Abstract
The topographical distribution of dural arteriovenous fistulas (DAVFs) was analyzed based on the embryological anatomy of the dural membrane. Sixty-six consecutive cases of intracranial and spinal DAVFs were analyzed based on the angiography, and each shunt point was identified according to the embryological bony structures. The area of dural membranes was categorized into three different groups: a ventral group located on the endochondral bone (VE group), a dorsal group located on the membranous bone (DM group) and a falcotentorial group (FT group) located in the falx cerebri, tentorium cerebelli, falx cerebelli, and diaphragm sellae. The FT group was designated when the dural membrane was formed only with the dura propria (meningeal layer of the dura mater) and not from the endosteal dura. Cavernous sinus, sigmoid sinus, and anterior condylar confluence was categorized to VE group, which had a female predominance, more benign clinical presentations, and a lower rate of cortical and spinal venous reflux. Transverse sinus, confluence, and superior sagittal sinus belonged to the DM group. Olfactory groove, falx, tent of the cerebellum, and nerve sleeve of spinal cord were categorized to the FT group, which presented later in life and which had a male predominance, more aggressive clinical presentations, and significant cortical and spinal venous reflux. The DAVFs was associated with the layers of the dural membrane characterized by the two different embryological bony structures. The FT group was formed only with the dura propria as an independent risk factor for aggressive clinical course and hemorrhage of DAVFs.
Keywords: dural arteriovenous fistulas, dura propria, osteal dura, endochondral bone, membranous bone
Introduction
Most popular classifications of dural arteriovenous fistulas (DAVFs) in the literature are hemodynamic classification based on the angiographic findings.1–3)
Geibprasert et al. reported a new classification for DAVFs based on the craniospinal epidural venous anatomy and concluded that there were significant differences between the groups with regard to biological and/or developmental characteristics according to epidural region.4)
They suggested that DAVFs had heterogeneous pathology and that susceptibility to shunt formation on the surface of dura mater varied according to this classification.
The shunt point of DAVFs is usually located on a certain area of dural membrane, such as transverse-sigmoid sinus, carotid cavernous sinus, cribriform plate of olfactory groove, falcotentorial surface and anterior condylar confluence,1–3) as these specific anatomical areas are vulnerable to DAVF formation.4) Embryologically, the intracranial dural membrane is derived from bony structures, and these bony structures consist of two types of bony tissue:5,6) endochondral bone with cartilaginous ossification and membranous bone based on the intramembranous ossification. By contrast, the falcotentorial dural membrane is independent from bony structures. This means there are at least three different anatomical domains of dural membrane.
This study retrospectively analyzed the correlation between distribution of DAVFs and the embryological domains of bony structures corresponding to these two different dural compartments.
Materials and Methods
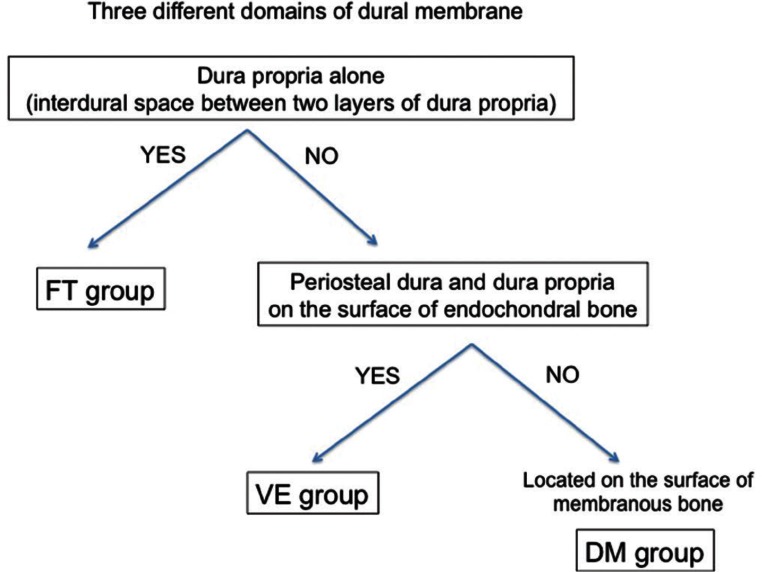
Sixty-six consecutive of DAVFs (32 men and 34 women; age range, 38–80 years; mean age, 68.4 years) were analyzed with selective and superselective digital subtraction angiography, three-dimensional (3D) rotational angiography and high-resolution cone beam computed tomography (CT). Based on these imaging modalities, each shunt point was identified and was categorized into one of three different dural compartments related to the embryologic bony structures, as follows:
The ventral group of endochondral bone from the dura propria and osteal dura (VE group)
The dorsal group of membranous bone from the dura propria and osteal dura (DM group)
The falx and tent of the cerebellum group only from the dura propria (FT group)
The patients were diagnosed in our hospital between January 2006 and December 2014. All patients underwent digital subtraction angiography with selective catheterization to identify the shunt points. 3D rotational angiography and/or high-resolution cone beam CT were performed when it was difficult to identify the precise location of the shunt point. Each shunt point was plotted on the map of the dural membrane to define the anatomical distribution on the surface of dural membrane. In cases of multiple shunts of DAVFs, superselective angiography from the dominant feeder was performed, and the highest flow compartment was defined as the primary shunt point.
Then, the topographical distribution was categorized into three different domains on the surface of dural membrane that were derived from three different embryological structures, as follows:
-
VE group: Ventral group on the surface of endochondral bone
The carotid cavernous sinus, sigmoid sinus and anterior condylar confluence belong to the VE group. This dural membrane consists of the osteal dura and the dura propria (Fig. 1, red-colored area).
-
DM group: Dorsal group on the surface of membranous bone
The transverse sinus, confluence (torcular Herophili), marginal sinus (dorsal portion), medial occipital sinus and accessory epidural sinuses on the dorsal surface of posterior fossa belong to the DM group. This dural membrane consists of the osteal dura and dura propria (Fig. 1, yellow-colored area).
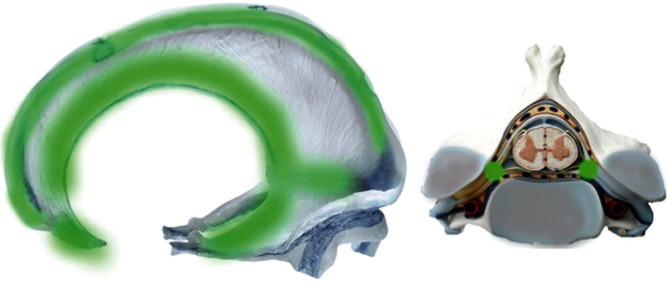
FT group: Falx and tent of the cerebellum group were defined as the dural membrane that was apart from the bony structures (Figs. 1, 2, green-colored area).
Fig. 1 .
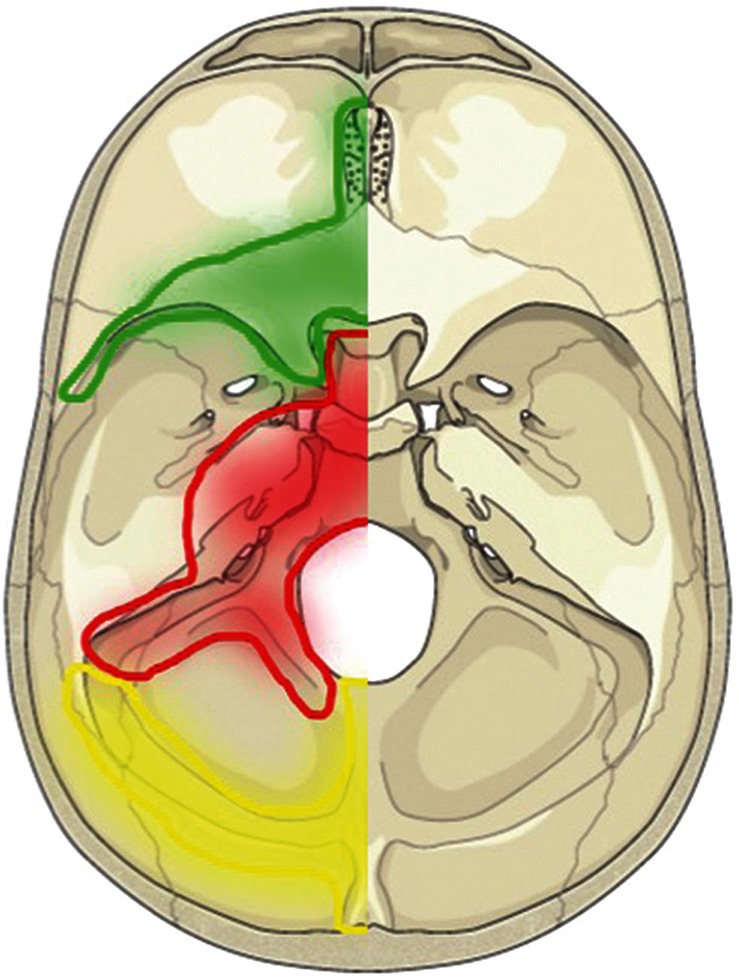
Topographical map of the three different groups of DAVFs in relation to the embryological domains and bony structures. The green area depicts the falx and the tent of the cerebellum (FT group) derived from neural crest cells. The red area reveals the ventral group on the surface of endochondral bone (VE group). The yellow area depicts the torcular, transverse sinus, medial occipital sinus, and posterior marginal sinus that are associated with the surface of membranous bone (DM group).
Fig. 2 .
The location and extent of the FT group. The green area shows the distribution of the marginal area of the falx and tent of the cerebellum. This embryological domain continues to the lateral surface of the spinal cord, corresponding to the nerve sheaths where the DAVFs develop. The domain of this territory is located on the border zone between the vertebral body of endochondral bone and the vertebral arch (lamina) of the membranous bone.
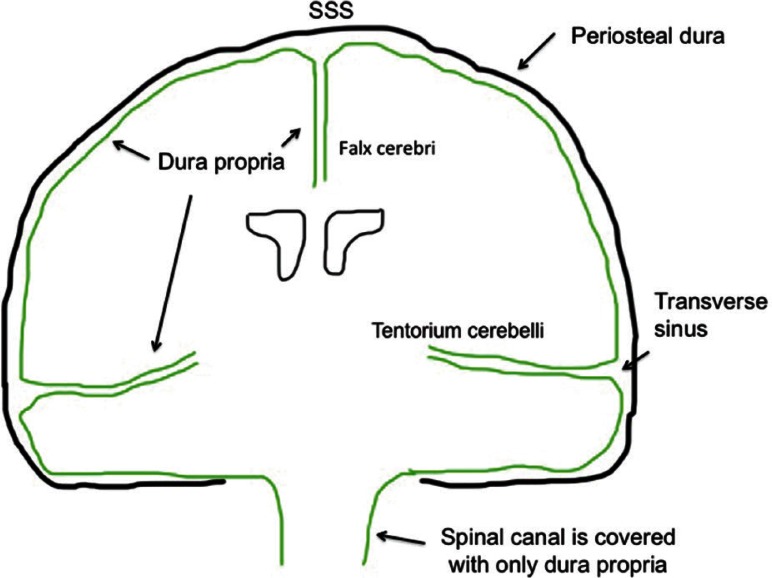
The olfactory groove (paramedian surface of crista galli), superior sagittal sinus, tent of the cerebellum, cerebral falx, falcine sinus and inferior sagittal sinus belong to the FT group. They are derived from part of the neural crest cells and form the dural membrane that is apart from the skull base and cranial vault. 7–10) Based on anatomical considerations, the falx and tent of the cerebellum arise from two folding layers of the dura propria (Fig. 3), which distinguished this group from the other two groups. Spinal cord DAVFs are also categorized to this group. The shunt point of spinal DAVF is located on the nerve sleeve that corresponds to the border zone between the vertebral body (endochondral bone) and the paired laminae of the vertebra arch (membranous bone). As the FT group is formed relatively apart from the major bony structures during the embryological stage and consists of dura propria alone, the FT group is independent from the both VE and DM groups in terms of embryological domain on the dural membrane. In fact, the spinal dura mater consists only of dura propria and lacks the periosteal layer of the cranial dura (Figs. 3, 4).
Fig. 3 .
Topographical scheme of the dura propria and osteal dural membrane. The green line indicates the surface of the dura propria associated with the FT group.
Fig. 4 .
Flow chart of group classification definitions
Clinical manifestations, existence of cortical venous reflux, angioarchitecture of the terminal feeding arteries, initial venous outlet, and types of Borden et al.’s classification were investigated retrospectively. Sixty-two of 66 patients underwent management via the endovascular approach. The other four patients underwent only diagnostic angiography, which showed no indication for intervention.
Statistical data were processed with Stat Plus R software (a free statistical analysis application for the Macintosh operating system). A P-value of <0.05 was used to indicate statistical significance.
Results
Distribution of DVFs and epidemiology
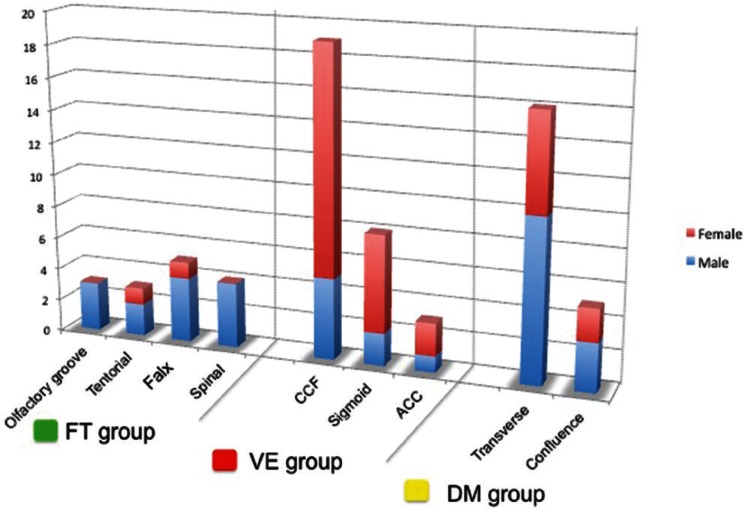
Thirty patients (45.5%) had lesions classified to the VE group. This group consisted of eight men and 22 women, indicating a female predominance (22 of 30 [73%]; P < 0.001). Mean age was 72.1 years. Shunt points were at the carotid cavernous sinus in 19 patients, at the sigmoid sinus in eight, and at the anterior condylar confluence in three.
For carotid cavernous lesions, the majority of shunt points were located at the level of the posterior clinoid processes that belonged to the endochondral bony structure of clivus. All these shunt points were localized at the paramedian unilateral posterior compartment of the cavernous sinus rather than at the midline.
Twenty-one patients (31.8%) had lesions classified to the DM group. These included 16 patients with transverse sinus DAVFs and five with confluence DAVFs. There was no marked sex predominance (male:female ratio = 13:8; P = 0.383), and mean age was 54.5 years.
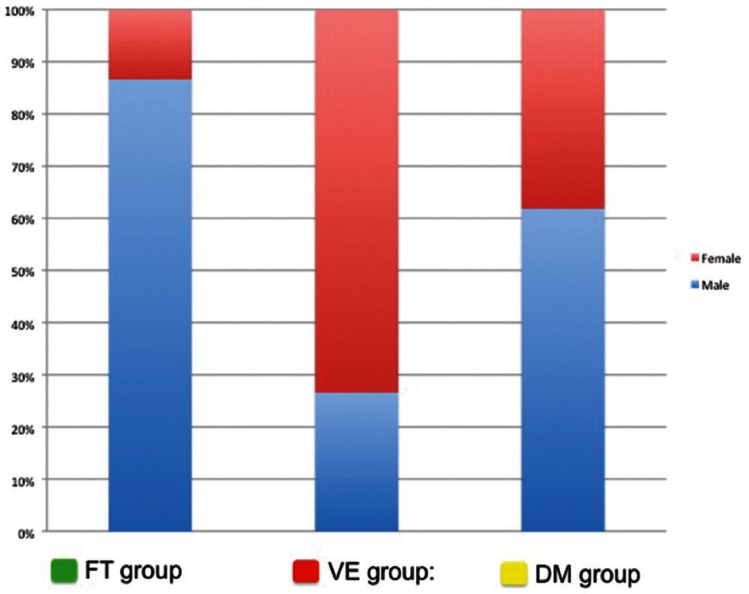
There were 15 patients (22.7%) who had lesions classified to the FT group. These included three patients with olfactory groove DAVFs, three with cerebellar tentorium DAVFs, five with superior sagittal sinus DAVFs, and four with spinal cord DAVFs. There was a strong male predominance (13 of 15 [87%]; P < 0.001), and mean age was 75.2 years, which was significantly older than the other two groups (Table 1, Figs. 5, 6).
Table 1.
Demographics and characteristics of the 66 patients
| FT: falx and tent of the cerebellum derived from neural crest cells | VE: ventral group derived from endochondral bone | DM: dorsal group derived from membranous bone | |
|---|---|---|---|
| N (male/female) | 15 (87% / 13%) | 30 (27% / 73%) | 21 (62% / 38%) |
| Mean age (years) | 65.1 | 78.4 | 59.8 |
| Cortical venous reflux | 93% | 37% | 52% |
| Mode of embolization | Headache, neurological deficit associated with venous infarction, hemorrhage, paraplegia (central myelopathy) | Diplopia, chemosis, bruit | Headache, tinnitus |
| Mode of embolization | TAE 93%, TAE + TVE 7% | TVE 60%, TAE 7% TAE + TVE 33% |
TVE 52%, TAE 19% TAE + TVE 29% |
Cortical venous reflux is predominantly observed in the FT group.
Fig. 5 .
Distributions of the shunt points
Fig. 6 .
Sex predominance among the groups
Clinical manifestations and cortical venous reflux
In the 30 patients of the VE group, major clinical symptoms were ophthalmoplegia, tinnitus and chemosis, which indicated a benign clinical course. There were two patients with neurological deficit associated with perifocal edema due to cortical venous reflux, and one patient experienced intracerebral hemorrhage because of the cortical venous reflux. The degree of cortical venous reflux varied depending on thrombosis and/or the intercompartmental architectures of the affected sinus.
In the 21 patients of the DM group, major symptoms were tinnitus. There were only two patients who presented ataxia as a cerebellar sign associated with parenchymal edema caused by cortical venous reflux. One patient experienced seizure due to the venous congestion of the occipital lobe. The severity of cortical venous reflux depended on the degree of thrombosis in the affected sinus as the major factor of venous outflow restriction (Fig. 7).
Fig. 7 .
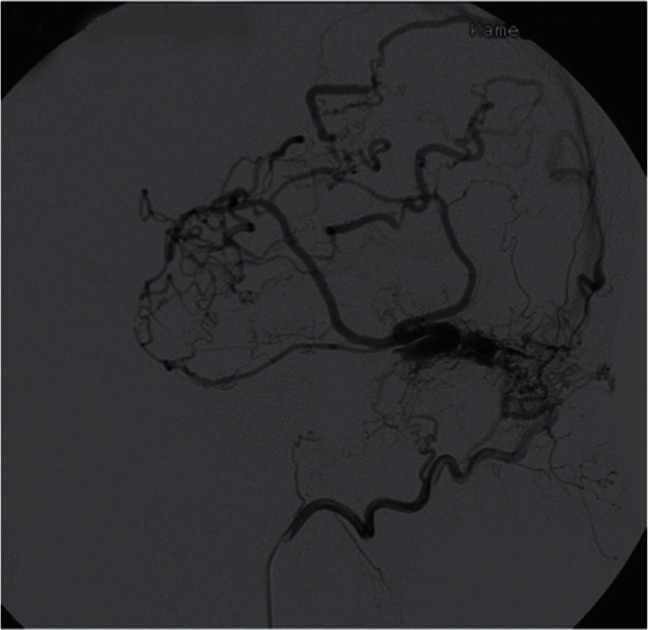
Selective occipital angiography of the transverse DAVF presenting as the severe cortical venous reflux (DM group). The degree of cortical venous reflux strongly correlates with the isolated sinus caused by the secondary-induced thrombosis.
In the FT group, 12 of 15 (80%) patients presented with aggressive clinical symptoms (P < 0.001). There were five patients with neurological deficit associated with perifocal edema due to the cortical venous reflux, three with intracerebral hemorrhage, and four with spinal cord DAVFs who presented with progressive myelopathy. Regarding the angioarchitecture of this group, venous outlet of the arteriovenous (AV) shunts was independent from the main sinus; therefore, 100% of shunt flow created reflux directly into the pial vein of the brain or spinal cord (Figs. 2, 8 and 9).
Fig. 8 .
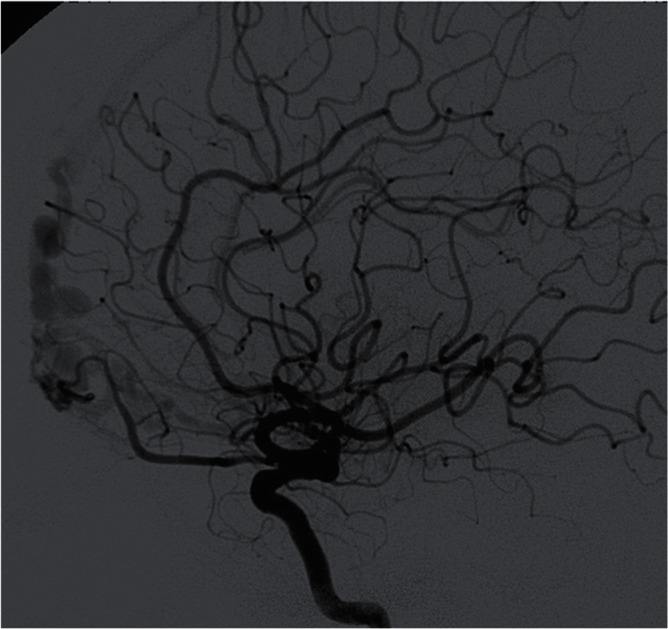
Typical angioarchitecture of the olfactory groove DAVF, showing the significant pial venous reflux through the olfactory vein (FT group). The shunt is located on the anterior part of the cribriform plate belonging to the basal part of the falx.
Fig. 9 .
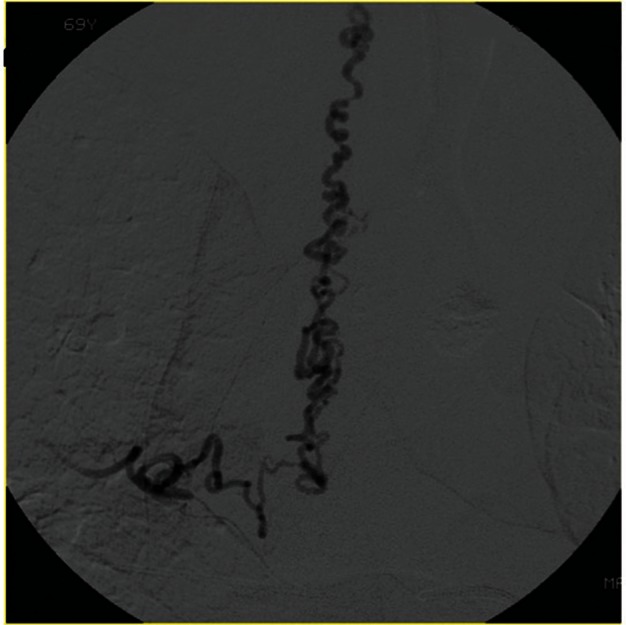
Selective angiography of the intercostal artery at the level of T6 showing spinal DAVF. Shunt is located on the dural surface of nerve sheath. Aggressive pial reflux is frequently observed in the group of FT.
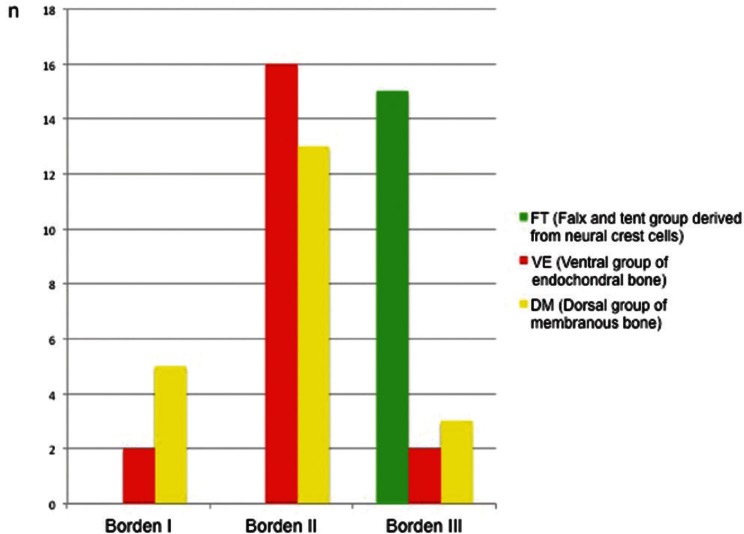
This was the main reason why the FT group showed an aggressive clinical course (i.e., Cognar types III, IV, and V, and Borden et al.’s classification type 3) (Fig. 10).
Fig. 10 .
The relation between Borden et al.’s classification and the three groups. FT group presents with a higher incidence of Borden type III.
Mode of embolization
The decision as to whether the transvenous and/or transarterial approach should be done was made according to the angioarchitecture of the lesions. This mode of embolization was fully influenced by the embryological domain of the dural membrane at which the shunt point was located. In the VE group, the majority of affected sinuses were cavernous sinus or sigmoid sinus; therefore, transvenous embolization was primarily indicated. In the FT group, 14 patients (93%) underwent management via the transarterial approach. All the shunt points of the FT group were independent from the main sinuses, except for some of the superior sagittal sinus DAVFs; therefore, the transarterial approach was predominantly selected. One case of superior sagittal sinus with multiple shunt points was embolized with both the transarterial and transvenous approach.
Discussion
The dural membrane is the most outer tough connective tissue covering the arachnoid membrane, and it attaches to the inner surface of the cranial vault and skull base.5,11) The dura mater, arachnoid mater, and pia mater develop from the meninx primitive that is one of the meningeal mesenchymes containing the mesodermal and neural crest.8,11) At the level of the skull, the outer dural layer forms the inner periosteum of the skull, and the inner dural layer forms the dural folds (falx and tentorium) that contain the dural sinuses.11) The cranial dura mater is a tough, fibrous membrane consisting of two connective tissue layers: an external periosteal layer and an inner meningeal layer. These two layers are fused together, except for where the dural venous sinuses are located (eg., superior sagittal sinus). The periosteal layer of the dura mater adheres to the inner surface of the skull bone and is highly vascular and innervated. The meningeal layer of the dura is smooth and avascular and is lined by mesothelium (a single layer of squamous-like, flattened cells) on its inner surface. At the foramen magnum (a large opening at the base of the occipital bone through which the medulla is continuous with the spinal cord), the meningeal layer of the cranial dura joins the spinal dura. The spinal dura mater consists of only the meningeal layer and lacks the periosteal layer of the cranial dura. At the level of the spinal cord, the dura mater is separated from the periosteum of the vertebral canal by an epidural space. This means there is no interdural space at the level of spinal cord. In fact, there are no dural sinuses in the spinal canal. The definition of the craniospinal epidural venous system by Geibprasert is the venous structures locating the epimeningeal layer of dural membrane that corresponds to dura propria, because there is no periosteal layer in the spinal canal.
The histology of the dural membrane is affected by the differences of bony structures.4,7,8) Both mesenchymal and neural crest-derived cells appear to be involved in the formation of the primary meninx that differentiates during embryonic development.5) The tent of cerebellar and falcine sinus (FT group) is formed in this early stage, but they develop relatively independently from the bony structure because the topographical location of the FT group is apart from the bony structures. The vulnerability of the dural membrane can be presumed and predicted from the process of development in the early stage of embryo in terms of shunt formation.
There are several classifications of DAVFs in the literature, and the majority are grading systems concerning the flow direction of the main sinuses and the presence or absence of cortical venous reflux based on angiographic findings.1,3) Geibprasert et al. reported a new classification of DAVFs according to the craniospinal epidural venous anatomical bases and clinical correlations.4) In that report, the investigators introduced three different types of epidural spaces at which the shunt points are located: the group of ventral epidural shunts, the group of dorsal epidural shunts, and the group of lateral epidural shunts. They showed that ventral epidural shunts were linked to the vertebral body, basioccipital, sigmoid sinus, petrous pyramid, basisphenoid (cavernous sinus) and adjacent sphenoid wings, and related dural structures. Dorsal epidural shunts were associated with the transverse sinus, occipital sinus, and superior sagittal sinus. Lateral epidural shunts were related to spinal dural AV shunts, marginal sinus (lateral portion of the foramen magnum) with the emissary-bridging vein to the condyloid vein, falcotentorial (vein of Galen), petrosal and basitentorial, sphenoparietal sinus, paracavernous region (embryonic tentorial sinus remnants), intraorbital shunts, and lamina cribriformis. Their ventral epidural shunts corresponded to our VE group, and their dorsal epidural shunts partly corresponded to our DM group. Their ventral epidural group included the sigmoid sinus, but the sigmoid sinus was surrounded with membranous bone, and therefore, it was categorized to the DM group in our classification.
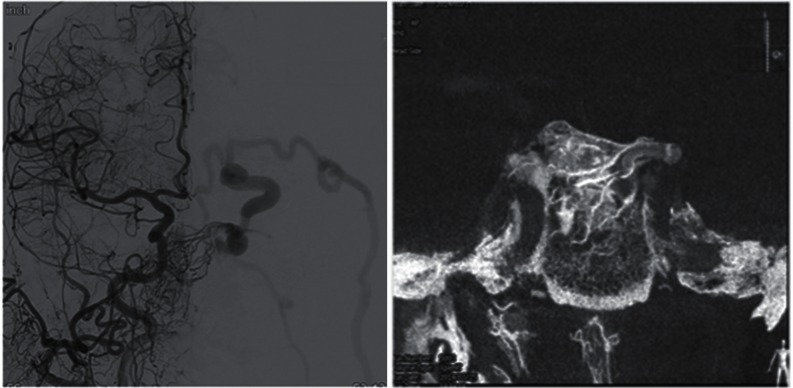
The main difference between their classification and ours was with regards to lateral epidural shunts. There was some controversy in that anterior condylar confluence DAVFs were defined as lateral epidural shunts despite the fact that the hypoglossal canal belongs to the basioccipital bone that was categorized as a ventral epidural shunt. We categorized the anterior condylar confluence DAVFs within the VE group simply because the shunt points were located at the level of the hypoglossal canal from endochondral bony structures. Additionally, there were some common characteristics between anterior condylar confluence DAVFs and carotid cavernous DAVFs. Both DAVFs had meningeal dural supply as well as intraosseous terminal feeding arteries that were not usually observed in the FT group (Fig. 11).12,13)
Fig. 11 .
Angiography and high resolution cone beam CT of carotid cavernous sinus DAVF. The shunt is located on the postero-medial part of the cavernous sinus that belongs to the domain of endochondral bone. Note the multiple terminal feeders passing through the clivus and converging to the left posterior clinoid process. This can be defined as dural shunts and as osseous shunts.
The FT group was defined as an embryological domain of the dural membrane that consisted of only dura propria and that was considered as the structures derived from neural crest cells.5–10) This topographical area contained the entire falx, the tent of the cerebellum, and the dural membrane covering the nerve sheaths of the spinal cord. The olfactory groove (lamina cribriformis) also belongs to this system as the most anterior part of the falx. This concept is consistent with the fact that there was a strong male predominance and that symptoms presented later in life in patients with spinal cord, olfactory groove, falx and tent of cerebellum among this FT group (Fig. 6).
Because of the aggressive clinical presentations, it was evident that transarterial embolization is indicated for the management of patients in the FT group.14)
There are two major weak points of this study. One was that the vulnerability of dural membrane at the level of interdural space has not yet been proven histologically. The other weak point was that the initial trigger of shunt formation and its mechanisms are still unknown. Regardless, characteristics of angioarchitecture and the natural history of DAVFs could be predicted according to classification based on the embryological domains of intracranial and spinal cord dural membrane.15,16) Further investigation of this concept may provide additional information to help understand the pathoetiology of DAVFs.
Conclusions
This reference to embryology enabled us to analyze intracranial and spinal DAVFs in terms of homologues. The presented classification based on the concept of embryological domain is useful to understand the pathoetiology and epidemiology of DAVFs. This principle can aid in decision making regarding the management of this disease. Segmental vulnerability of the dural membrane might be related to the biological and/or hormonal differences that are influenced by the embryological bony structures.
Acknowledgments
The author thanks Dr. Masaki Komiyama, Department of Neurosurgery, Osaka City General Hospital for his proposal to publish this article.
References
- 1).Borden JA, Wu JK, Shucart WA: A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82: 166–179, 1995 [DOI] [PubMed] [Google Scholar]
- 2).Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ: Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatr 65: 308–316, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ: Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194: 671–680, 1995 [DOI] [PubMed] [Google Scholar]
- 4).Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Toulgoat F, Pongpech S, Lasjaunias P: Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke 39: 2783–2794, 2008 [DOI] [PubMed] [Google Scholar]
- 5).Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA: The cranial dura mater: a review of its history, embryology, and anatomy. Child’s Nerv Syst 28: 827–837, 2012 [DOI] [PubMed] [Google Scholar]
- 6).Friede H: Normal development and growth of the human neurocranium and cranial base. Scand J Plast Reconstr Surg 15: 163–169, 1981 [DOI] [PubMed] [Google Scholar]
- 7).Mitsuhashi Y, Aurboonyawat T, Pereira VM, Geibprasert S, Toulgoat F, Ozanne A, Lasjaunias P: Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: possible homologs of spinal dural arteriovenous fistulas: clinical article. J Neurosurg 111: 889–899, 2009 [DOI] [PubMed] [Google Scholar]
- 8).Aurboonyawat T, Suthipongchai S, Pereira V, Ozanne A, Lasjaunias P: Patterns of cranial venous system from the comparative anatomy in vertebrates: part I, introduction and the dorsal venous system. Interv Neuroradiol 13: 335–344, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Aurboonyawat T, Pereira V, Kring T, Toulgoat F, Churojana A, Lasjaunias P: Patterns of the cranial venous system from the comparative anatomy in vertebrates: part II, the lateral–ventral venous system. Interv Neuroradiol 14: 21–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Aurboonyawat T, Pereira V, Krings T, Toulgoat F, Chiewvit P, Lasjaunias P: Patterns of the cranial venous system from the comparative anatomy in vertebrates: part III, the ventricular system and comparative anatomy of the venous outlet of spinal cord and its homology with the five brain vesicles. Interv Neuroradiol 14: 125–136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Opperman LA: Cranial sutures as intramembranous bone growth sites. Dev Dyn 219: 472–485, 2000 [DOI] [PubMed] [Google Scholar]
- 12).Baltsavias G, Parthasarathi V, Aydin E, Al Schameri RA, Roth P, Valavanis A: Cranial dural arteriovenous shunts: part 1: anatomy and embryology of the bridging and emissary veins. Neurosurg Rev 38: 253–264, 2014 [DOI] [PubMed] [Google Scholar]
- 13).Baltsavias G, Kumar R, Avinash KM, Valavanis A: Cranial dural arteriovenous shunts: part 2: the shunts of the bridging veins and leptomeningeal venous drainage. Neurosurg Rev 38: 265–272, 2014 [DOI] [PubMed] [Google Scholar]
- 14).Agid R, Terbrugge K, Rodesch G, Andersson T, Söderman M: Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg 110: 79–84, 2009 [DOI] [PubMed] [Google Scholar]
- 15).Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M: Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 64: 724–730, 1986 [DOI] [PubMed] [Google Scholar]
- 16).Krings T, Geibprasert S: Spinal dural arteriovenous fistulas. Am J Neuroradiol 30: 639–648, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]