Abstract
In Caucasians and Native Americans living at altitude, hemoglobin mass is increased in spite of erythropoietin concentrations ([Epo]) not markedly differing from sea level values. We hypothesized that a nocturnal decrease of arterial oxygen saturation (SaO2) causes a temporary rise of [Epo] not detected by morning measurements. SaO2 (continuous, finger oximeter) and [Epo] (ELISA, every 4 h) were determined in young highlanders (altitude 2600 m) during 24 h of usual daily activity. In Series I (six male, nine female students), SaO2 fell during the night with the nadir occurring between 01:00 and 03:00; daily means (range 92.4–95.2%) were higher in females (+1.7%, P < 0.01). [Epo] showed opposite changes with zenith occurring at 04:00 without a sex difference. Mean daily values (22.9 ± 10.7SD U/L) were higher than values obtained at 08:00 (17.2 ± 9.5 U/L, P < 0.05). In Series II (seven females), only SaO2 was measured. During follicular and luteal phases, SaO2 variation was similar to Series I, but the rhythm was disturbed during menstruation. While daily [Epo] variations at sea level are not homogeneous, there is a diurnal variation at altitude following changes in SaO2. Larger hypoventilation‐dependent decreases of alveolar PO2 decreases during the night probably cause a stronger reduction of SaO2 in highlanders compared to lowlanders. This variation might be enlarged by a diurnal fluctuation of Hb concentration. In spite of a lower [Hb], the higher SaO2 in women compared to men led to a similar arterial oxygen content, likely explaining the absence of differences in [Epo] between sexes.
Keywords: Circadian rhythm, female hormones, hypoxia, respiration, sex difference
Introduction
Erythropoietin concentration ([Epo]) rises during ascent to altitude, but soon decreases and returns to preceding or only slightly increased values after more than 30 days (Siebenmann et al. 2015; Böning et al. 1997; Lundby et al. 2014). The topic has been reviewed by Klausen (1998), Gunga et al. (2007), and Jelkmann (2011). Possible mechanisms might be down‐regulation of the hypoxia‐inducible factor alpha (HIFα) or degradation of Epo by the target cells in the bone marrow (Jelkmann 2011). In native highlanders, there are only few investigations on Epo. Winslow et al. (1989) and Schmidt et al. (1993) did not find an increase compared to lowlanders above 3500 m of altitude in the Himalaya as well as in the Andes. The [Epo] increase was also small or absent at 2600 m in Bogotá (Böning et al. 2001, 2004; Schmidt et al. 2002; Cristancho et al. 2007). In contrast to Tibetans, genetic adaptation of HIFα probably does not play a role in Epo secretion among Andean natives (Bigham et al. 2009). There are, however, changes in HIF‐targeted genes important for metabolism in an oxygen‐deprived cellular environment and for microcirculation.
In spite of the lack of rise in [Epo], Hb concentration as well as Hb mass (or the corresponding red cell volume) are increased in altitude visitors and in South American dwellers (reviewed in Sawka et al. 2000; West et al. 2007). Various investigations have shown that Hb mass in most visitors continues to rise in spite of the normalization of [Epo] after about 30 days at altitude (e.g., Milledge and Cotes 1985; Pugh 1964). Shaw and Simpson (1961) reviewed articles up to 1961 and found a rise of approximately 50% in red cell mass at 5000 m of altitude. Weil et al. (1968) measured a clear increase by nearly 5 mL/kg body mass (approx. 18%) at 3100 m in male North American highlanders. Various studies were performed on inhabitants of Bogotá using the CO rebreathing method to measure Hb mass. Hb mass was found to be increased by 12 to 22% in males (Böning et al. 2001; Schmidt et al. 2002) and by less than 7% in females (Böning et al. 2004) when compared to lowlanders.
The stimulus for Epo secretion is a decrease in arterial oxygen content leading to lowered tissue PO2 in the kidney (Jelkmann 2011). Weil et al. (1968) observed an inflection point on the arterial PO2 red cell mass curve in highlanders at approx. 67 mmHg PaO2 corresponding to 93–94% arterial oxygen saturation (SaO2). This indicates the “shoulder” of the oxygen dissociation curve (ODC) where a further small reduction of PO2 causes a marked fall of saturation (Sutton et al. 1980). In some subjects, the arterial PaO2 falls below the threshold already at 1600 m of altitude (Weil et al. 1968); at 2600 m, about half of the investigated young male and female inhabitants show values below the threshold (Böning et al. 2001, 2004).
In most investigations, [Epo] as well as SaO2 were measured once a day. But this can only be representative if there are no diurnal changes. Until now it is not clear whether a circadian rhythm for the secretion of the hormone exists (Cahan et al. 1992; Wide et al. 1989; Pasqualetti et al. 1996, 2000; Gunga et al. 2003, 2007); perhaps, physical activity and illness play a modifying role (Klausen 1998). While some authors (Gunga et al. 2003, 2007) observed no circadian rhythm during bed rest at sea level, others (Fitzpatrick et al. 1993; Klausen et al. 1996) detected a mostly small nocturnal [Epo] increase in subjects with usual daily activity.
Interestingly, during a short altitude stay (64 h at 4350 m), Klausen et al. (1996) measured an enlarged variation in [Epo] with peak values at 04:00 (+6 U/l compared to 08:00). The stimulation of ventilation by increased PCO2 and decreased PO2 in arterial blood is reduced during the night (reviewed by Buchanan 2013). It is conceivable that SaO2 decreases during night sleep and leads to a rise in mean 24‐h concentration of Epo causing the enlarged Hb mass. Unfortunately, Klausen et al. (1996) did not communicate SaO2 at various times of the day. But Fitzpatrick et al. (1993) as well as Gries and Brooks (1996) observed that already at sea level, saturation decreases during sleep in healthy subjects. This effect should be exaggerated at altitude when arterial PO2 reaches the “shoulder” of the ODC. Indeed, SaO2 decreases during sleep in healthy people living at 4380 m from approximately 86% in the awake state to a nadir of 83% between 01:00 and 03:00 (Spicuzza et al. 2004).
An additional interesting question is whether there are sex‐specific differences in SaO2 and correspondingly in [Epo]. Ventilatory stimulation by female sex hormones might cause a reduced decrease of SaO2 in women at altitude (Böning et al. 2001, 2004; Cristancho et al. 2007). Beall et al. (1997); Beall (2000b) measured daytime SaO2 in highlanders in Tibet and Bolivia. In Tibet, at an altitude of approx. 4000 m, SaO2 was significantly higher in fertile females than in males of equal age (Beall 2000a). For clarification, it seems useful to compare whole‐day profiles of SaO2 in both sexes.
This study addresses the following questions:
Is there a diurnal rhythm of [Epo] in the blood of highlanders with increased nocturnal values which might be the cause for increased Hb concentration as well as Hb mass?
Is this hypothetical rhythm caused by corresponding variations in arterial SaO2?
Are there sex differences in arterial SaO2 and correspondingly in [Epo] in highlanders?
Methods
The investigations were performed in Bogotá (2600 m above sea level) in subjects born at this altitude or living there for at least 3 years. Most inhabitants of this region are mestizos (Sandoval et al. 1993). Fifteen nonsmoking untrained medical students (six men, 19.4 ± 2.1 years, 66.7 ± 8.7 kg, 176.2 ± 6.8 cm, and nine women, 18.9 ± 0.7 years, 52.5 ± 6.2 kg, 156.7 ± 8.6 cm; means ± standard deviations) participated in Series I, in which [Epo] and SaO2 determinations were carried out. In Series II, only SaO2 was measured in another group of seven untrained nonsmoking female students (age 22.1 ± 1.64 years, body mass 52.2 ± 3.8 kg, height 163 ± 5 cm) during different phases of the menstrual cycle. Informed consent was obtained from all participants and experimental protocols were approved by the ethics committee of the Science Faculty of the Universidad Nacional de Colombia. All subjects were clinically healthy. They performed only lessons and laboratory work during the experimental time, heavy physical activity and sports were not allowed.
Study design
Series I
The subjects reported to the laboratory at 07:45 for preparation and anthropological measurements. SaO2 was measured continuously during 24 h with a finger oximeter (Wrist‐Ox 3100, Nonin, Minneapolis, MN). As the oximeter could not be checked continuously throughout the day, values were occasionally lost; in two females, sufficient data were not obtained. Blood samples were withdrawn from subjects in a seated position into EDTA vacutainer tubes every 4 h from 08:00 to 04:00 of the next day. In the first sample, [Hb] was measured with the cyanmethemoglobin method and hematocrit (Hct) values were calculated from [Hb] and mean red cell volume (ADVIA 120 hematology analyzer, Bayer Diagnostics, Tarrytown, NY). [Epo] was determined in plasma with a commercial ELISA kit (R&D systems, Minneapolis, MN). The samples were stored at −20°C maximally for 1 month. During the day, the subjects performed their usual tasks as students in the Universidad Militar, and at night, they slept from 22:00 to 07:00 in the university hospital to allow for the withdrawal of the blood samples. They took their meals after blood sampling.
Series II
SaO2 measurements were performed for 24 h during menstruation, luteal phase (14 days before the onset of menstruation) and follicular phase (remaining days). All subjects stated they were not taking contraceptives.
Calculations
Statistics
The data are presented as means ± standard deviations (SD) in the text, ± standard errors (SE) in the figures. Depending on the number of comparisons, t‐tests or analysis of variance (ANOVA) with subsequent Bonferroni corrected t‐tests were used for significance calculations (two‐tailed). Linear regression and correlation coefficients served as indicators for a relation between SaO2 and [Epo] considering the delay in secretion of the latter. Calculations were performed with SPSS (version 19).
Results
Diurnal changes of arterial oxygen saturation and erythropoietin concentration in male and female highlanders (Series I)
The male and female subjects presented normal sex differences and a slight altitude effect in hematological quantities (Males: [Hb] 16.4 ± 0.7 g/dL, Hct 46.6 ± 3.2%; females: [Hb] 14.8 ± 0.9 g/dl, Hct 45.1 ± 2.7%). Figure 1 shows SaO2 during 24 h (upper panel). There are two remarkable aspects. Firstly, SaO2 varied in both sexes with nadir occurring between 01:00 and 03:00 (ANOVA for all subjects: P < 0.001). Secondly, individual daily means differed markedly (range from 92.4 to 95.2%). At any time of the day, women had a higher oxygen saturation than men (overall P < 0.01). On average, the difference amounts to 1.7% but increases up to 3.2% during the night (P at least <0.05 from 21:00 to 03:00 for each hour). SaO2 decreased in men earlier in the evening (from 20:00 onwards); however, the maximal change was similar in both sexes (approx. 3.0%). From 04:00 to 06:00, SaO2 recovered with a similar pattern in both sexes and then varied little until 20:00.
Figure 1.
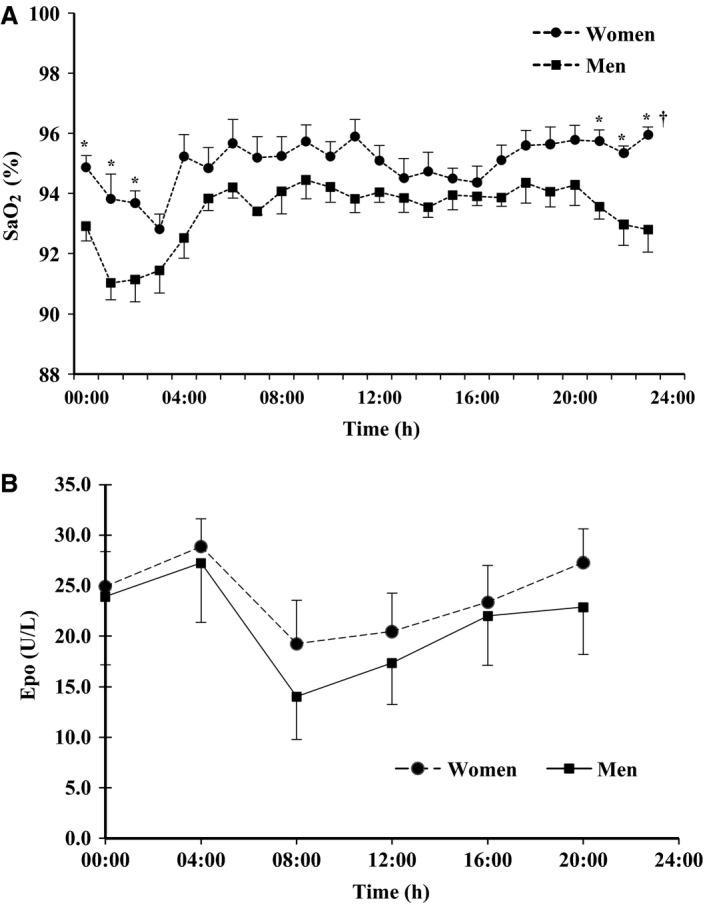
Diurnal changes of arterial oxygen saturation (SaO2, A) and erythropoietin (Epo) concentration (B) in six men (squares) and nine women (filled circles). Means and SE. SaO2 was only measured in five males and seven females. Asterisks indicate significant differences between sexes for single hours (P < 0.05 or better).
[Epo] (Fig. 1, lower panel) also showed diurnal changes (ANOVA P < 0.02 for all subjects), but no significant difference between sexes. The variations were inverse and slightly delayed with regard to those of arterial saturation in the period between 00:00 and 08:00. When SaO2 was decreased (00:00 to 03:00), [Epo] reached highest values at 04:00, and when SaO2 increased (04:00–06:00), [Epo] decreased to the lowest value at 08:00. In the following hours, while SaO2 variations were small, [Epo] slightly increased (not significant). A comparison of the mean value for all subjects at 08:00 (17.2 ± 9.5 U/L) with the rest of the day (mean: 24.0 ± 11.4 U/L) or the whole day (24 h mean: 22.9 ± 10.7 U/L) yielded a significant difference (all P < 0.05). Thus, the mean diurnal value is one‐third higher than the morning value.
Correlations
Correlations between SaO2 and [Epo] were calculated for the subgroups. Significant negative coefficients were obtained only for males with highest values for SaO2 1–3 h before blood sampling for Epo determination (r = −0.47, P < 0.01). For females, no significant values were found (best r = −0.02 for SaO2 1–2 h before blood sampling). However, when deviations from the daily means were calculated, the common regression for the sex‐specific nadir SaO2 was significant (Fig. 2).
Figure 2.
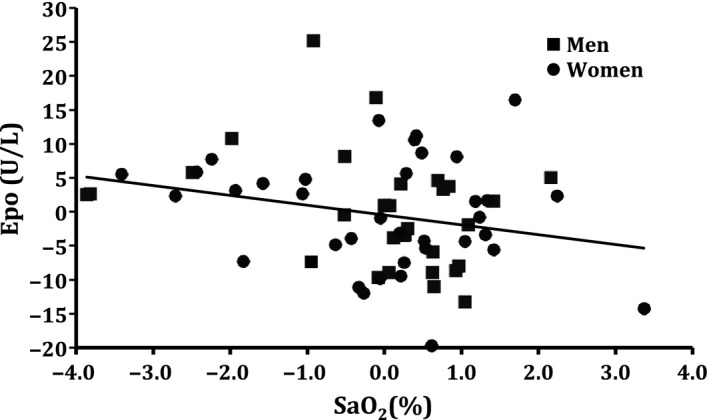
Correlations between changes in arterial oxygen saturation (SaO2) and [Epo]. SaO2 averaged 1–3 h before blood sampling in males, and 1 h in females. Common regression equation: [Epo] = −1.5 (SO 2) −0.5; r = −0.25 (P < 0.05).
Diurnal changes of arterial oxygen saturation in female highlanders in relation to the menstrual cycle (Series II)
The course of SaO2 was equal during the follicular and luteal phases and similar to that in Series I, but with a more marked and longer decrease during the night between 22:00 and 08:00 with lowest values around midnight (ANOVA P < 0.001). During the menses, however, the nocturnal depression was only moderate with the nadir at 04:00 (P < 0.01). The time course of the SaO2 variation was significantly different from those during the other phases (P < 0.01) (Fig. 3).
Figure 3.
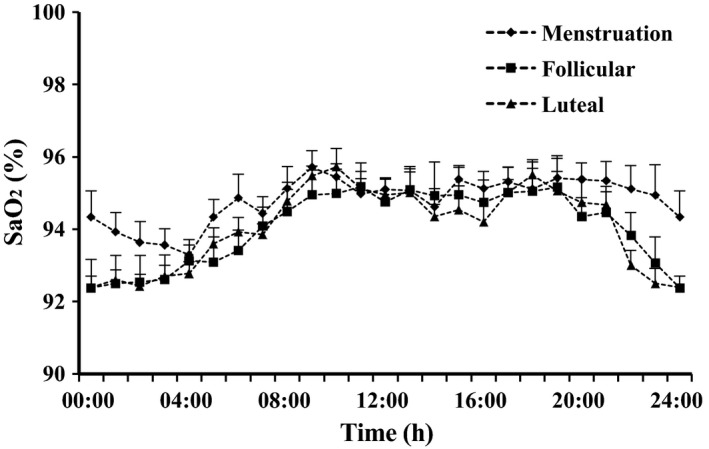
Diurnal changes of arterial oxygen saturation (SaO2) in seven women during the follicular phase (squares), the luteal phase (triangles), and menstruation (diamonds). Means and SE.
Discussion
General
As assumed, we found a diurnal rhythm of [Epo] in highlanders at moderate altitude following arterial oxygen saturation changes with peak values occurring after the nocturnal decrease of SaO2. Changes in SaO2 and [Epo] correlated significantly. In spite of a generally higher SaO2, there was no lower [Epo] in females.
Diurnal changes of arterial oxygen saturation and erythropoietin concentration in male and female highlanders (Series I)
The highlanders at 2600 m above sea level showed a clear nocturnal decrease of SaO2 with the nadir occurring between 01:00 and 03:00, similar to changes seen at 4380 m (Spicuzza et al. 2004). The values throughout 24 h varied between approximately 96 and 91%. According to Weil et al. (1968), the threshold for the stimulation of Epo secretion is located within this range of arterial saturations. Because of the “shoulder” of the ODC, equal diurnal differences in arterial PO2 cause larger SaO2 variations at altitude than at sea level. When the arterial PO2 decreases more markedly in chronic mountain sickness, [Epo] fluctuations are exaggerated, but a diurnal rhythm ceases to exist (Bernardi et al. 2003).
The suggested correlations between SaO2 and [Epo] were better in males than in females. The lower r values in females might be caused by larger scattering (perhaps caused by dysmenorrhea in some subjects, see below) and by the fact that saturation measurements were less complete leading to a loss of 13 pairs of data.
Decisive for Epo secretion, however, is the tissue PO2 in the kidneys which in turn depends on arterial PO2, [Hb], and oxygen affinity, while blood flow plays a minor role (Jelkmann 2011, 2013). These three quantities determine the oxygen content of arterial blood, which also can be calculated as SaO2 (as fraction) times [Hb] times 1.34 mL O2 /g Hb; the latter is the maximal binding capacity considering a small amount of CO‐Hb and MetHb. While arterial PO2 and affinity decrease at moderate altitude, [Hb] rises thus stabilizing the arterial oxygen content. When comparing residents in Berlin (30 m above sea level) and Bogotá (Böning et al. 2001, 2004), there is even a tendency for an increased arterial oxygen content in Bogotá at approximately 08:00: 20.1 and 21.7 mL/dL for males, 16.7 and 18.0 mL/dL for females. Additionally, the oxygen dissociation curve is slightly right‐shifted in Bogotá (approx. +2 mmHg for standard P50) causing a small increase in PO2 at any given SaO2 (Schmidt et al. 1990). This explains why no substantial increase in [Epo] was found for highlanders as compared with lowlanders in blood sampled during the morning in various studies in Bogotá but also at higher sites (Winslow et al. 1989; Böning et al. 2001, 2004; Schmidt et al. 2002; Cristancho et al. 2007).
At sea level also [Hb] shows a diurnal variation (Böning et al. 1974; Wisser and Breuer 1981) with lower values occurring during the night (amplitude approximately 4%), probably because of reduced filtration in the leg vessels in supine position. If such a rhythm exists at altitude as well, the [Epo] increase during the night should be larger than expected only from the mean saturation decrease of 3%. We have estimated the change in arterial O2 content assuming a variation in [Hb] as at sea level (Fig. 4). Calculation of the correlation between O2 content and [Epo] yielded significant dependencies for both males and females. For all subjects, the regression equation indicates an [Epo] increase of 3.6 U/L per 1 mL/dL decrease in O2 content during 1–3 h before blood sampling. As an acute hypoxic stimulus on Epo secretion needs 80–120 min to be effective (Eckardt et al. 1989), the diurnal variation probably is caused by the preceding changes of both SaO2 and [Hb].
Figure 4.
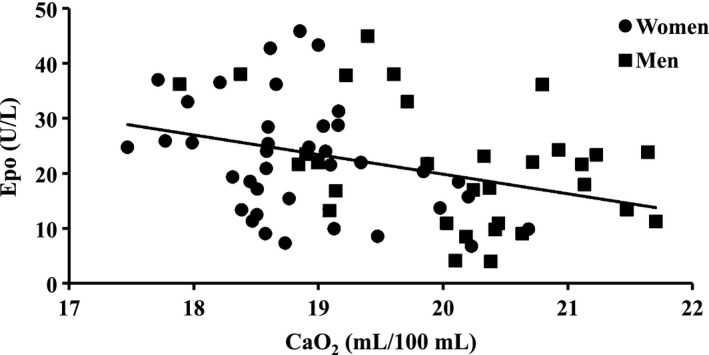
Correlation between calculated arterial oxygen content (CaO2) 1–3 h before venous blood sampling and [Epo]. Oxygen content is calculated assuming a diurnal [Hb] variation as at sea level (Böning et al. 1974). Regression equation: [Epo] = −3.6 CaO2 + 91 (r = 0.35, P < 0.02).
At first glance surprisingly, [Epo] is not decreased in females compared to males in spite of the increased SaO2 in the former group. But again the Hb concentration plays a role. Because of the lower [Hb] in women (−9%) the arterial O2 content is reduced, and thus the stimulus for Epo secretion is even slightly increased (women: arterial oxygen content at 08:00 18.9 vs. 20.4 mL/dL in males). As Hb mass is finally determined by production and destruction of erythrocytes, this quantity is lowered in young women compared to men partly by the regular loss of blood during menstruation in spite of a high [Epo]. Another mechanism for the general Hb mass difference between sexes is the erythropoietic effect of androgens (Murphy et al. 2010).
Diurnal changes of arterial oxygen saturation in female highlanders in relation to the menstrual cycle (Series II)
The nocturnal decrease of SaO2 lasted longer than in Series I except during menstruation. Possibly, the undisturbed sleep without blood sampling was the cause; the subjects had refused the use of indwelling cannulas in the series with [Epo] measurements. On the other hand, dysmenorrheal troubles might have reduced sleep quality during menstruation (Iacovides et al. 2015) and hindered the reduction of ventilation between 22:00 and 03:00. From these observations, one might suggest that the nocturnal increases of [Epo] are in reality larger than measured in Series I.
The curves for SaO2 during the follicular and luteal phases are indistinguishable corresponding to a study of Reeves et al. (2001) who did not find cycle‐dependent differences in SaO2 or [Epo]. This is not fully unexpected because a larger stimulating effect of progesterone rather than estrogen on respiration has been described occasionally, but was not uniformly observed (reviewed in Cristancho et al. 2007 and MacNutt et al. 1985). The mechanism of the altered ventilatory drive in females is still not known; the hormones possibly act on the carotid body and respiratory centers in the brain stem. MacNutt et al. (1985) have described the CO2 threshold for the stimulation of ventilation as being always lower in females than in males. A rise in energy metabolism during the luteal phase might also contribute to the increase in ventilation. As contraceptive drugs contain estrogen‐ and progesterone‐like substances, the sex difference should be observable in all women before menopause.
Conclusions
This study shows the presence of a diurnal rhythm in serum Epo levels with highest values at approx. 04:00 in highlanders with normal daily activity. Morning [Epo] is about one‐third lower than the mean value for 24 h. The mechanism causing the diurnal rhythmicity is a variation in arterial oxygen content. For assessment of serum Epo values, the time of day for collection of blood samples has to be taken into consideration.
Larger hypoventilation‐dependent arterial SO2 decreases during the night probably cause a stronger reduction of arterial O2 content in highlanders compared to lowlanders causing a higher Epo secretion and thus Hb mass in the former.
SaO2 is higher in females than males especially during sleep at night. The rhythm does scarcely vary during different phases of ovarian function, except during menstruation. In spite of the difference in SaO2, a difference between sexes in Epo concentration could not be detected because of the lowered [Hb] in women; this resulted in similar arterial O2 content in males and females.
Conflicts of Interest
None declared.
Acknowledgements
The investigation was performed with the support of the Universidad Nacional de Colombia in Bogotá, the Universidad Militar Nueva Granada in Bogotá and the German Academic Exchange Service. The authors thank all subjects for their willing cooperation.
Cristancho E., Riveros A., Sánchez A., Peñuela O., Böning D.. Diurnal changes of arterial oxygen saturation and erythropoietin concentration in male and female highlanders. Physiol Rep, 4 (17), 2016, e12901, doi: 10.14814/phy2.12901
Funding Information
None.
References
- Beall, C. M. 2000a. Oxygen saturation increases during childhood and decreases during adulthood among high altitude native Tibetans residing at 3800‐4200 m. High Alt Med Biol. 1:25–32. [DOI] [PubMed] [Google Scholar]
- Beall, C. M. 2000b. Tibetan and Andean contrasts in adaptation to high‐altitude hypoxia. Adv. Exp. Med. Biol. 475:63–74. [DOI] [PubMed] [Google Scholar]
- Beall, C. M. , Strohl K. P., Blangero J., Williams‐Blangero S., Almasy L. A., Decker M. J., et al. 1997. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am. J. Phys. Anthropol. 104:427–447. [DOI] [PubMed] [Google Scholar]
- Bernardi, L. , Roach R. C., Keyl C., Spicuzza L., Passino C., Bonfichi M., et al. 2003. Ventilation, autonomic function, sleep and erythropoietin. Chronic mountain sickness of Andean natives. Adv. Exp. Med. Biol. 543:161–175. [PubMed] [Google Scholar]
- Bigham, A. W. , Mao X., Mei R., Brutsaert T., Wilson M. J., Julian C. G., et al. 2009. Identifying positive selection candidate loci for high‐altitude adaptation in Andean populations. Hum Genomics 4:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böning, D. , Schweigart U., and Kunze M.. 1974. Diurnal variations of protein and electrolyte concentrations and acid‐base status in plasma and red cells of normal man. Eur. J. Appl. Physiol. 3:239–250. [DOI] [PubMed] [Google Scholar]
- Böning, D. , Maassen N., Jochum F., Steinacker J., Halder A., Thomas A., et al. 1997. After‐effects of a high altitude expedition on blood. Int. J. Sports Med. 18:179–185. [DOI] [PubMed] [Google Scholar]
- Böning, D. , Rojas J., Serrato M., Ulloa C., Coy L., Mora M., et al. 2001. Hemoglobin mass and peak oxygen uptake in untrained and trained residents of moderate altitude. Int. J. Sports Med. 22:572–578. [DOI] [PubMed] [Google Scholar]
- Böning, D. , E., Cristancho , Serrato M., Reyes O., Mora M., Coy l., et al. 2004. Hemoglobin mass and peak oxygen uptake in untrained and trained female altitude residents. Int. J. Sports Med. 25:1–8. [DOI] [PubMed] [Google Scholar]
- Buchanan, G. F. 2013. Timing, sleep, and respiration in health and disease. Prog Mol. Biol. Transl. Sci. 119:191–219. [DOI] [PubMed] [Google Scholar]
- Cahan, C. , Decker M. J., Arnold J. L., Washington L. H., Veldhuis J. D., Goldwasser E., et al. 1992. Diurnal variations in serum erythropoietin levels in healthy subjects and sleep apnea patients. J. Appl. Physiol. 72:2112–2117. [DOI] [PubMed] [Google Scholar]
- Cristancho, E. , Reyes O., Serrato M., Mora M. M., Rojas J. A., Robinson Y., et al. 2007. Arterial oxygen saturation and hemoglobin mass in postmenopausal untrained and trained altitude residents. High Alt Med Biol. 8:296–306. [DOI] [PubMed] [Google Scholar]
- Eckardt, K. U. , Boutellier U., Kurtz A., Schopen M., Koller E. A., and Bauer C.. 1989. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J. Appl. Physiol. 66:1785–1788. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, M. F. , Mackay T., Whyte K. F., Allen M., Tam R. C., Dore C. J., et al. 1993. Nocturnal desaturation and serum erythropoietin: a study in patients with chronic obstructive pulmonary disease and in normal subjects. Clin. Sci. (Lond.) 84:319–324. [DOI] [PubMed] [Google Scholar]
- Gries, R. E. , and Brooks L. J.. 1996. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest 110:1489–1492. [DOI] [PubMed] [Google Scholar]
- Gunga, H.‐C. , Kirsch K. A., Roecker L., and Schobersberger W.. 2003. Erythropoietin regulation in humans during exercise and in extreme environments Pp. 219–244 in Jelkmann W., ed. Erythropoietin: Molecular Biology and Clinical Use. FF Graham Publishing Co., Johnson City. [Google Scholar]
- Gunga, H. C. , Kirsch K. A., Roecker L., Kohlberg E., Tiedemann J., Steinach M., et al. 2007. Erythropoietin regulations in humans under different environmental and experimental conditions. Respir. Physiol. Neurobiol. 158:287–297. [DOI] [PubMed] [Google Scholar]
- Iacovides, S. , Avidon I., and Baker F. C.. 2015. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 21:762–778. [DOI] [PubMed] [Google Scholar]
- Jelkmann, W. 2011. Regulation of erythropoietin production. J. Physiol. 589:1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann, W. 2013. Physiology and pharmacology of erythropoietin. Transfus Med Hemother. 40:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen, T. 1998. The feed‐back regulation of erythropoietin production in healthy humans. Dan. Med. Bull. 45:345–353. [PubMed] [Google Scholar]
- Klausen, T. , Poulsen T. D., Fogh‐Andersen N., Richalet J.‐P., Nielsen O. J., and Olsen N. V.. 1996. Diurnal variations of serum erythropoietin at sea level and altitude. Eur. J. Appl. Physiol. 72:297–302. [DOI] [PubMed] [Google Scholar]
- Lundby, A. K. , Keiser S., Siebenmann C., Schaffer L., and Lundby C.. 2014. Kidney‐synthesized erythropoietin is the main source for the hypoxia‐induced increase in plasma erythropoietin in adult humans. Eur. J. Appl. Physiol. 114:1107–1111. [DOI] [PubMed] [Google Scholar]
- MacNutt, M. J. , De Souza M. J., Tomczak S. E., Homer J. L., and Sheel A. W.. 1985. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J. Appl. Physiol. 112:2012. [DOI] [PubMed] [Google Scholar]
- Milledge, J. S. , and Cotes P. M.. 1985. Serum erythropoietin in humans at high altitude and its relation to plasma renin. J. Appl. Physiol. 59:360–364. [DOI] [PubMed] [Google Scholar]
- Murphy, W. G. , Tong E., and Murphy C.. 2010. Why do women have similar erythropoietin levels to men but lower hemoglobin levels? Blood 116:2861–2862. [DOI] [PubMed] [Google Scholar]
- Pasqualetti, P. , Collacciani A., and Casale R.. 1996. Circadian rhythm of serum erythropoietin in multiple myeloma. Am. J. Hematol. 53:40–42. [DOI] [PubMed] [Google Scholar]
- Pasqualetti, P. , Collacciani A., and Casale R.. 2000. Circadian rhythm of serum erythropoietin in myelodysplastic syndromes. Eur. Rev. Med. Pharmacol. Sci. 4:111–115. [PubMed] [Google Scholar]
- Pugh, LGCE . 1964. Blood volume and haemoglobin concentration at altitudes above 18,000 ft. (5500 m). J. Physiol. 170: 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, J. T. , Zamudio S., Dahms T. E., Butterfield G. E., Braun B., Butterfield G. E., et al. 2001. Erythropoiesis in women during 11 days at 4,300 m is not affected by menstrual cycle phase. J. Appl. Physiol. 91:2579–2586. [DOI] [PubMed] [Google Scholar]
- Sandoval, C. , De la Hoz A., and Yunis E.. 1993. Estructura genética de la población colombiana. Revista de la Facultad de Medicina Universidad Nacional de Colombia 41:3–14. [Google Scholar]
- Sawka, M. N. , Convertino V. A., Eichner E. R., Schnieder S. M., and Young A. J.. 2000. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sport Exerc. 32:332–348. [DOI] [PubMed] [Google Scholar]
- Schmidt, W. , Dahners H. W., Correa R., Ramirez R., Rojas J., and Böning D.. 1990. Blood gas transport properties in endurance‐trained athletes living at different altitudes. Int J Sports Medicine 11:15–21. [DOI] [PubMed] [Google Scholar]
- Schmidt, W. , Spielvogel H., Eckardt K. U., Quintela A., and Penaloza R.. 1993. Effects of chronic hypoxia and exercise on plasma erythropoietin in high‐altitude residents. J. Appl. Physiol. 74:1874–1878. [DOI] [PubMed] [Google Scholar]
- Schmidt, W. , Heinicke K., Rojas J., Gomez J. M., Serrato M., Mora M., et al. 2002. Blood volume and hemoglobin mass in endurance athletes from moderate altitude. Med Sci Sport Exerc. 34:1934–1940. [DOI] [PubMed] [Google Scholar]
- Shaw, D. B. , and Simpson T.. 1961. Polycythaemia in emphysema. Quart J Med. 30:135–152. [Google Scholar]
- Siebenmann, C. , Cathomen A., Hug M., Keiser S., Lundby A. K., Hilty M. P., et al. 2015. Hemoglobin mass and intravascular volume kinetics during and after exposure to 3,454‐m altitude. J. Appl. Physiol. 119:1194–1201. [DOI] [PubMed] [Google Scholar]
- Spicuzza, L. , Casiraghi N., Gamboa A., Keyl C., Schneider A., Mori A., et al. 2004. Sleep‐related hypoxaemia and excessive erythrocytosis in Andean high‐altitude natives. Eur. Respir. J. 23:41–46. [DOI] [PubMed] [Google Scholar]
- Sutton, J. R. , Gray G. W., Houston C. S., and Powles A. C.. 1980. Effects of duration at altitude and acetazolamide on ventilation and oxygenation during sleep. Sleep 3:455–464. [DOI] [PubMed] [Google Scholar]
- Weil, J. V. , Jamieson G., Brown D. W., and Grover R. F.. 1968. The red cell mass‐arterial oxygen relationship in normal man. J Clin Invest. 47:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J. B. , Schoene R. B., and Milledge J. S.. 2007. High Altitude Medicine and Physiology. Hodder Arnold, London. [Google Scholar]
- Wide, L. , Bengtsson C., and Birgegard G.. 1989. Circadian rhythm of erythropoietin in human serum. Br. J. Haematol. 72:85–90. [DOI] [PubMed] [Google Scholar]
- Winslow, R. M. , Chapman K. W., Gibson C. C., Samaja M., Monge C. C., Goldwasser E., et al. 1989. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J. Appl. Physiol. 66:1561–1570. [DOI] [PubMed] [Google Scholar]
- Wisser, H. , and Breuer H.. 1981. Circadian changes of clinical chemical and endocrinological parameters. J. Clin. Chem. Clin. Biochem. 19:323–337. [PubMed] [Google Scholar]


