory arousal index, respectively) were computed.
Neurocognitive Assessments
The cognitive tests administered the morning following NPSG assessment consisted of the Differential Ability Scales (DAS) (30); the Peabody Picture Vocabulary Test, Third Edition (PPVT-III) (31); the Expressive Vocabulary Test (EVT) (32); and the NEPSY (a Developmental Neuropsychological Assessment) (33). Each of these tests or batteries of tests has been developed and standardized for assessment of aspects of broad cognitive function, as well as more specific domains of neuropsychological status, and are frequently used diagnostically in educational and clinical settings.
The DAS is a battery of cognitive tests developed to measure reasoning and conceptual ability in children ages 2–17 years. It was designed to provide specific information about an individual’s strengths and weaknesses across a wide range of intellectual activities. Children were administered either the preschool- or the school-age form of the DAS on the basis of their chronological age. The preschool form is divided into a Verbal Cluster (including two subtests) and a Nonverbal Cluster (including two spatial subtests and one nonverbal reasoning subtest) and yields a global composite score that is commensurate with the IQ, called the General Conceptual Ability (GCA) score. The school-age form yields a Spatial Cluster score in addition to the Verbal, Nonverbal, and global composite scores. The test was designed so that both the subtest and composite scores could be examined across forms and throughout the age range. Individual DAS subtests are designed to measure separate and distinct areas of cognitive function and thus have high specificity. The ability score for a subtest is expressed as a T score with a mean of 50 and an SD of 10. Subtest T scores are summed and converted to appropriate composite standard scores, while the sum of the core subtest T scores is converted to a total battery standard score (the GCA), with a mean of 100 and an SD of 15. The reliabilities of the subtests and indices are excellent (r = 0.90–0.95), and the DAS is highly correlated with other common measures of intellectual development, including the Wechsler and McCarthy scales (34).
The PPVT-III and the EVT together assess aspects of expressive and receptive language skills. Both of these tests have been developed to allow for the screening of language development by asking children to identify and name familiar objects from among an array of distracters. With the PPVT-III children are asked to point to the picture that represents a word said aloud by the examiner, while with the EVT the child articulates the word that is associated with a picture. Scores from these two measures are standardized with a mean of 100 and an SD of 15. Both of these measures have strong reliability and validity and are widely used, both clinically and in studies of cognitive and language development (35).
The NEPSY is also a well-established and validated neurobehavioral test battery. It is designed to assess neurobiological development in five functional domains and includes subtests comprising attention and/or executive function, language, sensorimotor function, visuospatial processing, and memory and learning domains, all normatively standardized with a mean score of 100 and an SD of 15. All of the subtests have good to excellent reliability (r = 0.77–0.91) (36). The NEPSY allows for comprehensive assessment of neuropsychological development in toddlers through early adolescence and is strongly correlated with measures of IQ such as the DAS.
As noted above, all assessments took place the morning following the child’s overnight sleep study, after the child had all biologic measures completed and had consumed a breakfast. The children included were all awake and engaged for the testing, based on trained assessors’ reviews of their behavior and participation. Potential fatigue was addressed through opportunities for breaks as the testing was completed. The results are acknowledged to be vulnerable to the potential impact of some sleep deprivation, although all children were documented to have slept similarly during the study; this is discussed further as a limitation in the Discussion section below.
Data Analysis
On the basis of questionnaires and NPSG findings, children enrolled in the study were subdivided into four categorical groups: group 1, nonsnoring with apnea–hypopnea index [AHI] less than 1 per hour of total sleep time (TST); group 2, habitual snoring with AHI less than 1 per hour of TST; group 3, habitual snoring with AHI greater than 1 but less than 5 per hour of TST; and group 4, habitual snoring with AHI greater than 5 per hour of TST. We also used respiratory arousal index in some of our analyses, owing to our previous findings illustrating divergent dynamics of spontaneous and respiratory arousals in children and the particular impact of respiratory arousal index on cognitive measures (37, 38). Comparisons of cognitive function across the four groups were conducted using subtests from the DAS, PPVT/EVT, and NEPSY. Appropriate, norm-based, standardized composite scores were calculated according to the procedures for the tests, and they were used for all analyses, with consideration of pertinent subtest scores as indicated by relevant significant composite score results.
Homogeneity of variance across conditions was supported in all comparisons but one; however, because of this exception and departures from normality in several cognitive measures, variables that failed to meet parametric assumptions were examined through the appropriate nonparametric test, and reported P values reflect nonparametric test results for these variables. Multivariate procedures were not used, owing to our interest in evaluating differences for each cognitive measure separately. Additionally, the amount of missing data across measures, given that there was variable completion of the evaluation measures by some children, as well as inclusion of total scores derived from subscores used, suggested that univariate measures were more appropriate here. Results were determined to be significant at the 0.05 significance level, though Bonferroni’s correction for multiple comparisons was applied to all omnibus analysis of variance (ANOVA) results and subsequent post hoc comparisons. This involves dividing 0.05 by the number of statistical tests to obtain an adjusted significance level for all comparisons. Unadjusted P values are reported for the results of omnibus ANOVA in the presented tables, and adjusted P values are reported for the results of post hoc comparisons. The Stata (StataCorp, College Station, TX) and IBM SPSS (IBM, Armonk, NY) software packages were used for statistical analyses, and SigmaPlot software (Systat Software, San Jose, CA) was used for creation of figures.
Results
Between October 2006 and October 2014, 1,010 children recruited from public schools and the broader communities where they resided completed the designated protocol. Specific subject characteristics are detailed in Table 1. An initial examination of BMI Z scores across these groups reached significance (P < 0.001). Post hoc comparisons indicated that group 4 children had significantly higher BMI Z scores than all lower AHI groups, though no other BMI Z score contrast reached significance. Neither asthma frequency (P = 0.447) nor sex frequency (P = 0.986) differed across groups. However, the proportion of white (non-Hispanic) and African American children was different across groups (P = 0.001), consistent with the differences in participants recruited between the two settings. Additionally, a greater number of African American children were noted to have more severe degrees of SDB pathology; this is consistent with the extant literature that addresses racial differences which occur in samples of children and adults with SDB. All statistical analyses using the race variable included only white (non-Hispanic) and African American children, owing to sample size limitations for other groups. Significant differences between sleep variables were expected and therefore uninformative, because groups were created on the basis of AHI values; however, values for these variables are included here for illustrative purposes.
Table 1.
General Characteristics of the Four Groups of Children Based on the Presence or Absence of Habitual Snoring and Their Polysomnographically Derived Apnea–Hypopnea Indices
| Group 1 |
Group 2 |
Group 3 |
Group 4 |
P Value | |
|---|---|---|---|---|---|
| Nonsnoring |
Snoring |
Mild OSA |
OSA |
||
| AHI <1/h TST |
AHI <1/h TST |
AHI ≥1/h and <5/h TST |
AHI ≥5/h TST* |
||
| (n = 90) | (n = 431) | (n = 348) | (n = 141) | ||
| Age, yr | 6.86 (0.70) | 6.81 (0.70) | 6.82 (0.82) | 6.69 (1.02) | 0.480 |
| Male sex, % | 56.67% | 54.99% | 53.16% | 56.03% | 0.899 |
| Race | <0.001† | ||||
| White, n | 67 (74.44%) | 273 (63.34%) | 200 (57.47%) | 48 (30.04%) | |
| African American, n | 13 (14.44%) | 119 (27.61%) | 100 (28.74%) | 72 (51.06%) | |
| BMI Z score | 0.46 (1.33) | 0.65 (1.26) | 0.73 (1.42) | 1.25 (1.53) | <0.001† |
| Total sleep duration, min | 469.17 (47.74) | 472.62 (43.79) | 472.03 (48.07) | 460.21 (55.16) | 0.055 |
| Sleep efficiency, % | 88.75 (8.09) | 89.25 (7.63) | 90.33 (7.54) | 89.63 (8.34) | 0.172 |
| Stage 1, % | 7.31 (4.98) | 6.92 (4.90) | 6.59 (6.41) | 7.85 (6.48) | 0.239 |
| Stage 2, % | 44.99 (7.81) | 45.87 (7.87) | 44.75 (8.56) | 44.38 (7.70) | 0.137 |
| Stage SWS, % | 28.53 (6.83) | 26.05 (9.05) | 28.96 (9.24) | 26.30 (8.64) | 0.163 |
| REM sleep, % | 24.43 (14.81) | 21.57 (8.78) | 20.56 (7.59) | 21.51 (13.93) | 0.059 |
| Sleep latency, min | 26.52 (28.84) | 23.61 (24.04) | 20.93 (21.53) | 18.04 (21.60) | 0.023 |
| REM latency, min | 147.86 (66.37) | 141.51 (59.24) | 150.22 (68.53) | 146.71 (67.62) | 0.300 |
| Obstructive AHI, events/h TST | 0.40 (0.34) | 0.40 (0.29) | 2.11 (1.03) | 14.87 (12.44) | <0.001† |
| SpO2 nadir,‡ % | 93.80 (2.35) | 92.72 (6.00) | 90.27 (5.00) | 82.65 (10.94) | <0.001† |
| Total arousal index, events/h TST | 8.56 (3.95) | 9.14 (5.77) | 10.78 (7.39) | 17.51 (11.24) | <0.001† |
| Asthma, % | 14.94 | 20.45 | 19.04 | 24.71 | 0.430 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; OSA = obstructive sleep apnea; SpO2 = oxygen saturation as measured by pulse oximetry; SWS = slow-wave sleep; TST = total sleep time.
AHI measured as number of events per hour of total sleep time.
Comparison is significant following Bonferroni’s correction.
SpO2 nadir refers to lowest levels of arterial oxygen saturation in the blood.
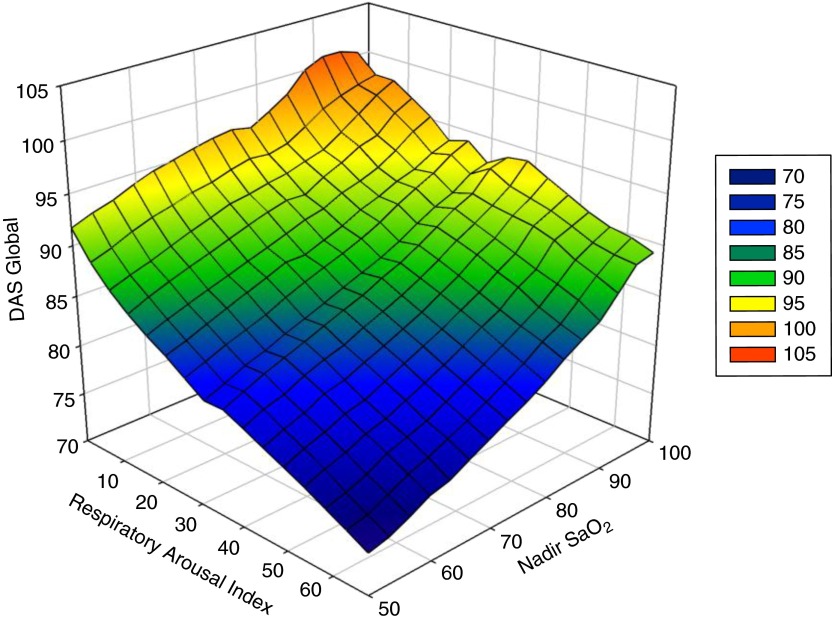
Omnibus ANOVA results suggested that, for both DAS Verbal (P < 0.001) and DAS Nonverbal (P = 0.002) performance, as well as for the GCA (P < 0.001), differences existed in sleep pathology groups (Tables 2 and 3). Additionally, four of the seven NEPSY subtest scores differed significantly across groups. No significant group differences were found in PPVT or EVT scores when we corrected for multiple comparisons. Post hoc tests on the significant DAS and NEPSY subtests revealed that group 4 (AHI >5/h of TST) was significantly more impaired on measures of cognition than the three lower AHI groups across nearly all measures that reached significance in omnibus analyses. Figure 1 illustrates DAS GCA trends across respiratory arousal index and nadir oxyhemoglobin saturation values. For nearly all significant cognitive measures, the most consistent and salient result was that group 4 was significantly more impaired than the other three groups, but differences among the three groups with less severe SDB were rarely significant.
Table 2.
Cognitive Function Differences across Snore Apnea–Hypopnea Levels
| Test | Number of Patients | F Test Statistic | P Value |
|---|---|---|---|
| DAS | |||
| Verbal | 977 | 6.58 | <0.001* |
| Nonverbal | 975 | 5.01 | 0.002* |
| Global | 985 | 7.51 | <0.001* |
| NEPSY | |||
| Design Copying | 1001 | 6.27 | 0.001* |
| Phonological Processing | 1001 | 3.38 | 0.019 |
| Tower | 1003 | 8.39 | <0.001* |
| Speed Naming | 958 | 2.45 | 0.048 |
| Arrows | 978 | 11.65 | <0.001* |
| Visual Attention | 968 | 4.68 | 0.004 |
| Comprehension | 977 | 7.14 | <0.001* |
| PPVT | 580 | 1.18 | 0.383 |
| EVT | 557 | 2.90 | 0.040 |
Definition of abbreviations: DAS = Differential Ability Scales; EVT = Expressive Vocabulary Test; NEPSY = a Developmental Neuropsychological Assessment; PPVT = Peabody Picture Vocabulary Test.
Omnibus comparison is significant after Bonferroni’s correction for multiple comparisons.
Table 3.
Cognitive Function Group Means and SDs
| Test | Group 1 (n = 90) | Group 2 (n = 431) | Group 3 (n = 348) | Group 4 (n = 141) |
|---|---|---|---|---|
| DAS | ||||
| Verbal | 99.45 (14.20) | 98.75 (15.08) | 98.02 (15.30) | 92.40 (12.49) |
| Nonverbal | 103.26 (15.10) | 99.35 (14.23) | 99.70 (14.65) | 95.63 (12.88) |
| Global | 102.05 (13.66) | 99.71 (13.62) | 98.79 (14.24) | 93.91 (11.93) |
| NEPSY | ||||
| Design Copying | 10.83 (3.22) | 10.12 (3.00) | 9.78 (3.13) | 9.14 (3.36) |
| Phonological Processing | 10.08 (3.34) | 9.67 (3.79) | 9.49 (3.75) | 8.66 (3.88) |
| Tower | 11.20 (2.88) | 11.32 (3.22) | 10.91 (3.34) | 9.77 (3.24) |
| Speed Naming | 9.85 (3.05) | 9.19 (3.33) | 9.04 (3.25) | 8.67 (3.20) |
| Arrows | 11.76 (3.02) | 10.48 (2.96) | 10.35 (2.87) | 9.47 (2.61) |
| Visual Attention | 10.69 (2.62) | 10.76 (3.00) | 10.14 (3.17) | 9.80 (3.35) |
| Comprehension | 10.69 (2.59) | 10.38 (2.98) | 10.03 (2.84) | 9.19 (3.05) |
| PPVT | 101.40 (18.58) | 99.08 (14.47) | 98.47 (14.78) | 96.44 (14.87) |
| EVT | 98.88 (14.78) | 98.33 (12.63) | 96.25 (13.01) | 94.01 (13.08) |
Definition of abbreviations: DAS = Differential Ability Scales; EVT = Expressive Vocabulary Test; NEPSY = a Developmental Neuropsychological Assessment; PPVT = Peabody Picture Vocabulary Test.
Figure 1.
Surface plot of the relationships between Differential Ability Scales (DAS) global composite score plotted against respiratory arousal index as a measure of fragmented sleep, and nadir oxyhemoglobin as an indicator of hypoxemic severity. Note: A negative exponential smoother was used with sampling rate at 0.8 and nearest neighbor bandwidth at intervals less than or equal to 16.
A few exceptions to this pattern existed. First, the higher AHI group (i.e., group 4) was significantly more impaired than all other groups except group 2 on the DAS Nonverbal scale (P = 0.07) and group 3 on the NEPSY Design subtest (P = 0.245). Second, on the NEPSY Visual Attention subtest, children in group 2 were more impaired than group 3 (P = 0.047) and significantly more impaired than group 4 (P = 0.009). No other differences were observed between groups in this subtest. Third, all comparisons, except the one between groups 2 and 3, were significant for the NEPSY Arrows subtest, with greater sleep pathology representing greater cognitive impairment in all comparisons. Other than these exceptions, no further differences between the three lower AHI groups (i.e., groups 1–3) were significant for any of the cognitive measures.
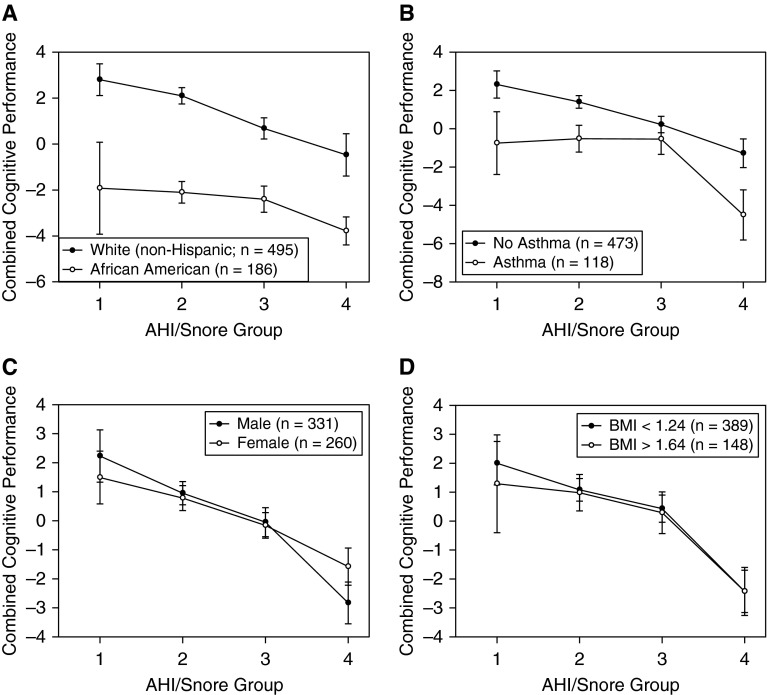
We next conducted separate analyses to assess whether the relationship between sleep pathology and overall cognitive function was significant when adjusting for race, asthma status, BMI, and sex. Because of the small numbers of children with asthma and African American children in group 1, which affected power for these comparisons, we used participants’ continuous AHI measurement as a covariate in a multiple regression analysis. This also allowed us to assess the sensitivity of overall cognitive performance to AHI as a continuous measure, because clinically relevant cutoffs were used to group sleep pathology in the above-described analysis. We conducted a multiple regression analysis to examine interactions between AHI and race, asthma status, BMI, and sex to predict overall cognitive performance, which was obtained by summing cognitive performance measure Z scores among those who completed all cognitive measures (n = 591). The results indicated that AHI was a significant predictor of overall cognitive performance (P < 0.011), suggesting that this relationship is robust despite adjusting for these potential confounders and using continuous measurement of AHI. Race (P < 0.001) was also a significant predictor of overall cognitive performance, as African American children performed more poorly. However, asthma status, BMI, and sex were not significant predictors of overall cognitive performance. Additionally, none of the interaction terms between these variables and AHI were significant, suggesting that race, BMI, asthma status, and sex do not appear to moderate any effects of AHI on cognition. Figure 2 clearly illustrates that differences in cognitive performance exist between white and African American children, but it also shows that the trend involving clear reductions in cognition at high AHI levels was consistent for both groups. This general decrease in cognitive function at the highest levels of sleep pathology is also clear across sex, asthma status, and BMI. Regarding the particular clinical interest in children with high and low BMI, as well as the common clinical use of these cutoffs, Figure 2D illustrates the similarity of trends for children with low (<1.34) and high (>1.64) BMI Z scores. Clearly, across demographic and anthropomorphic conditions, greater sleep pathology was associated with a pattern of lower overall cognitive function, with participants at the highest levels of AHI consistently demonstrating the greatest and most consistent patterns of impairment across cognitive domains.
Figure 2.
Mean total cognitive performance across snore apnea–hypopnea index (AHI) groups by (A) race and/or ethnicity, (B) asthma status, (C) sex, and (D) body mass index (BMI). Error bars represent SE. Regression analyses using continuous AHI as a covariate illustrated that only the main effect for race was significant (P < 0.001; all other P > 0.05). No interaction terms with these variables and AHI were significant (P > 0.05). Numbers in parentheses represent regression analysis sample.
Discussion
This study with a large, community-based cohort of young school-aged children not only indicates a clear relationship between SDB and multiple aspects of cognitive function but also shows that this reduction in cognitive performance is greatest at the highest levels of SDB, based on polysomnographic measures of SDB severity. Across measures of general intellectual development, as derived from indices in the DAS, our analyses show that, in agreement with previous findings across multiple studies, children who snore and show clear evidence of obstructive sleep apnea (OSA), either mild or more severe, perform less efficiently and with less capability across verbal and nonverbal cognitive demands. More significantly, we uncovered a clear pattern of increased impairment among those children with more significant OSA. These children often performed with a pattern of greater inefficiency, with scores generally falling 0.5 SD below those of their less affected peers. This difference, which was strongly statistically significant, references a likely vulnerability to clinically significant impact, given the demands of the educational environment. The SDB severity relationship with cognitive deficits, along with the relative heterogeneity of the effect at any given level of SDB severity, most likely underlies the sometimes contradictory findings in previous cohorts that lacked sufficient cohort size to detect such SDB-mediated effects.
In this context, it is important to note in regard to our findings that multiple aspects of broader neuropsychological status are affected among children with SDB. Using measures from the NEPSY that tap attention, executive function, language, memory, visuospatial planning and analysis, and processing speed, we found that the results were indicative of a possible impact of SDB on these aspects of higher function, with those with more severe SDB appearing to have the greatest reduction in function. However, we also identified that attention is more affected in habitually snoring children with an otherwise normal polysomnographic test (also called primary snorers [PS]) in comparison with nonsnoring children, while more generally the children with more severe SDB showed a profile of increased neurocognitive impact.
These alterations in attention highlight that even the mildest degree of SDB may begin to affect core capacities for learning and meeting adaptive demands, thereby confirming previous findings (37). Indeed, in published studies in which researchers have used highly sensitive methodologies such as evoked potential arrays for detection of subtle differences in attention between PS children and control subjects, PS children clearly exhibited enhanced recruitment of frontotemporal regions during a Stroop test, even though their neurocognitive function as tested with some of the batteries used herein revealed no significant deficits in this small group (38). Such subtle alterations in end-organ function among PS children have also been reported for cardiovascular and metabolic systems, suggesting that type II errors related to cohort size may account for the previously reported negative findings and that only expansion of the cohort size would enable the detection of such important yet difficult to detect morbidities (39–41).
The group of children participating in this study was broad in background, across both race and socioeconomic factors. Subanalyses taking race, asthma status, sex, and BMI Z score into account suggested lack of significant interactions with SDB for these potential confounders. Further, no effects involving sex, asthma status, or BMI Z score were significant. We believe that these results highlight that, despite previously observed and hence expected differences related to sociodemographic factors, our analyses with this diverse, community-derived sample show that there is a particularly uniform impact of SDB, whether more or less severe, on daily aspects of cognitive capability. Thus, even when we account for all potential confounders, our present results inform us that the potential effect of SDB may be a product of the interferences in neurocognitive function that are secondary to episodic decreases in oxyhemoglobin saturation and disruption of sleep continuity and architecture. With SDB, the underlying supportive and regulatory systems that are directing and guiding all aspects of learning are observably impacted, leading to alterations in the capacity to develop appropriate cognitive function required for adaptation and skill development.
The identification of a cutoff based on the observation of a dose–response impact of SDB is a clinically significant contribution to the field. Indeed, even though the extant literature has highlighted, albeit not uniformly (23), emergence of improvements in cognitive function secondary to treatment for SDB, our findings argue strongly for a substantial need for intervention among children whose sleep study results reveal the presence of moderate to severe OSA (AHI >5/h of TST). Notably, the study by Marcus and coworkers showed no evidence of improved cognitive measures within 7 months after adenotonsillectomy (23). However, their unique randomized controlled trial involved a clinical sample, and they imposed restrictions on specific SDB severity limits and did not report analyses of the effect of treatment in the subset of children with cognitive deficits (23). As shown herein, even in group 4 only a restricted proportion of children would manifest evidence of cognitive dysfunction, such that treatment in the whole cohort without addressing this particular point would unsurprisingly result in the absence of changes in cognitive performance. Although prospective randomized interventional studies are needed for conclusive demonstration of the veracity of such a recommendation, ethical considerations will likely preclude such trials (42) when researchers confront the challenge involved in leaving children without therapeutic intervention, because SDB-imposed reductions in children’s capacity to learn and achieve important cognitive milestones across the elementary years can serve to increasingly impair their successful movement toward adult independence. Thus, we propose that early identification and treatment of children with moderate to severe OSA should be a priority among primary care physicians to palliate and reverse the cognitive consequences identified herein. Notwithstanding, while there is a graded response according to the severity of SDB, a strong argument is made as well for initiating treatment for those children with lesser degrees of SDB severity, because our data indicate that visual attention and core aspects of early executive development are vulnerable, likely contributing to educational morbidity, including progressive delays in achievement.
Several limitations of this study require consideration. As mentioned above, differences exist across ethnicities that may contribute to the presence of both greater BMI Z score and associated SDB severity. These differences were variable but clearly present and require a more detailed examination, given the recognition that children of color often grow up in less advantaged socioeconomic circumstances with coexisting differences in educational opportunities. Similarly, although children in this sample differed across communities in terms of educational opportunity and engagement (43), because this study includes samples from two diverse communities in different sections of the United States who were assessed with a similar approach with consistent comparisons made using relevant normative data that includes similar samples, we believe that the findings are strongly representative of children living in a mixed urban setting. Nonetheless, there may be underlying variabilities that we have not successfully accounted for that serve as potential covariates to explore. Finally, there are considerations that are required with regard to the study design, including the testing of children following participation in an overnight sleep study. Differences in actual sleep duration and the potential resulting sleepiness experienced the following morning, during the testing, could have imposed some adverse influence for some if not all of the children; however, the unique precautions implemented in our study and the similarity of sleep duration in the study compared with ecologically assessed sleep duration in the homes for this age group suggest that this factor did not play any significant role. Notably, undergoing cognitive testing as done in this study, following a night with potentially less sleep duration than is desired for this age cohort, is highly comparable to daily life for many children with sleep disorders in the United States, who are then asked to attend school early in the morning and perform cognitively for periods that exceed by far the number of hours required for neurocognitive test batteries.
Nonetheless, it is important to recognize that this is the first large, population-based sample of early elementary school–age children that confirms a clear, graded impact of SDB on both broad and more specific aspects of neurocognitive development. Thus, we believe that this study not only confirms but also extends findings highlighting that SDB at any level of severity challenges underlying neural structures that direct attention, language, memory, and problem-solving abilities. Processing speed, engagement with information, and the capacity to effectively encode, consolidate, and then retrieve needed knowledge to meet day-to-day demands are all observed to be affected. Furthermore, more severe SDB contributes to increased likelihood of morbidity with foundational skills supporting academic and adaptive achievements. These SDB-imposed consequences further serve to limit both the ability to take in what is being taught and to form the appropriate foundation for later and ongoing learning. By establishing SDB severity–dependent relationships with cognitive function outcomes, this study advances intervention goal setting and should enable formulation of cogent healthcare policy in the near future. These findings are all the more relevant when considering the currently insufficient attention to SDB among primary care physicians, along with relatively ineffective implementation of existing guidelines for diagnosis and treatment of SDB in general pediatric practices (44–47), an issue that could certainly leave children experiencing SDB in a situation where their opportunities for success are likely much more limited.
Supplementary Material
Footnotes
Supported by National Institutes of Health grant HL-65270 (S.J.H., D.G., and L.K.-G.) and by a fellowship educational grant award from the Kingdom of Saudi Arabia (M.F.P.).
Author Contributions: S.J.H.: provided critical input regarding cognitive function interpretation and data analysis, and drafted the initial version of the manuscript; D.G.: conceptualized the study, was responsible for sleep study interpretation, performed data analysis, and edited the manuscript; D.L.S.: performed data analysis and contributed to manuscript editing; M.F.P.: participated in subject recruitment, performed sleep study scoring, compiled databases, and participated in data analysis. J.K.: participated in sample collection as well as cognitive battery scoring; and L.K.-G.: conceptualized the study, recruited subjects, scored sleep studies, coordinated the database, analyzed data, and drafted portions of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201510-2099OC on March 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kheirandish-Gozal L, Gozal D. Sleep disordered breathing in children: a comprehensive clinical guide to evaluation and treatment. Totowa, NJ: Humana Press; 2012. [Google Scholar]
- 2.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li AM, So HK, Au CT, Ho C, Lau J, Ng SK, Abdullah VJ, Fok TF, Wing YK. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65:991–997. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Ward SD, Sheldon SH, Shiffman RN, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 6.Weissbluth M, Davis AT, Poncher J, Reiff J. Signs of airway obstruction during sleep and behavioral, developmental, and academic problems. J Dev Behav Pediatr. 1983;4:119–121. doi: 10.1097/00004703-198306000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Chase C, Vezina RM, Heeren TC, Corwin MJ, Auerbach SH, Weese-Mayer DE, Lesko SM. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458–464. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Kheirandish-Gozal L, De Jong MR, Spruyt K, Chamuleau SA, Gozal D. Obstructive sleep apnoea is associated with impaired pictorial memory task acquisition and retention in children. Eur Respir J. 2010;36:164–169. doi: 10.1183/09031936.00114209. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol. 2008;15:100–106. doi: 10.1016/j.spen.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggs SN, Nixon GM, Horne RS. The conundrum of primary snoring in children: what are we missing in regards to cognitive and behavioural morbidity? Sleep Med Rev. 2014;18:463–475. doi: 10.1016/j.smrv.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Brockmann PE, Urschitz MS, Schlaud M, Poets CF. Primary snoring in school children: prevalence and neurocognitive impairments. Sleep Breath. 2012;16:23–29. doi: 10.1007/s11325-011-0480-6. [DOI] [PubMed] [Google Scholar]
- 13.Spruyt K, Capdevila OS, Kheirandish-Gozal L, Gozal D. Inefficient or insufficient encoding as potential primary deficit in neurodevelopmental performance among children with OSA. Dev Neuropsychol. 2009;34:601–614. doi: 10.1080/87565640903133566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44:417–422. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- 15.Giordani B, Hodges EK, Guire KE, Ruzicka DL, Dillon JE, Weatherly RA, Garetz SL, Chervin RD. Neuropsychological and behavioral functioning in children with and without obstructive sleep apnea referred for tonsillectomy. J Int Neuropsychol Soc. 2008;14:571–581. doi: 10.1017/S1355617708080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, McClimment MC, Gozal D. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13:165–172. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185:199–205. doi: 10.1164/rccm.201104-0721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33:1447–1456. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr. 1996;155:56–62. doi: 10.1007/BF02115629. [DOI] [PubMed] [Google Scholar]
- 21.Friedman BC, Hendeles-Amitai A, Kozminsky E, Leiberman A, Friger M, Tarasiuk A, Tal A. Adenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndrome. Sleep. 2003;26:999–1005. doi: 10.1093/sleep/26.8.999. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery-Downs HE, Crabtree VM, Gozal D. Cognition, sleep and respiration in at-risk children treated for obstructive sleep apnoea. Eur Respir J. 2005;25:336–342. doi: 10.1183/09031936.05.00082904. [DOI] [PubMed] [Google Scholar]
- 23.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, et al. Childhood Adenotonsillectomy Trial (CHAT) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galland B, Spruyt K, Dawes P, McDowall PS, Elder D, Schaughency E. Sleep disordered breathing and academic performance: a meta-analysis. Pediatrics. 2015;136:e934–e946. doi: 10.1542/peds.2015-1677. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien LM, Holbrook CR, Mervis CB, Klaus CJ, Bruner JL, Raffield TJ, Rutherford J, Mehl RC, Wang M, Tuell A, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111:554–563. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 26.Sans Capdevila O, Crabtree VM, Kheirandish-Gozal L, Gozal D. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based study. Pediatrics. 2008;121:e1208–e1214. doi: 10.1542/peds.2007-2049. [DOI] [PubMed] [Google Scholar]
- 27.Spruyt K, Gozal D. Screening of pediatric sleep-disordered breathing: a proposed unbiased discriminative set of questions using clinical severity scales. Chest. 2012;142:1508–1515. doi: 10.1378/chest.11-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 29.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott CD. Differential Ability Scales: introductory and technical handbook. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 31.Dunn LM, Dunn LM. Examiner’s manual for the PPVT-III, Peabody Picture Vocabulary Test. 3rd ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 32.Williams KT. AGS Expressive Vocabulary Test manual. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 33.Korkman M, Kirk U, Kemp S. NEPSY: a developmental neuropsychological assessment: manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 34.Elliott CD. The nature and structure of children’s abilities: evidence from the Differential Ability Scales. J Psychoeduc Assess. 1990;8:376–390. [Google Scholar]
- 35.Restrepo MA, Schwanenflugel PJ, Blake J, Neuharth-Pritchett S, Cramer SE, Ruston HP. Performance on the PPVT-III and the EVT: applicability of the measures with African American and European American preschool children. Lang Speech Hear Serv Sch. 2006;37:17–27. doi: 10.1044/0161-1461(2006/003). [DOI] [PubMed] [Google Scholar]
- 36.Ahmad SA, Warriner EM. Review of the NEPSY: a developmental neuropsychological assessment. Clin Neuropsychol. 2001;15:240–249. doi: 10.1076/clin.15.2.240.1894. [DOI] [PubMed] [Google Scholar]
- 37.Tauman R, O’Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004;27:274–278. doi: 10.1093/sleep/27.2.274. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep. 2004;27:279–282. doi: 10.1093/sleep/27.2.279. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, Raffield TJ, Gozal D. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–49. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Barnes ME, Huss EA, Garrod KN, Van Raay E, Dayyat E, Gozal D, Molfese DL. Impairments in attention in occasionally snoring children: an event-related potential study. Dev Neuropsychol. 2009;34:629–649. doi: 10.1080/87565640903133632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li AM, Au CT, Chook P, Lam HS, Wing YK. Reduced flow-mediated vasodilation of brachial artery in children with primary snoring. Int J Cardiol. 2013;167:2092–2096. doi: 10.1016/j.ijcard.2012.05.108. [DOI] [PubMed] [Google Scholar]
- 42.Li AM, Au CT, Ho C, Fok TF, Wing YK. Blood pressure is elevated in children with primary snoring. J Pediatr. 2009;155:362–368.e1. doi: 10.1016/j.jpeds.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 43.Khalyfa A, Gharib SA, Kim J, Capdevila OS, Kheirandish-Gozal L, Bhattacharjee R, Hegazi M, Gozal D. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011;34:153–160. doi: 10.1093/sleep/34.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redline S, Amin R, Beebe D, Chervin RD, Garetz SL, Giordani B, Marcus CL, Moore RH, Rosen CL, Arens R, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–1517. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blair C, Raver CC. Child development in the context of adversity: experiential canalization of brain and behavior. Am Psychol. 2012;67:309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erichsen D, Godoy C, Gränse F, Axelsson J, Rubin D, Gozal D. Screening for sleep disorders in pediatric primary care: are we there yet? Clin Pediatr (Phila) 2012;51:1125–1129. doi: 10.1177/0009922812464548. [DOI] [PubMed] [Google Scholar]
- 47.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Sheldon SH, Spruyt K, Ward SD, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.