Abstract
Most of cerebral aneurysms (CAs) are incidentally discovered without any neurological symptoms and the risk of rupture of CAs is relatively higher in Japanese population. The goal of treatments for patients with CAs is complete exclusion of the aneurysmal rupture risk for their lives. Since two currently available major treatments, microsurgical clipping and endovascular coiling, have inherent incompleteness to achieve cure of CAs with some considerable treatment risks, and there is no effective surgical or medical intervention to inhibit the formation of CAs in patients with ruptured and unruptured CAs, new treatment strategies with lower risk and higher efficacy should be developed to prevent the formation, growth, and rupture of CAs. Preemptive medicine for CAs should be designed to prevent or delay the onset of symptoms from CAs found in an asymptomatic state or inhibit the de novo formation of CAs, but we have no definite methods to distinguish rupture-prone aneurysms from rupture-resistant ones. Recent advancements in the research of CAs have provided us with some clues, and one of the new treatment strategies for CAs will be developed based on the findings that several inflammatory pathways may be involved in the formation, growth, and rupture of CAs. Preemptive medicine for CAs will be established with specific biomarkers and imaging modalities which can sensor the development of CAs.
Keywords: cerebral aneurysm, prevention, pathogenesis, preemptive medicine
Introduction
Cerebral aneurysms (CAs) is a common disease in general public with prevalence between 1% and 5%,1) and subarachnoid hemorrhage (SAH) mostly caused by rupture of CAs is still a serious disease and a relevant health problem in the middle- and old-aged populations. Although clinical outcomes in patients with ruptured CAs have improved in recent advances of microsurgical and endovascular techniques and intensive care and management, nearly half of cases suffer from severe morbidity and mortality. Most of the CAs are incidentally discovered without any neurological symptoms through imaging studies such as magnetic resonance imaging (MRI) and indication of surgical intervention to unruptured CAs are still a matter of debate considering several factors including each patient’s background, nature of the lesion, and risk of surgical intervention. In Japan, because of widespread brain dock, many unruptured CAs are detected and the number of surgically treated patients has been increasing. Intriguingly, the risk of rupture of CAs is relatively higher in Japanese and Finnish population than other peoples,2) making the treatment of CAs important especially in our country.
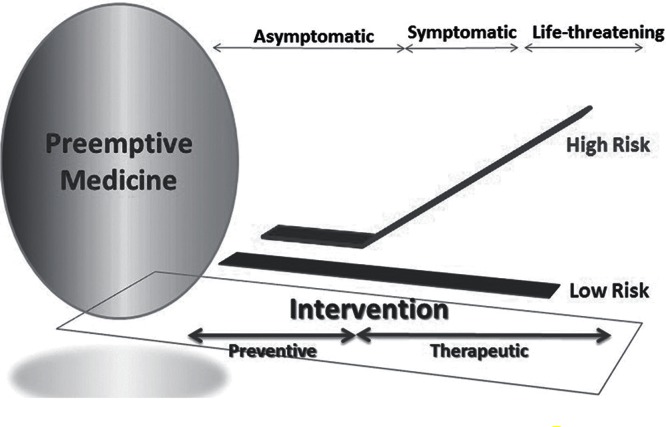
Preemptive medicine is a new idea of interventional therapy to prevent or delay the onset of diseases with predictive and precise diagnosis and adequate treatments on people who are in an asymptomatic state in which clinical symptoms or severe tissue damages have not occurred or who are free from diseases (Fig. 1). Therefore, the treatment to achieve preemptive medicine should be based on pathogenesis and triggering/promoting mechanisms of diseases considering disease-related risks, and its goal is to maintain health and to prevent onset or progression of the disease by focusing on not only individual but also community health. To date, there is no non-surgical treatment available for cerebral aneurysmal growth and rupture except control of systemic blood pressure and cessation of smoking, and therefore microsurgical clipping and endovascular coiling are performed to prevent first and repeated rupture and resultant SAH. Available and apparently effective preventive therapy for CAs is surgical intervention for unruptured lesions to reduce the risk of first rupture and for ruptured lesions to reduce the risk of re-rupture, and preemptive medicine for CAs has not been put in practice and its development depends on future advancements in cerebral aneurysmal research. Thereby, considering the relatively small number of rupture events among unruptured cases, the intrinsic risk of complication in any surgical intervention, the loss of social productivity due to SAH after rupture of CAs, and high prevalence of CAs in adult population, a new treatment options with lower risk and higher efficacy should be developed to prevent the formation, growth, and rupture of CAs.
Fig. 1 . Idea of preemptive medicine .
The objective of this article is to overview the current standards in the management of CAs and to discuss the possible interventions in the future including preemptive medicine considering current progress in basic and clinical research.
Risk Factors
Annual incidence of SAH is 5–20 per 100,000 people, and the number is higher in Japan and Finland as compared with other countries. The estimated prevalence of unruptured CAs in a healthy population with a mean age of 50 years old is calculated to be 3.2% with higher prevalence in women and an increased prevalence with age,1) and almost 30% of all unruptured CAs in people of working age seem to rupture during a lifelong follow-up.3) Because all detected unruptured CAs do not necessarily rupture during their lives and SAH may occur from aneurysms which are not detected, unruptured CAs should be stratified according to the risk of rupture and growth. The size of unruptured CAs is a strong risk of rupture,4) and effective interventions to reduce rupture risk by controlling growth and rupture of unruptured CAs are to be developed.
There are modifiable and non-modifiable risk factors for CAs.1,5,6) These factors can affect the formation, growth (morphological changes), and rupture of CAs. In modifiable risk factors, smoking appears to increase the risk in the formation of CA, and also is an independent and important risk factor for SAH. Although the effectiveness of antihypertensive medication in prevention is not proven yet, adequate control of systemic blood pressure seems to exert against aneurysmal rupture because untreated hypertension is seen frequently in patients with ruptured aneurysms. Excessive alcohol use may also be a risk factor for aneurysm development and rupture. Other possible factors for growth include female sex, younger age, aneurysm location, multiplicity of aneurysms, specific genetic background; family history of SAH, specific disorders such as polycystic kidney disease, type IV Ehlers-Danlos syndrome, Marfan syndrome, coarctation of the aorta, pseudoxanthoma elasticum, hereditary hemorrhagic telangiectasia, and neurofibromatosis type 1. Other risk factors for SAH include age (greater than 70 years old), female sex, race (particularly Japanese and Finnish), size/location/ shape of the CA, previous history of SAH, family history of SAH, and familial aneurysms (at least one first-degree family member with CA). The risk estimation and stratification of unruptured CAs considering each patient’s risk factors is useful to individualize CA managements. Prospective studies to assess the effectiveness of intervention by dealing with risk factors are needed either on general population or high-risk population basis to evaluate the burden of modifiable risk factor.
Risk factors for aneurysm development, growth, and rupture: recommendations
Given that smoking appears to increase the risk of unruptured intracranial aneurysm (UIA) formation, patients with UIA should be counseled regarding the importance of smoking cessation (Class I; Level of Evidence B).
Given that hypertension may play a role in growth and rupture of intracranial aneurysms (IAs), patients with UIA should monitor blood pressure and undergo treatment for hypertension (Class I; Level of Evidence B).
Aneurysmal growth may increase the risk of rupture, and intermittent imaging studies to follow those UIAs managed conservatively should be considered (Class I; Level of Evidence B).
(Guidelines for the Management of Patients with Unruptured Intracranial Aneurysms : A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association, 2015).
Diagnosis
Imaging studies for CAs have developed and refined with advancements in magnetic resonance angiography (MRA), three-dimensional (3D) computed tomography angiography (CTA), and digital subtraction angiography (DSA). Each modality has been adapted in different purposes such as initial screening imaging, pre-interventional surgical view, and follow-up imaging. MRA is usually used for screening in patients with no or minimal symptoms, and 3D-CTA is used to assess pre-interventional precise assessment of surgical anatomy of the aneurysm and surrounding vessels. DSA, a rather invasive examination, is also performed as pre- and post-interventional assessment of the treatment to obtain not only arterial but also venous information, but further improvement of CTA and MRA technology may reduce the necessity of DSA in the management of CAs. Although MRI seems to be a good tool in the follow-up of endovascular-treated aneurysms, it is not suitable for clipped aneurysms. In the future, specific anatomical or biological features of the aneurysm should be evaluated to properly estimate the risk of each aneurysm and select appropriate management. Subarachnoid blood is detected in computed tomography (CT) in the early phase after SAH, but the sensitivity decreases with time after the onset of SAH. Fluid-attenuated inversion recovery (FLAIR) magnetic resonance (MR) seems to be comparable with CT in the acute phase of SAH and superior to CT in a few weeks following SAH.
Morphological risk factors for growth or rupture of CAs are size/location/shape of the CA.1–6) However, there is no specific diagnostic tool to assess the nature of being prone to rupture. Recently, vascular wall imaging has been developed in high magnetic field MR using high resolution sequences. Identification of the rupture site in patients with multiple CAs has been demonstrated using T1-weighted black blood vessel wall sequence (turbo spin echo acquisition) before and after intravenous administration of contrast agent, gadolinium.7) Nagahata et al. also reported that rupture site could be detected in high specificity and sensitivity using the 3D turbo spin echo sequence with motion-sensitized driven equilibrium imaging after gadolinium injection in patients with CA.8) New diagnostic modalities for preemptive medicine should be aimed to identify “dangerous” CAs with new imaging techniques or specific biomarkers.
I. Diagnosing/imaging: recommendations
DSA can be useful compared with non-invasive imaging for identification and evaluation of CAs if surgical or endovascular treatment is being considered (Class IIa; Level of Evidence B).
DSA is reasonable as the most sensitive imaging for follow-up of treated aneurysms (Class IIa; Level of Evidence C).
CTA and MRA are useful for the detection and follow-up of UIA (Class I; Level of Evidence B).
It is reasonable to perform MRA as an alternative for follow-up for treated aneurysms, with DSA used as necessary when deciding on therapy (Class IIa; Level of Evidence C).
Coiled aneurysms, especially those with wider neck or dome diameters or those that have residual filling, should have follow-up evaluation (Class I; Level of Evidence B). The timing and duration of follow-up is uncertain, and additional investigation is necessary.
The importance of surveillance imaging after endovascular treatment of UIAs lacking high-risk features for recurrence remains unclear, but surveillance imaging is probably indicated (Class IIa; Level of Evidence C).
II. Screening: recommendations
Patients with ≥ 2 family members with IA or SAH should be offered aneurysmal screening by CTA or MRA. Risk factors that predict a particularly high risk of aneurysm occurrence in such families include history of hypertension, smoking, and female sex (Class I; Level of Evidence B).
Patients with a history of autosomal dominant polycystic kidney disease, particularly those with a family history of IA, should be offered screening by CTA or MRA (Class I; Level of Evidence B), and it is reasonable to offer CTA or MRA to patients with coarctation of the aorta and patients with microcephalic osteodysplastic primordial dwarfism (Class IIa; Level of Evidence B).
(Guidelines for the Management of Patients with Unruptured Intracranial Aneurysms : A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association, 2015).
Treatment Modalities
Microsurgical clipping introduced by Walter Dany in 1937 excludes aneurysmal sac from the parent artery and blocks blood flow into the lesion. Improvements of microsurgical instruments and intraoperative imaging studies such as indocyanine green (ICG) angiography and endoscopic inspection further reinforce its efficacy. First introduced in 1991, endovascular coiling has been an alternative to microsurgical clipping, and its use for ruptured and unruptured CAs has been increasing over the years along with the improvements and wide availability of endovascular techniques and devices. The superiority of each treatment modality should be assessed depending on several aspects including overall morbidity and mortality, completeness and durability of aneurysmal occlusion, necessity of retreatment, post-treatment management, rehemorrhage (intra- and post-procedural aneurysm rupture), and both treatments should be tested if they have a long-term treatment effect.
The International Subarachnoid Aneurysm Trial (ISAT) and Cerebral Aneurysm Rerupture After Treatment (CARAT) studies reported rerupture risk once after the treatment of ruptured CAs.9–13) The ISAT study showed that the risk of rebleeding at 1 year was low in both groups (2 per 1,276 patient years and 0 per 1,081 patient years for endovascular and microsurgical treatment, respectively), and that the rate of occlusion was higher in clipped aneurysms (82%) as compared to coiled ones (66%), and that endovascular coiling resulted in a significantly decreased rate of death or dependency as compared to clipping (23.7% vs. 30.6%).9) Rebleeding rates were low but more frequent in the coiled group and the rate of epilepsy was higher in microsurgical patients.9,10) Nine-year follow-up data revealed 24 rehemorrhages, 13 from the treated aneurysms (10 treated by coiling and 3 by clipping).11) Eighteen-year follow-up data showed that at 10 years, 83% of coiled patients and 79% of clipped patients were still alive, 82% of coiled patients and 78% of clipped patients were independent, and although the risk of rebleeding was higher in the coiled group, the probability of disability-free survival was significantly greater as compared to the clipped group.12)
Long-term data from the CARAT study showed that there were 19 reruptures during 4 years of follow-up in 1,001 treated patients (3.4% risk of rerupture for coiling and 1.3% for clipping), and concluded that the degree of aneurysmal occlusion after initial treatment in both microsurgical clipping and endovascular coiling is a strong predictor of the risk of rebleeding; 1.1% risk of rehemorrhage in complete occlusion as compared with a 2.9% risk for small residual neck, a 5.9% risk for residual neck, and a 17.6% risk for partial occlusion.13)
The International Study of Unruptured Intracranial Aneurysms (ISUIAs) reported that overall morbidity and mortality (defined as death and modified Rankin Scale 3–5 or impaired cognitive status) at 1 year was 12.6% (if no prior history of SAH) and 10.1% (if prior history of SAH) in 1,917 patients who received microsurgical clipping and 9.8% (if no prior history of SAH) and 7.1% (if prior history of SAH) in 451 patients who received endovascular coiling.14) Complete obliteration was accomplished in 55% of patients, incomplete obliteration in 24%, and no obliteration in 3% in endovascular group, but the degree of aneurysmal obliteration was not determined in microsurgical group.
Based on the clinical trials above, microsurgical clipping and endovascular coiling have inherent incompleteness to achieve cure of CAs with some considerable treatment risks. It is true that larger the aneurysm, higher the chance of rupture and the higher the risk of treatments. Thereby, procedural and rupture risks and long-term benefit should be precisely assessed in the decision for or against intervention in each case, taking into account each patient’s background, intrinsic factors of each lesion, and burden of the intervention. Ruptured CAs causing SAH should be treated as early as logistically and technically possible to reduce the risk of rebleeding, and any treatment should be done at least within 72 h after onset. Generally, microsurgical clipping is in favor of patients with younger age, presence of space-occupying intracerebral hematoma, relatively superficially located aneurysms, wide-neck aneurysms, and aneurysms with arterial branches exiting directly out of the aneurysmal sac. Endovascular coiling is in favor of patients with age above 70 years old, no space-occupying intracerebral hematoma, deep-located aneurysms, and small neck aneurysms. From ISAT data, if the aneurysm appears to be equally effectively treated by either coiling or clipping, coiling is the preferred treatment, but the decision should be based on several confounding factors in each case including patient’s age, SAH grade, comorbidity, presence of intracerebral hematoma, aneurysm size, shape, and location.
Recommendations
Several factors should be considered in the selection of the optimal management of an UIA, including the size, location, and other morphological characteristics of the aneurysm; documented growth on serial imaging; the age of the patient; a history of prior aneurysmal SAH; family history of CA; the presence of multiple aneurysms; or the presence of concurrent pathology such as an arteriovenous malformation or other cerebrovascular or inherited pathology that may predispose to a higher risk of hemorrhage (Class I; Level of Evidence C).
Patients with unruptured CAs who are considered for treatment should be fully informed about the risks and benefits of both endovascular and microsurgical treatment as alternatives to secure the UIAs and prevent bleeding (Class I; Level of Evidence B).
The results of UIA treatment are inferior at low-volume centers, and hence treatment is recommended to be performed at higher-volume centers (Class I; Level of Evidence B).
Data from prospective and retrospective studies from multiple national and international investigations indicate that microsurgical clip ligation may confer more durable protection against aneurysm regrowth, but coil embolization may be superior to surgical clipping with respect to procedural morbidity and mortality, length of stay, and hospital costs. So it may be reasonable to choose endovascular therapy over surgical clipping in the treatment of select UIAs, particularly in cases for which surgical morbidity is high, such as at the basilar apex and in the elderly (Class IIb; Level of Evidence B).
The treatment risk of patients with UIAs is related to advancing age, medical comorbidities, and aneurysm location and size, so in older patients (> 65 years of age) and those with associated medical comorbidities with small asymptomatic UIAs and low hemorrhage risk by location, size, morphology, family history, and other relevant factors, observation is a reasonable alternative (Class IIa; Level of Evidence B).
(Guidelines for the Management of Patients with Unruptured Intracranial Aneurysms : A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association, 2015).
Prevention
The goal of treatments for patients with CAs is complete exclusion of the aneurysmal rupture risk for their lives. Available surgical interventions for unruptured and ruptured CAs include microsurgical clipping or trapping, endovascular coiling or stenting, and obliterate CAs in morphological aspects. However, they do not seem to have reasonable preventive effects on the recurrence of the lesion at the same or remote sites. Microsurgical neck clipping mechanically closes the orifice of aneurysmal sac and interfaces endothelial cell layers which promote reconstruction and modification of histological endothelium. It seems to reconstruct normal vascular layers, but surgical clipping itself cannot promote the normalization of pathological vascular wall or modify hemodynamic stress to the site of lesion. Endosaccular coiling induces flow stasis-mediated luminal thrombosis of CAs for possible promotion of endothelialization of aneurysmal orifice and serves as scaffold for recruitment of cells that synthesize extracellular matrix and induce fibrosis. Recent developments in several kinds of coil materials and new stents such as flow diverters have changed endovascular treatments for CAs, but optimal modification or reconstruction of normal vascular wall has never been obtained by endovascular techniques. Any available surgical intervention itself cannot modify or reduce the risk of CA formation in the same location or other locations of cerebral arterial trees in patients with unruptured or ruptured CAs.
Surgical intervention effectively reduces further rupture, rerupture, or growth of treated CAs, but both endovascular and microsurgical interventions had a risk of rupture or rerupture in treated patients (Table 1). The occurrence of SAH in treated patients with CAs is attributable to incomplete obliteration in treated aneurysms or formation of de novo aneurysms.9–25) It should be noted that both unruptured and ruptured CAs carry a risk of rupture in a long term follow-up even after apparently effective and complete treatments, and patients with CAs have an inherent risk of de novo aneurysms which may rupture during their lives. Endovascular coiling may not reduce the risk of growth and rupture of thrombosed aneurysms, particularly giant lesions, and it has been also reported that endovascular deployment of flow diverters has a considerable risk of rupture.26) There is no effective surgical or medical intervention to inhibit the de novo formation of CAs in patients with ruptured and unruptured CAs. Smoking cessation might reduce the formation of CAs, and antihypertensive medication or changes in life style may reduce the risk of rupture, but the effects have not been proven. Recently, possible medical intervention has been investigated in CA animal models to evaluate the effect of several drugs on the formation and rupture of CAs.
Table 1.
Recurrence of subarachnoid hemorrhage/cerebral aneurysms after surgical intervention
| Author (year) | Study design | Mean follow-up period | Risk of recurrence (microsurgery) | Endovascular surgery |
|---|---|---|---|---|
| Tsutsumi et al. (1998) | 220 ruptured cases | 9.9 y (3–17 y) | Recurrent SAH 2.7% (6/220) (de novo 2) | |
| retrospective | (2.2% at 10 y, 9.0% at 20 y) | |||
| Tsutsumi et al. (1999) | 115 unruptured cases | 8.8 y (median) | SAH 4/115 (regrowth 2, de novo 2) | |
| retrospective | (1.4% in 10 y, 12.4% in 20 y) | |||
| Tsutsumi et al. (2001) | 140 clipped aneurysms, 112 cases | 9.0 y (3–21 y) | Recurrent aneurysm 2.9% (4/140) | |
| including ruptured and unruptured | (3/125 complete clipping, 1/14 incomplete clipping) | |||
| retrospective | De novo 8.0% (9/112 cases) (0.89%/y) | |||
| Sluzewski et al. (2005) | 393 consecutive ruptured cases | Late rebleeding 1.27% (5/393) | ||
| retrospective | (0.32%/y) | |||
| CARAT (2006) | 1010 ruptured cases prospective cohort | Rerupture after 1 y; 0/711 | Rerupture after 1 y; 1/299 (0.11%/y) | |
| Aikawa et al. (2007) | 227 ruptured cases retrospective | 4.2 y | Late rebleeding 2.6% (6/227) | |
| Johnston et al. (2008) | 1,001 ruptured cases | 4.0 y | Rerupture 1.3% | Reruptured 3.4% |
| ambidirectional cohort | (median time to rerupture 3 d) | |||
| Molyneux et al. (2009) | 1,582 ruptured cases | 9 y (6–14 y) | 7/769 (de novo 3) | 17/813 (de novo 3) |
| prospective cohort | 3/769 occurred in 4–7 y from treated aneurysms | 10/813 occurred in 2–5 y from treated aneurysms | ||
| Schaafsma et al. (2009) | 283 coiled cases (> 90% occlusion) | 6.3 y (1.0–12.2 y) | 17/748 (4 from treated aneurysms, de novo 13) | 3/283 (1 confirmed, 2 possible SAH) |
| 748 clipped cases all ruptured, retrospective | 7.6 y (0.04–19.5 y) | |||
| Gonda et al. (2014) | 2,509 unruptured cases | 7 y (median; 4–12 y) | Incidence of non-traumatic intracranial hemorrhage | |
| observational analysis of a database | 5.9% (92/1,565) | 4.8% (45/944) | ||
| Molyneux et al. (2015) | 1,644 ruptured cases | 10.0–18.5 y | Recurrent SAH after 1 y; 33 cases (17 from treated aneurysms, 16 from another source) | |
| prospective cohort | 0.64% | 2.16% |
SAH: subarachnoid hemorrhage
Preemptive Intervention
Preemptive medicine for CAs should be designed to prevent or delay the onset of symptoms from CAs found in an asymptomatic state or to inhibit the de novo formation of CAs (Fig. 1). In order to establish the preemptive medicine in the treatment of CAs, new principles of treatments based on the pathogenesis of CAs should be developed and, at the same time, specific biomarkers and adequate imaging modalities are mandatory. Although most of CAs which are incidentally detected show rather stable clinical courses and reveal less inflammatory or degenerative changes in their walls, and their risk of rupture is relatively low, some of the unruptured CAs follow the rather unstable course with significant changes in size and shape seemingly with inherent high risk of rupture.6,27) These aneurysms may rupture even if they are small in size in the early phase of development or enlarge in a short time because of wall thinning by advancement of degenerative changes. It is of clinical relevance to estimate accurately the rupture risk of individual CA, but we have no definite methods to distinguish rupture-prone aneurysms from rupture-resistant ones.
I. Pathogenesis
Molecular mechanisms of the occurrence, development, or rupture of CAs have been experimentally investigated. The formation of CAs initiates in response to excessive hemodynamic stress to intracranial arterial wall of vascular bifurcation. The successive common pathway involves endothelial dysfunction/injury, infiltration of inflammatory cells, phenotypic modulation and degeneration of smooth muscles, remodeling of extracellular matrix, and subsequent cell death and vessel wall degeneration. In this process, hemodynamic stress, inflammatory reaction by activated macrophages, and vascular smooth muscle cell death are presumably crucial for the formation of CAs.28,29) The pathological findings in CAs are characterized by protrusion of vascular wall at bifurcations with disruption of elastic laminae, thinning of smooth muscle cell layer, and vascular remodeling, most often degenerative remodeling, coupled with massive inflammatory infiltrates is considered to be an essential part in the pathogenesis of CAs. The infiltration of T cells, neutrophils, and macrophages in particular, is a hallmark of CAs, and the role of nuclear factor-kappa B (NF-kB)-mediated monocyte chemotactic protein-1 (MCP-1) expression seems therefore pivotal in recruiting macrophages into the wall of CAs, triggering and exacerbating the inflammatory reaction and thus contributing to aneurysm formation and progression. Histopathological studies show that the ruptured CAs manifest significant endothelial damage, structural change of the wall, and massive inflammatory cell invasion compared with unruptured CAs.30) The wall of ruptured CAs is found to be fragile, possibly because infiltration of inflammatory cells, mainly macrophages, into the aneurysmal wall resulting in loss of smooth muscle cells and excessive degeneration of matrix proteins. Symptomatic unruptured CAs resemble ruptured aneurysms in their pathology and show significant endothelial cell damage, structural changes of the wall, and inflammatory cell infiltration.
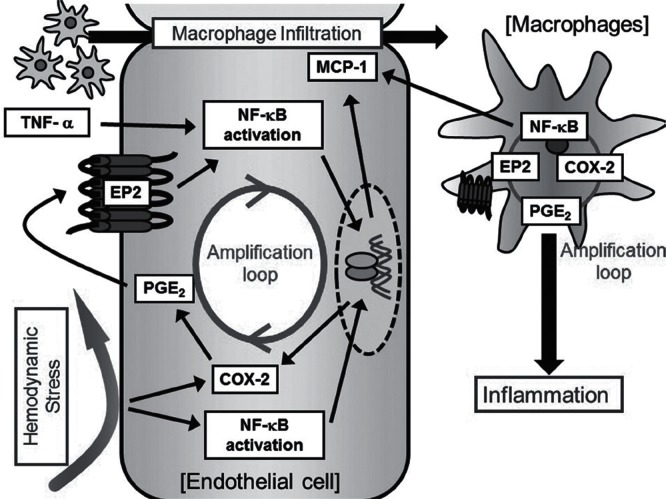
From recent experimental studies using animal models, several pro-inflammatory cascades seem to be activated during aneurysmal progression including NF-κB, tumor necrosis factor-α (TNF-α, prostaglandin, myeloperoxidase, and reactive oxygen species. Thereby, CA is one of the chronic inflammatory diseases like atherosclerosis, cancer, or insulin intolerance. In these pathways, contribution of prostaglandin pathway and NF-κB signaling to CA formation and progression is well studied and supported by several studies (Fig. 2). In brief, hemodynamic stress activates endothelial cells at the bifurcation and prostaglandin producing enzyme, cyclooxygenase (COX), is induced and NF-κB signaling is activated in these cells. Prostaglandin E2 produced by COX-2 then acts on prostaglandin E2 receptor 2 (EP2), one of the prostaglandin E receptor subtype, to evoke inflammation including NF-κB activation. Intriguingly, because NF-κB transcriptionally induces COX-2 expression as a master transcription factor, amplification loop consisting of COX-2-prostaglandin E2-EP2-NF-κB forms to maintain/exacerbate inflammation once triggered by hemodynamic stress loaded, resulting in long-lasting chronic inflammation. Same amplification loop is formed in macrophages, which are recruited by NF-κB-mediated MCP-1 induction in endothelial cells and macrophages themselves and becomes a major type of inflammatory cells in CAs, contributing to further exacerbation of inflammation in lesions. Here, because TNF-α can activate NF-κB, TNF-α presumably functions with prostaglandin signaling to further enhance inflammatory responses leading to CA progression.
Fig. 2 . Inflammatory process in the development of cerebral aneurysms. COX-2: cyclooxygenase-2, EP2: prostaglandin E2 receptor 2, MCP-1: monocyte chemotactic protein-1, NF-kB: nuclear factor-kappa B, PGE2; prostaglandin E2, TNF-α: tumor necrosis factor-α .
II. Biomarkers
Several studies reported possible biomarkers for formation, growth, and rupture of CAs, but there has not been proven available markers to monitor the pathological condition of CAs yet. Hussain et al. performed literature review for biomarkers of CAs and found that CAs were associated with an increase in levels of immunologic markers such as complement C3 and C9, immunoglobulins IgG and IgM, M1/M2 macrophages, monocytes, B and T lymphocytes, cell adhesion molecules, and other cerebral proteins related to brain and vascular damage such as neurofilament heavy chain SM135 and S-100, in both the formation and progression to rupture of CAs.31) However, it is not clarified whether each biomarkers has clinical importance in the management of CAs. Recently Chalouhi et al. demonstrated the increase of some cytokines, such as regulated on activation, normal T cell expressed and secreted (RANTES), IL-8 and IL17, in plasma from CA lesion compared with one from femoral artery.32) Currently, it is technically impossible to detect changes of these cytokines in plasma from peripheral vessels because the change is of course too tiny, but technical advancement may overcome the limitation. Xu et al. applied lectin-affinity enrichment of glycoproteins coupled with quantitative isobaric Tag for Relative and Absolute Quantitation (iTRAQ) labeling proteomic techniques and identified cerebrospinal fluid (CSF) proteins either distinctively changed in ruptured or unruptured CAs or commonly changed in ruptured and unruptured CAs, and found that both CSF and plasma Axl (a tyrosine kinase membrane receptor) levels are significantly elevated in patients with ruptured CAs, and concluded that Axl might serve as a promising biomarker to predict the rupture of CAs.33) Since Axl has oncogenic potential and interacts with other membrane receptors and the involvement of Axl in the process of CA development is not clarified, the role of Axl as a biomarker of CAs is to be elucidated.
Li et al. carried out microarray assays in a screening cohort of 40 patients with CAs (20 unruptured and 20 ruptured) and 20 healthy volunteers to clarify whether circulating micro ribonucleic acids (microRNAs) could be used as biomarkers for CAs, and found that miR-16 and miR-25 were independent factors for CA occurrence.34) Jin et al. investigated warning effect of serum miRNAs for rupture of CAs through microarray hybridization, and showed that several differently expressing serum microRNAs are detected in different levels between normal people and aneurysm patients, as well as among different groups of aneurysms such as daughter aneurysms group, aneurysm without daughter aneurysms group, ruptured aneurysms group, and angiography negative group.35)
Although it is not plausible that serum or CSF molecules act as a sensitive monitor for small intracranial lesions such as CAs, future investigations are necessary to confirm the clinical relevance of each candidate as a new diagnostic/prognostic biomarker of CAs.
III. Imaging
Imaging of molecules or cells may provide us with important information regarding active remodeling, progression, and even risk of rupture of CAs. However, molecular imaging of cerebral arteries is challenging because of many aspects including anatomic variability, tortuosity, small wall thickness, relatively large motion artifact, and insufficient contrast from surrounding brain parenchyma and CSF. Black blood MRI with additional CSF suppression at higher tesla MR with several modified sequences has been applied to vascular diseases including atherosclerosis, dissection, and vasculitis. Gounis et al. reported the use of myeloperoxidase-specific MR signal enhancement for imaging inflammation in a rabbit model of carotid artery aneurysm.36) Ultrasmall superparamagnetic particles of iron oxide (USPIO) uptake into inflammatory cells has been investigated in major arterial wall such as aortic aneurysms and cervical carotid plaques. Early accumulation of USPIO within hours is not necessarily associated with inflammatory cells, but USPIO uptake revealed on T2*-weighted MR images seems to be useful in human CAs to monitor the condition of each lesion.37,38) Because USPIO-mediated contrast in aneurysmal wall detected by MR images could be partly ameliorated in patients with aspirin uptake,39) a clinical study is ongoing to prove the usefulness of USPIO-mediated MR contrast imaging for rupture risk assessment of human CAs. Molecular imaging of cerebral arterial wall may exert as a useful detector of CA progression and biological marker of its growth and rupture. Further study is warranted to visualize molecular and cellular events in cerebral vessels with thin wall.
Image-based computational fluid dynamics (CFD) have been applied to intraluminal flow analysis in CAs and may be a promising role in the evaluation of CAs as a tool to stratify rupture risk and to perform pre- and post-interventional simulation to obtain optimal results.40) Previous reports indicated that both high and low wall shear stress sometimes accompanied with turbulent flow to the wall of CAs have been related to growth and rupture and the results may be influenced by preset parameter definitions, hypothesized calculations, and the time at which flow data are obtained. Although wall shear stress and related analyses among several parameters seem to be a predictor of growth and rupture of CAs, the interpretation of CFD should be carefully performed before determining its clinical utility. Since most of CFD studies have been simulated in steady, non-pulsatile flow with phase contrast magnetic resonance-derived volumetric inflow rate, the findings by CFD should be proven in clinical situations to validate the risk of unruptured CAs, and hemodynamic-biologic mechanisms should be clarified before establishing reliable models to predict growth and rupture risk of CAs by CFD.
IV. Cell therapy
In order to achieve complete cure of CAs, reconstruction of vascular wall with normal endothelialization is necessary. The aim of endovascular coiling and stenting is to form intraluminal thrombus and sequestration of aneurysmal sac from circulation. Since metal materials themselves may not recruit effective cell populations which repair and reconstruct normal vasculature, cell-mediated remodeling process is necessary to achieve permanent occlusion of the aneurysm and reconstitution of the luminal surface of the arterial segment harboring the aneurysm. Several cell therapy modalities with endothelial progenitor cells (EPCs), mesenchymal stem cells (MSCs), vascular smooth muscle cells (VSMCs), and fibroblasts have been shown to be effective and feasible in animal studies (Table 2).41–53)
Table 2.
| Author (year) | Creation of aneurysm | Site of aneurysm | Animal | Cell type | Delivery |
|---|---|---|---|---|---|
| Raymond (1999) | Venous side-wall pouch | Common carotid artery | Dog | VSMC | Fibrin glue |
| Marbacher et al. (2014) | Arterial side-wall pouch | Infrarenal abdominal aorta | Rat | VSMC | Fibrin glue |
| Kawakami (2005) | Venous pouch on artery | Common carotid artery | Rabbit | Fibroblast | Coil |
| Dai et al. (2007) | End sac + local elastase | Common carotid artery | Rabbit | Fibroblast | Coil |
| Marx (2001) | End sac + local elastase | Common carotid artery | Rabbit | Fibroblast | Coil |
| Aronson (2012) | End sac + local elastase | Common carotid artery | Rabbit | EPC | Fibrin glue |
| Li (2013) | End sac + local elastase | Common carotid artery | Rabbit | EPC | Systemic intravenous |
| Zhang (2014) | Arterial side-wall pouch | Infrarenal abdominal aorta | Rat | EPC | Via abdominal aorta |
| Rouchaud (2013) | End sac + local elastase | Common carotid artery | Rabbit | MSC | Intraluminal |
| Kuwabara et al. (2014) | Hypertension + CSF elastase | Cerebral artery | Mouse | MSC | Systemic intravenous |
| Gao et al. (2014) | End sac | Common carotid artery | Rat | EPC | Intraluminal |
CSF: cerebrospinal fluid, EPC: endothelial progenitor cell, MSC: mesenchymal stem cell, VSMC: vascular smooth muscle cell
It is well known that circulating EPCs, as committed linage specific stem cells to endothelial cells, play an important role in maintaining vascular integrity by replacing injured and dysfunctional endothelial cells. Li et al. examined the potential of endothelial colony-forming cells (ECFCs) transfusion on vascular degeneration after CA induction in a rat model and found that ECFCs transfusion confers protection against degeneration of aneurysmal wall by inhibiting inflammatory cascades and SMCs apoptosis.49) Gao et al. tested the efficacy of stromal cell-derived factor-1α (SDF-1α)-coated coils together with EPCs transplantation in mechanically-produced common carotid artery aneurysms and showed that SDF-1α-coated coils plus EPCs transplantation produced a well-organized fibrous tissue bridging the orifice of aneurysms and concluded that combination of these treatments is a safe and effective treatment for rat aneurysms.50) Kuwabara et al. studied the effect of intravenous infusion of xenogeneic MSCs on rupture rate of elastase-hypertension induced IAs in mice, and showed statistically significant decrease in aneurysmal rupture rate in the MSC-treatment group as compared with the control group.51) Marbacher et al. showed the aggravation of degeneration of aneurysmal wall by removing VSMCs from the aneurysmal tissue and the reduction of angiographic recurrence by implanting VSMCs inside occluded aneurysms in decellularized aneurysms and better histologic outcomes in both decellularized and non-decellularized aneurysms.52) Dai et al. applied fibroblasts for aneurysm treatment using platinum coils in a rabbit elastase aneurysm model, and showed that the treatment group showed significant histologic improvement over the control group at 2 weeks but the difference did not persist at later time points.53)
Although aneurysm cell therapy studies seem to be promising, the precise mechanisms of cell therapy should be clarified and the study should be well-designed including the selection of cells and their counts, route of administration, and criteria of outcome measurement.
V. Drug therapy
In the accumulation of knowledge about the involvement of inflammatory process in the development of CAs, several medical therapeutic strategies have been investigated in animal models (Table 3).54–81) The candidate drugs include several anti-inflammatory agents, compounds with anti-NF-κB effect, Ca2+ channel blockers, some protease inhibitors including matrix metalloproteinase (MMP) inhibitors, mast cell degranulation inhibitors, free radical scavengers, and mineralocorticoid receptor antagonists. One of the promising drugs among these agents is a hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitor (statins) presumably through their potent inhibitory action on NF-κB.54–57) Previous experimental data indicate that statins may exert as both protective and deleterious effects on the development and rupture of CAs depending on drugs and their doses. In human studies, Marbacher et al. conducted a case-control study to examine whether statins reduced the risk of aneurysm development, and found no overall association between statin use and incidence of CA formation, nor when dichotomized into hydrophilic and lipophilic user, or between short (≤ 12 months) and long (≥ 36 months) duration of intake.82) Yoshimura et al. conducted another hospital-based case-control study and suggested that there was an inverse relationship between the usage of any kind of statins and CA rupture.83) To confirm the preventive effect of atorvastatin against progression and rupture of small unruptured CAs, a randomized clinical trial is on-going with primary endpoints as aneurysm enlargement over 0.5 mm, aneurysm morphological change, and rupture in Japan.
Table 3.
Tested drugs in animal models against the development of cerebral aneurysms
| Agent | Drug | Delivery | Animal | Aneurysm | Author (year) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Formation | Growth | Rupture | |||||
| HMG-CoA reductase inhibitor | Simvastatin | p.o. | rat | ↓at 25 mg/kg/day | Aoki et al. (2008) | ||
| rat | ↑at 5 mg/kg/day | Tada et al. (2011) | |||||
| Pravastatin | p.o. | rat | ↓at 50, 100 mg/kg/day | Kimura et al. (2010) | |||
| rat | ↑at 25, 50 mg/kg/day | ↑at 25, 50 mg/kg/day | Tada et al. (2011) | ||||
| Pitavastatin | p.o. | rat | ↓at 5 mg/kg/day | Aoki et al. (2009) | |||
| Angiotensin II receptor antagonist | Valsartan | s.c. | rat | no change | Aoki et al. (2009) | ||
| Candesartan | p.o. | rat | ↓at 0.5 mg/kg/day | Tamura et al. (2009) | |||
| Olmesartan | p.o. | rat | ↓at 3, 10 mg/kg/day | Kimura et al. (2010) | |||
| Ca channel blocker | Nifedipine | i.p. | rat | no change | ↓at 10 mg/kg/day | Aoki et al. (2008) | |
| ACE inhibitor | Imidapril | i.p. | rat | ↓at 3 mg/kg/day | Ishibashi et al. (2012) | ||
| Phosphodiesterase inhibitor | Ibudilast | p.o. | rat | ↓at 60 mg/day | Yagi et al. (2010) | ||
| COX-2 inhibitor | Celecoxib | p.o. | rat | ↓at 150 mg/kg/day | ↓at 150 mg/kg/day | Aoki et al. (2011) | |
| Cathepsin inhibitor | NC-2300 | p.o. | rat | ↓ at 50 mg/kg/day | Aoki et al. (2008) | ||
| Protease inhibitor | Tolylsam | p.o. | rat | ↓ at 50 mg/kg/day | Aoki et al. (2007) | ||
| MCP-1 inhibitor | 7ND | i.m. | rat | ↓500 ug of plasmid/body | Aoki et al. (2009) | ||
| Mast cell degranulation inhibitor | Tranilast | p.o. | rat | ↓ at 200 mg/kg/day | Ishibashi et al. (2010) | ||
| Emedastine difumarate | p.o. | rat | ↓ at 25 mg/kg/day | Ishibashi et al. (2010) | |||
| Rho-kinase inhibitor | Fasudil | p.o. | rat | ↓at 0.5 mg/ml in water | ↓at 0.5 mg/ml in water | Eldawoody et al. (2010) | |
| Mineralocorticoid receptor blocker | Eplerenone | p.o. | rat | ↓at 100 mg/kg/day | ↓at 100 mg/kg/day | Tada et al. (2009) | |
| Free radical scavenger | Edaravone | p.o. | rat | ↓at 100 mg/day | Aoki et al. (2009) | ||
| TNF-α inhibitor | Etanercept | s.c. | rat | ↓at 2.5, 25 ug/week/body | Yokoi et al. (2014) | ||
| i.p. | mouse | ↓at 56 mg/kg/day | ↓at 56 mg/kg/day | Starke et al. (2014) | |||
| Tetracycline derivative | Doxycycline | p.o. | mouse | no change | ↓at 40 mg/kg/day | Makino et al. (2012) | |
| Doxycycline | p.o. | rat | no change | Kaufmann et al. (2006) | |||
| Minocycline | i.p. | mouse | no change | ↓at 45 mg/kg/day | Makino et al. (2012) | ||
| MMP inhibitor | SB-3CT | i.p. | mouse | no change | Makino et al. (2012) | ||
| NOS inhibitor | Amnoguanidine | i.p. | rat | ↓at 100, 200 mg/kg/day | Fukuda et al. (2000) | ||
| Endothelin receptor antagonist | K-8794 | p.o. | rat | ↓at 30 mg/day | Sadamasa et al. (2007) | ||
| PPARγ agonist | Pioglitazone | i.p. | mouse | no change | ↓at 10 mg/kg/day | Shimada et al. (2015) | |
| PPARγ antagonist | GW9662 | i.p. | mouse | no change | no change | Shimada et al. (2015) | |
| Angiotensin | Ang-(1–7) | i.p. | mouse | no change | ↓at 0.5 mg/kg/day | Shimada et al. (2015) | |
| Estrogen receptor β agonist | Diarylpropionitrile | by implanting pellet | mouse | ↓at 2.5 mg/body | Tada et al. (2014) | ||
| Diarylpropionitrile | by implanting pellet | mouse | no change | ↓at 0.17 mg/body | Tada (2014) | ||
| Estrogen receptor α agonist | Propyl pyrazole triol | by implanting pellet | mouse | no change | Tada et al. (2014) | ||
| Propyl pyrazole triol | by implanting pellet | mouse | no change | no change | Tada et al. (2014) | ||
| Estrogen | 17 β-estradiol | by implanting pellet | mouse | no change | ↓at 0.0017 mg/kg/day | Tada et al. (2014) | |
| Prostaglandin F receptor antagonist | AS604872 | p.o. | rat | no change | no change | Fukuda et al. (2014) | |
ACE: angiotension converting enzyme, COX-2: cyclooxygenase-2, HMG-CoA: hydroxymethylglutaryl-CoA, MMP: matrix metalloproteinase, i.m.: intramuscular, i.p.: intraperitoneal, MCP-1: monocyte chemotactic protein-1, NOS: nitric oxide synthase, p.o.: per os, PPARγ: peroxisome proliferator-activated receptor γ, SB-3CT; 2-[(4-phenoxyphenyl)sulfonylmethyl]thiirane, s.c.: subcutaneous, TNF-α: tumor necrosis factor, 7ND: an N-terminal deletion variant of MCP-1
Another anti-inflammatory agent is aspirin, a widely-used non-selective inhibitor of COX. A case-control study from subjects enrolled in ISUIA showed that patients with unruptured CAs who used aspirin three times weekly or more had a lower risk of hemorrhage compared with those who never used aspirin.84) Gross et al. also demonstrated that the rate of hemorrhagic event was significantly greater in patients without aspirin usage, supporting a preventive effect of aspirin on CA rupture.85) This protective effect of aspirin may be plausible because of its anti-inflammatory effects, through inhibiting prostaglandin production, in the development of CA, but its adverse effect of bleeding tendency due to the non-specific inhibition of prostaglandin synthesis may be the problem in the setting of clinical trials particularly in Asian people because of their high incidence of intracranial hemorrhage. Indeed, presumably reflecting these two sides of aspirin, anti-inflammatory effect and anti-thrombotic effect, another study by Garbe et al. reported the increased risk of SAH in patients with aspirin usage through nested case-control study in a cohort of the German Pharmacoepidemiological Research Database.86) A clinical trial is planned in the United States to determine the effectiveness of aspirin on reducing the incidence of aneurysmal growth and rupture.
Future Perspectives
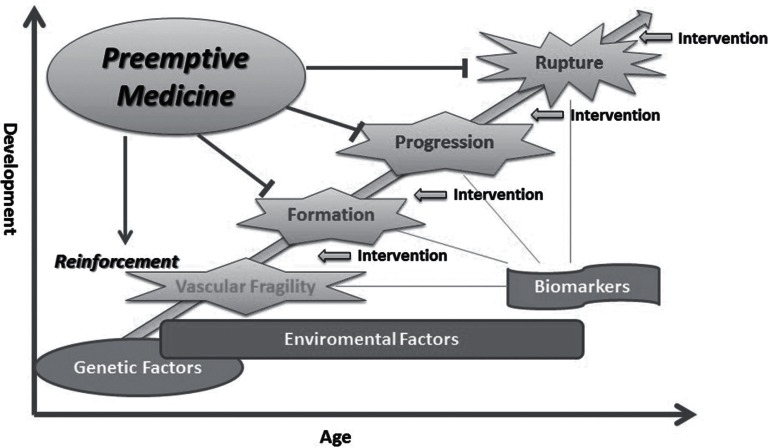
Rupture of the CA is the major cause of catastrophic SAH and the goal of treatment for patients with CAs is the prevention of its rupture/re-rupture during their lives. Although microsurgical clipping and endovascular coiling/stenting are currently the main but only available treatment options, they only modify morphological aspects in identified aneurysms and cannot completely prevent further rupture from the targeted lesions or de novo aneurysmal formation in other cerebral arterial bifurcations. Due to considerable complications by these invasive surgical options, it is necessary to develop more effective and less risky treatment strategies which may modify the risk of cerebral aneurysmal development including rupture. Growing knowledge has been gathered on the mechanisms underlying formation, growth, and rupture of CAs from epidemiological analysis of risk factors, radiological identification of vascular wall biology, and pharmacological intervention to aneurysmal development in animal models. One of the new treatment strategies for CAs will be developed based on the findings that several inflammatory pathways may be involved in the formation, growth, and rupture of CAs. Preemptive medicine is to be established with specific biomarkers and imaging modalities which can sensor the development of a disease. Its application to CAs may include reinforcement of vascular structures, control of the formation by modifying genetic/environmental risk factors, and structural fragility and inhibition of the progression resulting in the prevention of their rupture in high-risk populations for aneurysmal development (Fig. 3). Such intervention should be performed considering the risk-benefit of each patient and the cost-effectiveness.
Fig. 3 . Cerebral aneurysm development and preemptive medicine .
References
- 1). Thompson BG, Brown RD, Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Jr, Duckwiler GR, Harris CC, Howard VJ, Johnston SC, Meyers PM, Molyneux A, Ogilvy CS, Ringer AJ, Torner J, American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Epidemiology and Prevention. American Heart Association; American Stroke Association : Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 46: 2368– 2400, 2015. [DOI] [PubMed] [Google Scholar]
- 2). Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ: Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 38: 1404– 1410, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Korja M, Lehto H, Juvela S: Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45: 1958– 1963, 2014. [DOI] [PubMed] [Google Scholar]
- 4). UCAS Japan Investigators. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T: The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474– 2482, 2012. [DOI] [PubMed] [Google Scholar]
- 5). Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, European Stroke Organization : European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 35: 93– 112, 2013. [DOI] [PubMed] [Google Scholar]
- 6). Morita A: Current perspectives on the unruptured cerebral aneurysms: origin, natural course, and management. J Nippon Med Sch 81: 194– 202, 2014. [DOI] [PubMed] [Google Scholar]
- 7). Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R, Johnson MH, Mikulis DJ, Minja FJ: Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 72: 492– 496; discussion 496, 2013. [DOI] [PubMed] [Google Scholar]
- 8). Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S, Sato S, Mouri W, Saito S, Kayama T: Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9). Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group : International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 11: 304– 314, 2002. [DOI] [PubMed] [Google Scholar]
- 10). Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group : International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366: 809– 817, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J, ISAT Collaborators : Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 8: 427– 433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS: The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 385: 691– 697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR, CARAT Investigators : Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke 39: 120– 125, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362: 103– 110, 2003. [DOI] [PubMed] [Google Scholar]
- 15). Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T: Risk of recurrent subarachnoid hemorrhage after complete obliteration of cerebral aneurysms. Stroke 29: 2511– 2513, 1998. [DOI] [PubMed] [Google Scholar]
- 16). Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T: Risk of subarachnoid hemorrhage after surgical treatment of unruptured cerebral aneurysms. Stroke 30: 1181– 1184, 1999. [DOI] [PubMed] [Google Scholar]
- 17). Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T: Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke 32: 1191– 1194, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D: Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34: 1398– 1403, 2003. [DOI] [PubMed] [Google Scholar]
- 19). Wermer MJ, Greebe P, Algra A, Rinkel GJ: Incidence of recurrent subarachnoid hemorrhage after clipping for ruptured intracranial aneurysms. Stroke 36: 2394– 2399, 2005. [DOI] [PubMed] [Google Scholar]
- 20). Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC: Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 26: 2542– 2549, 2005. [PMC free article] [PubMed] [Google Scholar]
- 21). CARAT Investigators : Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke 37: 1437– 1442, 2006. [DOI] [PubMed] [Google Scholar]
- 22). Aikawa H, Kazekawa K, Nagata S, Onizuka M, Iko M, Tsutsumi M, Kodama T, Nii K, Matsubara S, Etou H, Tanaka A: Rebleeding after endovascular embolization of ruptured cerebral aneurysms. Neurol Med Chir (Tokyo) 47: 439– 445; discussion 446–447, 2007. [DOI] [PubMed] [Google Scholar]
- 23). Schaafsma JD, Sprengers ME, van Rooij WJ, Sluzewski M, Majoie CB, Wermer MJ, Rinkel GJ: Long-term recurrent subarachnoid hemorrhage after adequate coiling versus clipping of ruptured intracranial aneurysms. Stroke 40: 1758– 1763, 2009. [DOI] [PubMed] [Google Scholar]
- 24). Gonda DD, Khalessi AA, McCutcheon BA, Marcus LP, Noorbakhsh A, Chen CC, Chang DC, Carter BS: Long-term follow-up of unruptured intracranial aneurysms repaired in California. J Neurosurg 120: 1349– 1357, 2014. [DOI] [PubMed] [Google Scholar]
- 25). Lecler A, Raymond J, Rodriguez-Régent C, Al Shareef F, Trystram D, Godon-Hardy S, Ben Hassen W, Meder JF, Oppenheim C, Naggara ON: Intracranial aneurysms: recurrences more than 10 years after endovascular treatment—a prospective cohort study, systematic review, and meta-analysis. Radiology 277: 173– 180, 2015. [DOI] [PubMed] [Google Scholar]
- 26). Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F: Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J 28: 365– 375, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Sonobe M, Yamazaki T, Yonekura M, Kikuchi H: Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 41: 1969– 1977, 2010. [DOI] [PubMed] [Google Scholar]
- 28). Aoki T: Inflammation mediates the pathogenesis of cerebral aneurysm and becomes therapeutic target. Neuroimmunol Neuroinflammation 2: 86– 92, 2015. [Google Scholar]
- 29). Kataoka H, Aoki T: Molecular basis for the development of intracranial aneurysms. Expert Rev Neurother 10: 173– 187, 2010. [Google Scholar]
- 30). Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R: Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 30: 1396– 1401, 1999. [DOI] [PubMed] [Google Scholar]
- 31). Hussain S, Barbarite E, Chaudhry NS, Gupta K, Dellarole A, Peterson EC, Elhammady MS: Search for biomarkers of intracranial aneurysms: a systematic review. World Neurosurg 84: 1473– 1483, 2015. [DOI] [PubMed] [Google Scholar]
- 32). Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D: Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke 44: 2594– 2597, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Xu J, Ma F, Yan W, Qiao S, Xu S, Li Y, Luo J, Zhang J, Jin J: Identification of the soluble form of tyrosine kinase receptor Axl as a potential biomarker for intracranial aneurysm rupture. BMC Neurol 15: 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Li P, Zhang Q, Wu X, Yang X, Zhang Y, Li Y, Jiang F: Circulating microRNAs serve as novel biological markers for intracranial aneurysms. J Am Heart Assoc 3: e000972, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Jin H, Li C, Ge H, Jiang Y, Li Y: Circulating microRNA: a novel potential biomarker for early diagnosis of intracranial aneurysm rupture a case control study. J Transl Med 11: 296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Gounis MJ, van der Bom IM, Wakhloo AK, Zheng S, Chueh JY, Kühn AL, Bogdanov AA, Jr: MR imaging of myeloperoxidase activity in a model of the inflamed aneurysm wall. AJNR Am J Neuroradiol 36: 146– 152, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Hasan DM, Mahaney KB, Magnotta VA, Kung DK, Lawton MT, Hashimoto T, Winn HR, Saloner D, Martin A, Gahramanov S, Dósa E, Neuwelt E, Young WL: Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol 32: 1032– 1038, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Winn HR, Heistad D: Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke 43: 3258– 3265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, Young WL, Hashimoto T, Richard Winn H, Heistad D: Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc 2: e000019, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Xiang J, Tutino VM, Snyder KV, Meng H: CFD: computational fluid dynamics or confounding factor dissemination? The role of hemodynamics in intracranial aneurysm rupture risk assessment. AJNR Am J Neuroradiol 35: 1849– 1857, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Adibi A, Sen A, Mitha AP: Cell therapy for intracranial aneurysms: a review. World Neurosurg 86: 390– 398, 2016. [DOI] [PubMed] [Google Scholar]
- 42). Raymond J, Desfaits AC, Roy D: Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke 30: 1657– 1664, 1999. [DOI] [PubMed] [Google Scholar]
- 43). Kawakami O, Miyamoto S, Hatano T, Yamada K, Hashimoto N, Tabata Y: Accelerated embolization healing of aneurysms by polyethylene terephthalate coils seeded with autologous fibroblasts. Neurosurgery 56: 1075– 1081; discussion 1075–1081, 2005. [PubMed] [Google Scholar]
- 44). Marx WE, Cloft HJ, Helm GA, Short JG, Do HM, Jensen ME, Kallmes DE: Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 22: 323– 333, 2001. [PMC free article] [PubMed] [Google Scholar]
- 45). Aronson JP, Mitha AP, Hoh BL, Auluck PK, Pomerantseva I, Vacanti JP, Ogilvy CS: A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms. J Neurosurg 117: 546– 554, 2012. [DOI] [PubMed] [Google Scholar]
- 46). Li ZF, Fang XG, Yang PF, Huang QH, Zhao WY, Liang C, Zhao R, Liu JM: Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther 19: 352– 357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Zhang S, An Q, Li Q, Huang J, Chen X, Chen X, Zhang J, Wang Y, Yang GY, Zhu W: Therapeutic benefit of bone marrow-derived endothelial progenitor cell transplantation after experimental aneurysm embolization with coil in rats. PLoS One 9: e90069, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Rouchaud A, Journé C, Louedec L, Ollivier V, Derkaoui M, Michel J-B, Mazighi M: Autologous mesenchymal stem cell endografting in experimental cerebrovascular aneurysms. Neuroradiology 55: 741– 749, 2013. [DOI] [PubMed] [Google Scholar]
- 49). Li S, Tian Y, Huang X, Zhang Y, Wang D, Wei H, Dong J, Jiang R, Zhang J: Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain Res 1593: 65– 75, 2014. [DOI] [PubMed] [Google Scholar]
- 50). Gao Y, Wang Q, Cui X, Liu Y, Zheng T, Chen C, Sun C, Huang S, Wang X, Liu Y, Jiang X, Zeng C, Quan D: Controlled release of stromal cell-derived factor-1α from silk fibroin-coated coils accelerates intra-aneurysmal organization and occlusion of neck remnant by recruiting endothelial progenitor cells. Int J Clin Exp Pathol 7: 8366– 8380, 2014. [PMC free article] [PubMed] [Google Scholar]
- 51). Kuwabara A, Liu J, Lee JW, Hashimoto T: Human mesenchymal stem cells reduce the rupture rate of intracranial aneurysm. Paper presented at Anesthesiology 2014, Meeting of the American Society of Anesthesiologists New Orleans, LA, 2014, October 11–15. [Google Scholar]
- 52). Marbacher S, Frösén J, Marjamaa J, Anisimov A, Honkanen P, von Gunten M, Abo-Ramadan U, Hernesniemi J, Niemelä M: Intraluminal cell transplantation prevents growth and rupture in a model of rupture-prone saccular aneurysms. Stroke 45: 3684– 3690, 2014. [DOI] [PubMed] [Google Scholar]
- 53). Dai D, Ding YH, Danielson MA, Kadirvel R, Hunter LW, Zhan WZ, Helm GA, Lewis DA, Cloft HJ, Sieck GC, Kallmes DF: Endovascular treatment of experimental aneurysms by use of fibroblast-coated platinum coils: an angiographic and histopathologic study. Stroke 38: 170– 176, 2007. [DOI] [PubMed] [Google Scholar]
- 54). Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N: Simvastatin suppresses the progression of experimentally induced cerebral aneurysms in rats. Stroke 39: 1276– 1285, 2008. [DOI] [PubMed] [Google Scholar]
- 55). Tada Y, Kitazato KT, Yagi K, Shimada K, Matsushita N, Kinouchi T, Kanematsu Y, Satomi J, Kageji T, Nagahiro S: Statins promote the growth of experimentally induced cerebral aneurysms in estrogen-deficient rats. Stroke 42: 2286– 2293, 2011. [DOI] [PubMed] [Google Scholar]
- 56). Kimura N, Shimizu H, Eldawoody H, Nakayama T, Saito A, Tominaga T, Takahashi A: Effect of olmesartan and pravastatin on experimental cerebral aneurysms in rats. Brain Res 1322: 144– 152, 2010. [DOI] [PubMed] [Google Scholar]
- 57). Aoki T, Kataoka H, Ishibashi R, Nakagami H, Nozaki K, Morishita R, Hashimoto N: Pitavastatin suppresses formation and progression of cerebral aneurysms through inhibition of the nuclear factor kappaB pathway. Neurosurgery 64: 357– 365; discussion 365–366, 2009. [DOI] [PubMed] [Google Scholar]
- 58). Aoki T, Nishimura M, Kataoka H, Ishibashi R, Miyake T, Takagi Y, Morishita R, Hashimoto N: Role of angiotensin II type 1 receptor in cerebral aneurysm formation in rats. Int J Mol Med 24: 353– 359, 2009. [DOI] [PubMed] [Google Scholar]
- 59). Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, Nagahiro S: Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens 27: 1284– 1292, 2009. [DOI] [PubMed] [Google Scholar]
- 60). Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N: Nifedipine inhibits the progression of an experimentally induced cerebral aneurysm in rats with associated down-regulation of NF-kappa B transcriptional activity. Curr Neurovasc Res 5: 37– 45, 2008. [DOI] [PubMed] [Google Scholar]
- 61). Ishibashi R, Aoki T, Nishimura M, Miyamoto S: Imidapril inhibits cerebral aneurysm formation in an angiotensin-converting enzyme-independent and matrix metalloproteinase-9-dependent manner. Neurosurgery 70: 722– 730, 2012. [DOI] [PubMed] [Google Scholar]
- 62). Yagi K, Tada Y, Kitazato KT, Tamura T, Satomi J, Nagahiro S: Ibudilast inhibits cerebral aneurysms by down-regulating inflammation-related molecules in the vascular wall of rats. Neurosurgery 66: 551– 559; discussion 559, 2010. [DOI] [PubMed] [Google Scholar]
- 63). Aoki T, Nishimura M, Matsuoka T, Yamamoto K, Furuyashiki T, Kataoka H, Kitaoka S, Ishibashi R, Ishibazawa A, Miyamoto S, Morishita R, Ando J, Hashimoto N, Nozaki K, Narumiya S: PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol 163: 1237– 1249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N: Cathepsin B, K, and S are expressed in cerebral aneurysms and promote the progression of cerebral aneurysms. Stroke 39: 2603– 2610, 2008. [DOI] [PubMed] [Google Scholar]
- 65). Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N: Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 38: 162– 169, 2007. [DOI] [PubMed] [Google Scholar]
- 66). Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N: Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 40: 942– 951, 2009. [DOI] [PubMed] [Google Scholar]
- 67). Ishibashi R, Aoki T, Nishimura M, Hashimoto N, Miyamoto S: Contribution of mast cells to cerebral aneurysm formation. Curr Neurovasc Res 7: 113– 124, 2010. [DOI] [PubMed] [Google Scholar]
- 68). Eldawoody H, Shimizu H, Kimura N, Saito A, Nakayama T, Takahashi A, Tominaga T: Fasudil, a Rho-kinase inhibitor, attenuates induction and progression of cerebral aneurysms: experimental study in rats using vascular corrosion casts. Neurosci Lett 470: 76– 80, 2010. [DOI] [PubMed] [Google Scholar]
- 69). Tada Y, Kitazato KT, Tamura T, Yagi K, Shimada K, Kinouchi T, Satomi J, Nagahiro S: Role of mineralocorticoid receptor on experimental cerebral aneurysms in rats. Hypertension 54: 552– 557, 2009. [DOI] [PubMed] [Google Scholar]
- 70). Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N: Reactive oxygen species modulate growth of cerebral aneurysms: a study using the free radical scavenger edaravone and p47phox(−/−) mice. Lab Invest 89: 730– 741, 2009. [DOI] [PubMed] [Google Scholar]
- 71). Yokoi T, Isono T, Saitoh M, Yoshimura Y, Nozaki K: Suppression of cerebral aneurysm formation in rats by a tumor necrosis factor-α inhibitor. J Neurosurg 120: 1193– 1200, 2014. [DOI] [PubMed] [Google Scholar]
- 72). Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, Medel R, Dumont AS: Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture. Transl Stroke Res 5: 269– 277, 2014. [DOI] [PubMed] [Google Scholar]
- 73). Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T: Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke 43: 2450– 2456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Kaufmann TJ, Marx WF, Kallmes DF: A failure of matrix metalloproteinase inhibition in the prevention of rat intracranial aneurysm formation. Neuroradiology 48: 190– 195, 2006. [DOI] [PubMed] [Google Scholar]
- 75). Fukuda S, Hashimoto N, Naritomi H, Nagata I, Nozaki K, Kondo S, Kurino M, Kikuchi H: Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 101: 2532– 2538, 2000. [DOI] [PubMed] [Google Scholar]
- 76). Sadamasa N, Nozaki K, Takagi Y, Moriwaki T, Kawanabe Y, Ishikawa M, Hashimoto N: Cerebral aneurysm progression suppressed by blockage of endothelin B receptor. J Neurosurg 106: 330– 336, 2007. [DOI] [PubMed] [Google Scholar]
- 77). Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T: Protective role of peroxisome proliferator-activated receptor-γ in the development of intracranial aneurysm rupture. Stroke 46: 1664– 1672, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, Nagahiro S, Hashimoto T: Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab 35: 1163– 1168, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, Hasan DM, Kanematsu Y, Nagahiro S, Hashimoto T: Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension 63: 1339– 1344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T: Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery 75: 690– 695; discussion 695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Fukuda M, Aoki T, Manabe T, Maekawa A, Shirakawa T, Kataoka H, Takagi Y, Miyamoto S, Narumiya S: Exacerbation of intracranial aneurysm and aortic dissection in hypertensive rat treated with the prostaglandin F-receptor antagonist AS604872. J Pharmacol Sci 126: 230– 242, 2014. [DOI] [PubMed] [Google Scholar]
- 82). Marbacher S, Schläppi JA, Fung C, Hüsler J, Beck J, Raabe A: Do statins reduce the risk of aneurysm development? A case-control study. J Neurosurg 116: 638– 642, 2012. [DOI] [PubMed] [Google Scholar]
- 83). Yoshimura Y, Murakami Y, Saitoh M, Yokoi T, Aoki T, Miura K, Ueshima H, Nozaki K, SSS Research Group : Statin use and risk of cerebral aneurysm rupture: a hospital-based case-control study in Japan. J Stroke Cerebrovasc Dis 23: 343– 348, 2014. [DOI] [PubMed] [Google Scholar]
- 84). Hasan DM, Mahaney KB, Brown RD, Jr, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators : Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 42: 3156– 3162, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Gross BA, Rosalind Lai PM, Frerichs KU, Du R: Aspirin and aneurysmal subarachnoid hemorrhage. World Neurosurg 82: 1127– 1130, 2014. [DOI] [PubMed] [Google Scholar]
- 86). Garbe E, Kreisel SH, Behr S: Risk of subarachnoid hemorrhage and early case fatality associated with outpatient antithrombotic drug use. Stroke 44: 2422– 2426, 2013. [DOI] [PubMed] [Google Scholar]