In the healthy adult, the primary energy source for both the left and right ventricles (LVs and RVs) are fatty acids, accounting for 60–90% of the substrate used for ATP generation (1, 2). In disease, both the LV and RV revert to a more fetal-like metabolic state, with a shift in substrate use to increase glucose and decrease fatty acid metabolism (1, 2). Teleologically, such a shift would presumably be adaptive, with increased oxygen efficiency in an oxygen-limited environment, as fatty acid β-oxidation requires 12% more O2 per mol of ATP generated than glucose oxidation (3). Indeed, in both humans and animal models of pulmonary hypertension (PH), there is a shift toward glucose oxidation in the pathologic response of the RV. In contrast, in “good” or “homeostatic” hypertrophy, such as that observed in athletes, predominant fatty acid oxidation is preserved (4). In the failing RV, the scope of metabolic alterations remain unclear, and key questions remain on whether glucose oxidation is adaptive or maladaptive to the stressed RV, if the shift in substrate use (from fatty acid to glucose) occurs as a consequence of the increase in afterload and/or lung vascular disease, and the specific role of bone morphogenetic protein receptor type 2 (BMPR2) mutations in the responses of the RV resulting from PH.
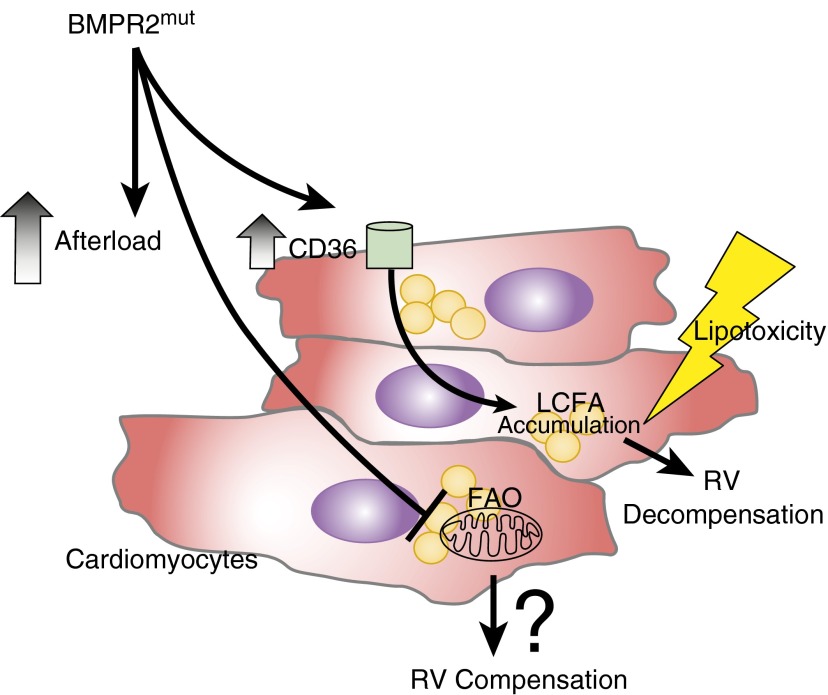
In this issue of the Journal, Talati and colleagues (pp. 719–728) provide some insights into these questions (Figure 1) (5). The present study builds on the prior work by Hemnes and colleagues, who used transgenic mice with a globally expressing conditional BMPR2 mutant, which abrogated BMP signaling in a dominant-negative fashion. These mice developed RV dysfunction with increased triglyceride and pro-apoptotic ceramide deposits in RV myocytes (6). Lipid deposition was also observed in human pulmonary arterial hypertension RV tissue (6). Their current work further parses the RV lipid content, finding an increase in long-chain fatty acids (LCFAs), specifically palmitate, in BMPR2 mutant RV myocytes, coupled with impaired fatty acid oxidation (FAO), both of which were exacerbated by a Western diet. Interestingly, they found an association between impaired BMPR2 signaling and increased sarcolemma-associated CD36 expression. CD36, in addition to being the scavenger receptor for oxidized LDL and promoting binding of thrombospondin, is a well-described transporter of LCFA, facilitating its uptake in adipocytes and cardiac and skeletal muscle (7, 8). As a consequence, these data implicate increased LCFA uptake and decreased use by FAO as resulting in the lipid deposition observed.
Figure 1.
Defective bone morphogenetic protein receptor type 2 (BMPR2) signaling effects on right ventricle (RV) myocytes. Aberrant BMPR2 signaling is associated with increased CD36 sarcolemma expression (independent of the effects of increased RV afterload in pulmonary hypertension), contributing to long-chain fatty acid (LCFA) accumulation and lipotoxicity, resulting in RV decompensation. In addition, disordered BMPR2 signaling is associated with impaired fatty acid oxidation (FAO), which could be associated with RV compensation in pulmonary hypertension.
The observation that dysfunctional BMPR2 signaling results in increased sarcolemma CD36 expression has parallels in other biologic systems. Transforming growth factor β family signaling has been previously implicated in regulating CD36 expression in macrophages via both noncanonical mitogen-activated protein kinase–mediated phosphorylation of peroxisome proliferator-activated receptor gamma, as well as canonical Smad-2 signaling (9, 10). As recognized by the authors, the exact biologic underpinnings of this potential BMPR2-CD36 axis remain unclear in cardiac myocytes. However, these alterations were reproduced in cultured myocytes transfected with mutant BMPR2, suggesting the metabolic alterations are cell autonomous, rather than the result of pulmonary vascular disease and/or increased afterload.
An area of uncertainty is the extent to which accumulation of triglycerides within myocytes causes RV dysfunction. Insights into cardiac lipotoxicity have been gained from studies of LV dysfunction in type 2 diabetes and heart failure: increased lipid accumulation in these diseases correlates with heightened expression of CD36 (11), as was also observed here. Pathogenic molecules in LV lipotoxicity include ceramide, diacylglycerol, acylcarnitine, unesterified cholesterol, and lysolecithin; their effects include inflammation, apoptosis, mitochondria dysfunction, and defective insulin signaling (12). Although lipotoxicity has been linked to accumulation of lipid droplets in myocytes, proper triglyceride storage (as within fat vacuoles) may rather protect LV myocytes against oxidative stress; supporting this protection is the key role of the lipid-binding protein perilipin 5 in properly storing saturated fatty acids away from oxidant generation (13). As in metabolic syndrome, diets that are enriched for fat and fructose lead to LV dysfunction in rodent models (11) and also resulted in lipid accumulation in the BMPR2 mutant mice. Detailed studies of substrate flux are thus warranted to more conclusively dissect the pathogenesis of BMPR2 mutation-related RV lipotoxicity and failure.
Last, it is currently unclear how pharmacologically modulating fatty acid metabolism in the RV will be of clinical benefit. In advanced LV disease, two fatty acid catabolic inhibitors with clinical benefit are perhexiline (14), which likely functions by inhibiting the mitochondrial fatty acid transporter carnitine palmitoyltransferase 1, and trimetazidine (15), which likely functions by blocking the β-oxidation enzyme 3-ketoacyl-CoA thiolase. These medications may confer benefit by promoting a further shift toward glucose oxidation, but there is a possibility that, as suggested in the study by Talati and colleagues, blocking FAO could detrimentally increase lipid accumulation and lipotoxicity. The phase 1 clinical trial of dichloroacetate in pulmonary arterial hypertension (ClinicalTrials.gov: NCT01083524) may provide insight into the functional consequence of decreased fatty acid β-oxidation in the RV. As an inhibitor of pyruvate dehydrogenase kinase, dichloroacetate will augment glucose oxidation by increasing the flux of pyruvate into the citric acid cycle. This will also increase acetyl-CoA and malonyl-CoA concentrations; malonyl-CoA, in turn, is an inhibitor of carnitine palmitoyltransferase 1, likely resulting in partial inhibition of FAO (an effect also known as the Randle cycle [16]). As an alternative approach, the author’s prior work reported a protective effect of the 5′ AMP-activated protein kinase activator metformin in rodents (6); decreased 5′ AMP-activated protein kinase, which would favor fatty acid synthesis over oxidation, may participate in the increased fatty acid accumulation in the RV. With continued granular dissection of the metabolic abnormalities of the diseased RV in PH, it is likely that targeted therapeutic strategies will result.
Supplementary Material
Footnotes
Supported by K08HL105536 (B.B.G.) and P01HL014985 (B.B.G. and R.M.T.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 2.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions: potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 4.Takala TO, Nuutila P, Katoh C, Luotolahti M, Bergman J, Mäki M, Oikonen V, Ruotsalainen U, Grönroos T, Haaparanta M, et al. Myocardial blood flow, oxygen consumption, and fatty acid uptake in endurance athletes during insulin stimulation. Am J Physiol. 1999;277:E585–E590. doi: 10.1152/ajpendo.1999.277.4.E585. [DOI] [PubMed] [Google Scholar]
- 5.Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, Grueter C, Jerome WG, Freeman M, Newman JH, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med. 2016;194:719–728. doi: 10.1164/rccm.201507-1444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Nieuwenhoven FA, Verstijnen CP, Abumrad NA, Willemsen PH, Van Eys GJ, Van der Vusse GJ, Glatz JF. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun. 1995;207:747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]
- 8.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael DR, Salter RC, Ramji DP. TGF-β inhibits the uptake of modified low density lipoprotein by human macrophages through a Smad-dependent pathway: a dominant role for Smad-2. Biochim Biophys Acta. 2012;1822:1608–1616. doi: 10.1016/j.bbadis.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wende AR, Symons JD, Abel ED. Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep. 2012;14:517–531. doi: 10.1007/s11906-012-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosatos K, Schulze PC. Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep. 2013;10:109–121. doi: 10.1007/s11897-013-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole PL, Beamer AD, McGowan N, Cantillon CO, Benfell K, Kelly RA, Hartley LH, Smith TW, Antman EM. Efficacy and safety of perhexiline maleate in refractory angina: a double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation. 1990;81:1260–1270. doi: 10.1161/01.cir.81.4.1260. [DOI] [PubMed] [Google Scholar]
- 15.Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, Hasan EJ, Cao X, Boueiz A, Damico R, et al. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol Sin. 2010;31:175–183. doi: 10.1038/aps.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.