Abstract
Antidiabetic medication may modify the incidence of hepatocellular carcinoma (HCC). We aimed to compare the use of different antidiabetic strategies and the incidence of HCC. PubMed, Embase.com and Cochrane Library databases were searched up to 31 October 2015 and randomized controlled trials (RCTs), cohort studies or case-control studies were included for our analyses. A total of thirteen studies enrolling 481358 participants with 240678 HCC cases who received at least two different strategies were retrieved in this analysis. Direct comparisons showed that use of metformin (risk ratio [RR] 0.49, 95% CI 0.25–0.97) was associated with a significant risk reduction of HCC, while insulin (RR = 2.44, 95% CI 1.10- 5.56) may significantly increase the risk. Indirect evidence also suggested that insulin (RR = 2.37, 95% CI 1.21–4.75) was associated with a significantly increased risk of HCC. Additionally, metformin was effective in reducing the risk of HCC when compared with sulphonylurea (RR = 0.45, 95% CI 0.27–0.74) and insulin (RR = 0.28, 95% CI 0.17–0.47). Notably, metformin was hierarchically the best when compared with other antidiabetic therapies for the prevention of HCC. In summary, available evidence suggests that metformin was the most effective strategy to reduce HCC risk when compared with other antidiabetic interventions.
The incidence and mortality rate of hepatocellular carcinoma (HCC) has significantly increased over recent decades. With a poor 5-year survival rate, HCC has become the third most common cause of cancer death worldwide1. Although established risk factors for HCC are recognized as including hepatitis C and hepatitis B viral infection and excessive alcohol consumption, there are at least 15–50% of HCC cases without specifically recognized aetiology2.
Diabetes mellitus (DM) has been proposed as a potential risk factor for HCC3. As part of metabolic syndrome, DM is believed to share many risk factors with a variety of cancers4 and epidemiological evidence linking DM to HCC has been reported in recent studies around the globe5,6,7,8,9. The underlying mechanisms responsible for the increased risk for developing HCC in patients with DM is complex and remains a matter of debate. Emerging evidence suggests that it is likely to be related to the interplay between obesity, DM and tumorigenesis, with insulin resistance and hyperinsulinaemia playing critical roles10.
On this basis, recent research has suggested that the use of antidiabetic medication may modify the risk for developing HCC in several different ways11,12. Evidence from in vitro and in vivo experimental studies has shown that insulin, it’s analogs and oral insulin secretagogues (such as sulfonylureas), which may contribute to hyperinsulinemia, are suggested to increase the incidence and progression of cancer13. In contrast, metformin, an insulin sensitizer, not only reduces levels of circulating glucose and insulin but also has potential protective effects on carcinogenesis in patients with insulin resistance and hyperinsulinemia. Observational studies from different countries and areas support the belief that the use of antidiabetic drugs which increase insulin sensitivity, such as metformin or thiazolidinediones (TZDs), may decrease the incidence of liver cancer, while exposure to insulin and sulphonylurea has been associated with an excess HCC risk14,15,16. Two large randomized controlled trials (RCTs), namely, ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes), failed to demonstrate that metformin offered any chemopreventive effect on the development of HCC when compared with TZDs, but remained potentially advantageous over sulphonylurea17.
Currently, there is still no high-quality RCT assessing the potential impact of different classes of antidiabetic pharmacotherapies on modifying the risk of developing HCC in DM patients. Due to the discrepant results in the literature and limited epidemiological evidence for the precise relationship between DM treatment and the risk of HCC, this study sought to systematically review the literature to evaluate, quantify, and summarize the association between the use of different anti-diabetic medications and the development of HCC. To obtain a better understanding of this issue, we performed a network meta-analysis of available clinical studies to investigate the association between antidiabetic medications and the risk of developing HCC.
Results
Study characteristics
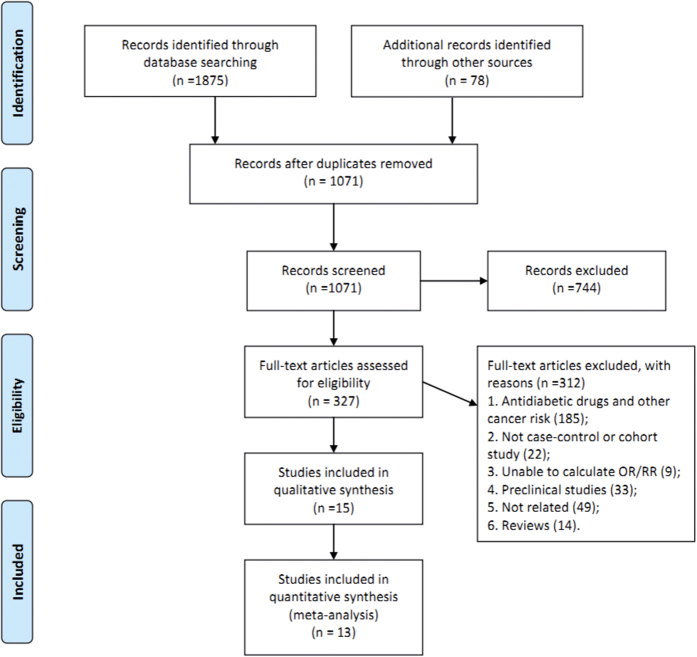
The participant flow diagram for study inclusion in the meta-analysis is shown in Fig. 1. A total of thirteen studies were retrieved, which were all published in English15,16,17,18,19,20,21,22,23,24,25,26,27. Of the 1935 potentially relevant references identified by electronic and manual searches, 744 publications were excluded according to title and abstract. After detailed assessment of the full text, a further 312 were excluded because they did not satisfy the inclusion criteria. Overall, a total of 13 studies enrolling 481358 participants with 240678 HCC cases who received at least two different treatment strategies were included in this analysis (Fig. 2), with one RCT, four cohort studies, and eight case-control studies. Table 1 summarizes the characteristics of studies which qualified for this network meta-analysis. The 13 studies were from different countries and published between 1991 and 2015. Among the 13 studies, which were mostly multiple-arm trials, patients were treated with insulin in 12 studies, metformin in 11 studies, sulphonylurea in 10 studies, and TZDs in 7 studies. Table 2 depicts the methodological quality and scores of included studies. For the cohort and case-control studies, the median score was 7 on the Newcastle–Ottawa quality assessment scale, which showed that the quality of included studies was reliable. Additionally, the quality of the RCT was assessed as moderate by the Cochrane risk of bias tool.
Figure 1. Literature search and selection.
Figure 2. Network of the comparisons for the Bayesian network meta-analysis.
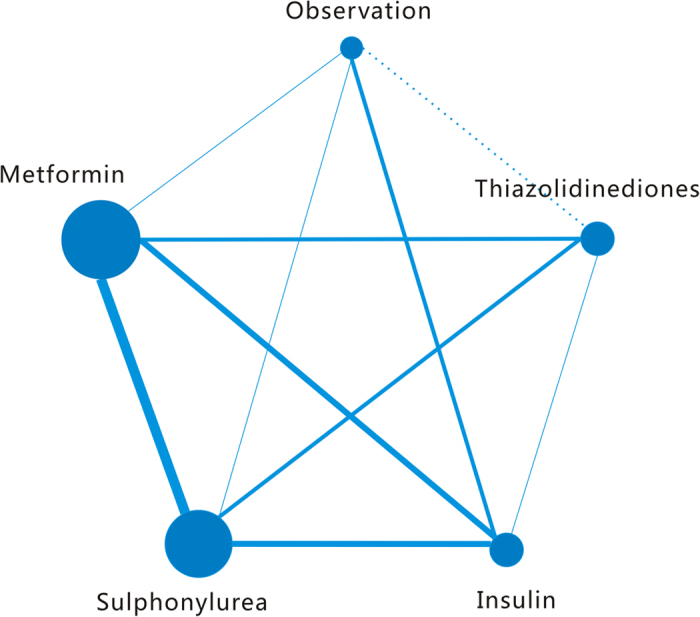
The size of every node is proportional to the number of patients. The width of the lines is proportional to the number of trials or pairs of trial arms.
Table 1. Characteristics of included studies.
| Studies | Design | Location | Study population | Study period | Mean follow-up (years) | Cases defined | Total no. of subjects | No. of HCC cases | Adjusted confoundersa | Antidiabetic typesb |
|---|---|---|---|---|---|---|---|---|---|---|
| Yu et al.18 | Case-control | USA | Cases of newly diagnosed hepatocellular carcinoma in Los Angeles County and controls from the neighborhood | 1984–1990 | NR | histologically confirmed | 32 | 18 | 1–4, 7–10, 13 | I |
| Oliveria et al.19 | Cohort | USA | Diabetes patients | 2000–2004 | 3.9 | ICD-9 | 16705 | 9 | 4, 5, 7–10 | M,T,S,I |
| Donadon et al.15 | Case-control | Italy | Diabetes patients with chronic liver disease | 1994–2008 | NR | NR | 549 | 190 | 1, 2, 5, 7–9 | M,S,I |
| Hassan et al.20 | Case-control | USA | Cases of newly diagnosed HCC in the Cancer Center and controls from genetically unrelated family. | 2000–2008 | NR | NR | 217 | 124 | 1–4, 6–9 | M,T,S,I |
| Home et al.17 | RCT (ADOPT) | UK | Diabetes patients | 2000–2006 | 4 | NR | 4351 | 4 | 1–6, 13 | M,T,S,I |
| Home et al.17 | RCT (RECORD) | UK | Diabetes patients | 2001–2008 | 5.5 | NR | 2225 | 2 | 1–6, 13 | M,T,S,I |
| Home et al.17 | RCT (RECORD) | UK | Diabetes patients | 2001–2008 | 5.5 | NR | 2222 | 2 | 1–6, 13 | M,T,S,I |
| Kawaguchi et al.21 | Nested case-control | Japan | Hepatitis C patients with diabetes mellitus | 2004–2008 | NR | NR | 143 | 96 | 1, 2, 5, 7, 10 | M,T,S,I |
| Nkontchou et al.22 | Cohort | France | Diabetes patients | 1988–2007 | 5 | histologically confirmed or noninvasive criteria | 100 | 39 | 1, 2, 4, 5, 9, 12, 13 | M,S,I |
| Chang et al.16 | Nested case-control | Taiwan | Diabetes patients | 2000 | NR | ICD-9 | 115183 | 25236 | 1, 2, 4, 14 | M,T,S,I |
| Ruiter et al.23 | Cohort | Netherlands | All individuals with prescription for any hypoglycemic drug based on hospital record database | 1998–2008 | 2.8 | ICD-9 | 85289 | 31 | 1, 2, 14 | M,S |
| Schlesinger et al.24 | Cohort | UK | General people | 1992–2000 | 8.5 | ICD-10 | 8324 | 2089 | 1, 2, 4–7, 13 | I |
| Hagberg et al.25 | Nested case-control | USA | Diabetes patients | 1988–2011 | NR | Read codes B150300, B150z00, and B152.00 | 690 | 121 | 1, 2, 5–9, 14 | M,I |
| Bosetti et al.26 | Nested case-control | Italy | Diabetes patients | 2005–2007 | 6 | ICD-9 | 4477 | 209 | 1, 2, 14 | M,S,I |
| Miele et al.27 | Case-control | Italy | General people | 2005–2012 | NR | AASLD guidelines | 171 | 102 | 1, 2, 6, 7 | M,S,I |
a1, age; 2, sex; 3, race; 4, socioeconomic status; 5, body mass index; 6, smoking; 7, ethanol intake; 8, HBV infection; 9, HCV infection; 10, cirrhosis; 11, alcoholic liver disease; 12, on-alcoholic liver disease; 13, diabetes mellitus duration; 14. medications taken (unspecified).
bM: Metformin, T: Thiazolidinediones, S: Sulphonylurea, I: Insulin.
Table 2. Quality assessment of included studies.
| Studies | Observational studiesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection |
Comparability | Outcome or exposure |
Score | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Yu et al.18 | * | * | * | * | ** | * | ******* | ||
| Oliveria et al.19 | * | * | * | * | ** | * | * | * | ********* |
| Donadon et al.15 | * | * | * | ** | * | * | ******* | ||
| Hassan et al.20 | * | * | * | ** | * | * | ******* | ||
| Kawaguchi et al.21 | * | * | * | ** | * | * | ******* | ||
| Nkontchou et al.22 | * | * | ** | * | * | ****** | |||
| Chang et al.16 | * | * | * | * | ** | * | * | ******** | |
| Ruiter et al.23 | * | * | * | ** | * | * | * | ******** | |
| Schlesinger et al.24 | * | * | * | ** | * | * | * | ******** | |
| Hagberg et al.25 | * | * | * | * | ** | * | * | ******** | |
| Bosetti et al.26 | * | * | * | * | ** | * | * | ******** | |
| Miele et al.27 | * | * | * | ** | * | * | ******* | ||
| Randomized clinical trialb | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Home et al.17 | L | L | L | L | H | U | L | ||
aThe quality of the observational studies was performed using the Newcastle–Ottawa Quality Assessment Scale.
bThe quality of the RCT was assessed by Cochrane risk of bias tool (L = low risk, H = high risk, U = unclear risk). 1: random sequence generation; 2: allocation concealment; 3: blinding of participants and researchers; 4: blinding of outcome assessment; 5: incomplete outcome data; 6: selective reporting; 7: other bias.
Results from pair-wise comparisons
Five different comparisons including observation were performed in pairwise meta-analysis, with a lack of direct comparison between TZDs and observation alone. The weighted RRs for the occurrence of liver cancer were calculated for each comparison, the geometric distribution of which is displayed in Fig. 2. Compared with observation, meta-analysis of the direct comparisons showed that the use of metformin (RR 0.49, 95% CI 0.25–0.97) was associated with a significant risk reduction of HCC, while insulin (RR 2.44, 95% CI 1.10–5.56) appeared to increase the risk of liver cancer. Additionally, in comparisons between active interventions, metformin appeared to be significantly superior to insulin (RR 0.30, 95% CI 0.18–0.50), together with sulphonylurea (RR 0.44, 95% CI 0.27–0.72), and TZDs (RR 0.97, 95% CI 0.91–1.02), albeit with no statistical significance achieved. Despite a lack of direct comparison with observation, TZDs showed borderline significance when compared with insulin (RR 0.33, 95% CI 0.14–0.78). These results were derived from 9 independent analyses, with other comparisons failing to show results of statistical significance (Table 3).
Table 3. Outcomes and assessment of heterogeneity and publication bias in traditional meta-analysis.
| Treatment comparisons | Results of pair-wise meta-analysis | H (I2%) | P values of Begg’s test | P values of Egger’s test |
|---|---|---|---|---|
| M vs I | 0.30 (0.18, 0.50) | 38.92 (79.4) | 0.81 | 0.57 |
| S vs I | 0.68 (0.46, 1.02) | 27.81 (74.8) | 0.62 | 0.28 |
| T vs I | 0.33 (0.14, 0.78) | 6.12 (51.0) | 1 | 0.28 |
| I vs O | 2.44 (1.10, 5.56) | 10.97 (63.5) | 0.62 | 0.45 |
| M vs O | 0.49 (0.25, 0.97) | 3.78 (47.1) | 0.11 | 0.11 |
| M vs S | 0.44 (0.27, 0.72) | 64.12 (86.0) | 0.25 | 0.01 |
| M vs T | 0.97 (0.91, 1.02) | 4.12 (0) | 0.19 | 0.24 |
| S vs O | 1.30 (0.34, 4.97) | 2.85 (64.9) | 1 | NA |
| T vs S | 0.50 (0.15, 1.68) | 21.52 (76.8) | 0.57 | 0.91 |
Notes: NA: not available; O: Observation; M: Metformin; T: Thiazolidinediones; S: Sulphonylurea; I: Insulin.
With respect to statistical heterogeneity, it was estimated in two of the comparisons by the I2 statistic. Overall, statistical heterogeneity in our analysis was moderate, although for some comparisons 95% confidence intervals (CIs) were wide and included values indicating very high or no heterogeneity. As illustrated in Table 3, I2 values higher than 75% were recorded for 3 comparisons, that is, metformin versus insulin, metformin versus sulphonylurea, TZDs versus sulphonylurea, with remaining comparisons lower than 40%. In addition, no publication bias was found for Begg’s rank correlation test and Egger’s test among these comparisons of different antidiabetic therapies.
Results from the network meta-analysis
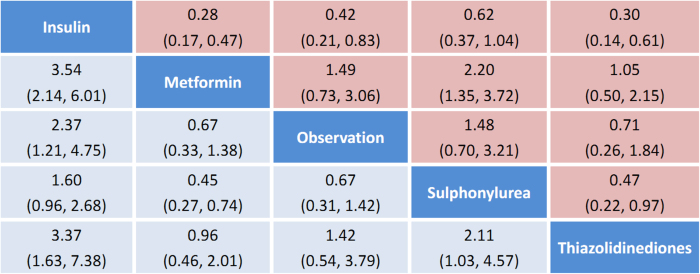
We summarize the results of the random-effects network meta-analysis for HCC rates in Fig. 3, which illustrates the RRs for HCC occurrence with 95% CIs obtained from the indirect comparisons of the included regimens. Compared with observation alone, insulin (RR 2.37, 95% CI 1.21–4.75) was associated with a significant increased risk of HCC, in line with the results of direct evidence. According to network meta-analysis, metformin was significantly effective in reducing the risk of HCC when compared with sulphonylurea (RR 0.45, 95% CI 0.27–0.74) and insulin (RR 0.28, 95% CI 0.17–0.47). Likewise, TZDs showed a similar trend in more beneficial effects against HCC incidence when compared with sulphonylurea (RR 0.47, 95% CI 0.22–0.97) and insulin (RR 0.30, 95% CI 0.14–0.61).
Figure 3. Pooled odds ratios for HCC incidence.
The column treatment is compared with the row treatment. Numbers in parentheses indicate 95% confidence intervals.
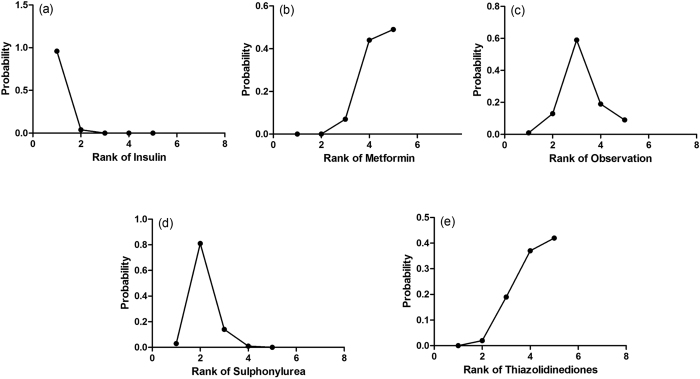
The probabilities of best treatment for each strategy were ranked at each of the five possible parameters (Fig. 4). In agreement with aforementioned results, metformin was hierarchically shown the best to decrease the risk of liver cancer according to the estimated surface under the cumulative curve values. Conversely, insulin was ranked the lowest for the prevention of HCC, which may suggest that it was the least effective in reducing HCC occurrence. As the comparison-adjusted funnel plot showed in Fig. 5, there was no evidence of asymmetry.
Figure 4. Rankograms showing probability of each strategy have each rank (1–5) for HCC incidence.
Ranking indicates the probability to be the best treatment, the second best, the third best and so on. Specifically, rank 1 is worst and rank N is best.
Figure 5. Comparison-adjusted funnel plot.
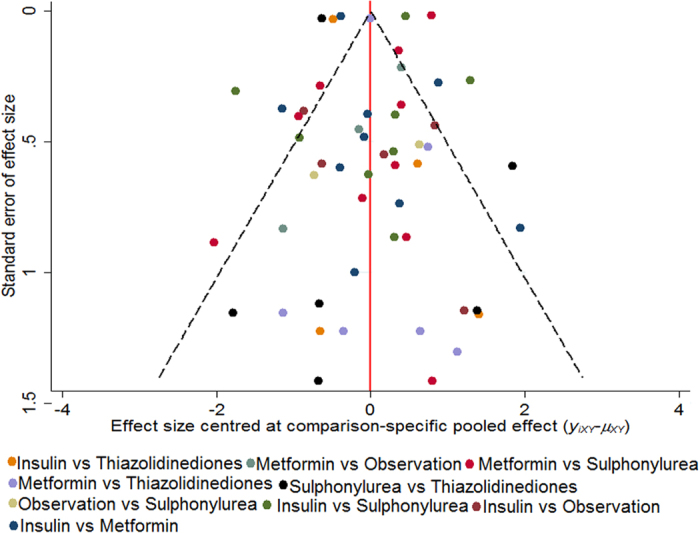
The red dotted line represents the null hypothesis that the study-specific effect sizes cannot differ from the respective comparison-specific pooled effect estimates. The two black dashed lines represent a 95% CI for the difference between study-specific effect sizes and comparison-specific summary estimates. Different colors correspond to different comparisons. Yixy is the noted effect size in study i that compares x with y. μxy is the comparison-specific summary estimate for x versus y.
Comparisons between traditional pairwise and network meta-analyses
According to the results of traditional pairwise (Table 3) and network meta-analyses (Fig. 3), the pooled estimates showed slight differences. The CIs from traditional pairwise meta-analyses and the Bayesian network meta-analyses in general overlapped, which suggests that the evidence derived from both methods is consistent. Overall, the P values of the node-splitting method show no significant difference between direct and indirect effects, suggesting no inconsistency within the networks for any of the 5 outcomes (Table 4).
Table 4. Asseessment of inconsistency between direct and indirect evidence.
| Treatment comparisons | Direct effect | Indirect effect | Overall | P-Value of Node-Splitting Method |
|---|---|---|---|---|
| I vs M | −1.24 (−1.79, −0.72) | −0.88 (−2.17, 0.34) | −1.26 (−1.79, −0.76) | 0.57 |
| I vs S | −0.34 (−0.84, 0.16) | −1.19 (2.38, −0.00) | −0.47 (−0.99, 0.44) | 0.18 |
| I vs T | −1.23 (−2.17, −0.37) | −0.51 (−2.11, 1.03) | −1.21 (−2.00, −0.49) | 0.43 |
| M vs O | 0.68 (−0.13, 1.54) | −0.75 (−2.13, 0.65) | 0.40 (−0.32, 1.12) | 0.07 |
| M vs S | 0.78 (0.33, 1.27) | −0.03 (−3.93, 3.62) | 0.79 (0.30, 1.31) | 0.65 |
| M vs T | −0.19 (−0.94, 0.57) | 0.78 (−3.02, 4.84) | 0.05 (−0.70, 0.77) | 0.58 |
| O vs S | 0.38 (−0.88, 1.63) | 0.29 (−0.57, 1.10) | 0.39 (−0.35, 1.17) | 0.89 |
| S vs T | −0.68 (−1.54, 0.12) | −1.69 (−5.22, 1.31) | −0.75 (−1.52, −0.03) | 0.52 |
Notes: O: Observation, M: Metformin, T: Thiazolidinediones, S: Sulphonylurea, I: Insulin.
Discussion
This analysis, based on one RCT and 12 observational studies, showed that metformin had a chemopreventive effect on the incidence of HCC when compared with observation alone, while insulin was associated with a statistically significant increase in HCC risk. In addition, the probabilities of best treatment for each strategy suggested that metformin was the best, TZDs were the second best, sulphonylurea was the third best, and insulin was ranked the lowest in the prevention of HCC. Moreover, there was no inconsistency or publication bias in our network meta-analysis.
According to our current knowledge and available data, glucose lowering drugs can variably modify the incidence of HCC. Our finding is consistent with the current understanding that exogenous insulin therapy or insulin secretagogues may be associated with an increased incidence of hepatoma and a higher mortality because of cirrhosis and HCC13,21,28. As a recent meta-analysis of seven observational studies showed, insulin and sulphonylurea conferred a total of 161% and 62% increase in HCC incidence, respectively29. In contrast, metformin, as a first-line anti-hyperglycemic drug, has recently gained more attention for its potential to reduce cancer incidence and improve prognosis of DM patients with solid tumor14,30,31. In agreement with our results, DM patients taking metformin were reported to have a 76% reduction in HCC risk compared with those receiving other standard hypoglycemic therapies when data was subjected to a meta-analysis32. In addition, the association of exposure to metformin with a 35% reduction of cancer-related mortality was also noted33,34.
Notably, the association of DM and cancer is implicated in insulin resistance and hyperinsulineamia10. The administration of insulin or insulin secretogogues such as sulfonylureas, leads to exogenous or endogenous hyperinsulinemia35. As an important mitogen, insulin may promote cancer progression through its binding to the insulin receptor (IR) and/or IGF-1R (insulin-like growth factor receptor), which results in auto-phosphorylation of insulin-receptor substrate-1 (IRS-1) and activation of the phosphatidylinositol 3-kinase (PI3K)/Akt (serine/threonine kinase)/mammalian target of rapamycin (mTOR) pathway and the mitogen-activated protein kinase (MAPK) pathway36. Additionally, hyperinsulinemia increases hepatic growth hormone receptor (GHR) levels and down-regulates the level of IGF-binding protein 1, raising the bioavailablility of IGF-1 on cellular proliferation and inhibition of apoptosis37,38.
Conversely, metformin, together with other insulin sensitizers, may counteract insulin resistance and consequent hyperinsulinemia, and appears to be associated with a lower cancer risk39. This finding may in part be explained considering that metformin acts to inhibit hepatic gluconeogenesis, inhibits glucose uptake in the muscle and leads to an improvement of insulin sensitivity in peripheral tissue40,41. In addition, metformin may impede carcinogenesis through indirect mechanisms, which include induction of cell cycle arrest and/or apoptosis, activation of the immune system, inhibition of the unfolded protein response (UPR), leading to a possible eradication of cancer stem cells42. Intracellularly, metformin, when highly concentrated in the liver, may prevent protein synthesis, cell proliferation and angiogenesis through activation of AMPK (adenosine monophosphate activated protein kinase) pathway42,43. AMPK, a key mediator of tumor suppressor serine-threonine liver kinase B1 (LKB1), mechanistically serves as a cellular energy sensor essential for metabolic processes. In addition, TZDs, as peroxisome proliferator-activated receptor-γ agonists, may not only increase the sensitivity of insulin, but also trigger cell cycle arrest, apoptosis, anti-proliferative, anti-angiogenic, and pro-differentiation pathways and thus contribute to down-regulation of carcinogenesis44. It has been suggested that PPAR-γ activation by TZDs is able to induce an inhibitory effect on HCC metastasis. Increasing evidence from both in vitro and in vivo studies supports the concept that PPAR-γ deficiency produce an environment prone to tumorigenesis, while PPAR-γ overexpression may inhibit HCC growth. Thus, PPAR-γ could exert beneficial effects against HCC and may represent as an anti-tumorigenic and therapeutic target45.
Some methodologic issues should be acknowledged which could be regarded as limitations in our study. First, our findings are primarily based on data derived from observational studies, which are inevitably prone to bias and confounders. Given the retrospective or hospital-based nature of some included studies that could lead to an overestimate of effect, further prospective studies with high quality analyses are needed to validate the effect of antidiabetics on such outcomes. Secondly, details on dosage, duration of antidiabetic therapy as well as full information on other residual confounders were incomplete. Thus, the findings provided by this meta-analysis should be viewed with some caution. Thirdly, statistical heterogeneity was moderate in the meta-analyses of direct comparisons, while no substantial inconsistency was found in the network meta-analysis. The diversity in study populations, comparators, and study design is responsible for a substantial heterogeneity in effect estimates across studies. Lastly, as the number of included studies is small, a bias (including bias of indication) should be considered in interpreting the results described.
Besides these limitations, our meta-analysis has several strengths. Our study is the first Bayesian network meta-analysis performed on the potential tumor-modifying effect of antidiabetics. Based on the available literature and current knowledge, we have constructed a comprehensive and complete picture of the relationship between different antidiabetic medications and the incidence of HCC. In the absence of available studies directly comparing all eligible treatments, we have to rely on indirect comparisons of multiple treatments (such as the comparison arm of observation vs. TZDs in these studies). For instance, an indirect comparison of A with B can be made by integrating the information derived from studies of A vs. C and studies of B vs. C. Thus, the method of network meta-analysis may increase statistical power by incorporating evidence from both direct and indirect comparisons across all interventions. To ensure the accuracy and reliability of our results, this study compared the pool effect estimates of all available antidiabetic therapies, even when no head-to-head studies existed. For the included studies, major confounding factors have been controlled which include age, sex, race, socioeconomic status, body mass index, smoking, ethanol intake, hepatitis C virus infection, hepatitis B virus infection, cirrhosis, and DM duration. Data quality was reliable and the total statistical heterogeneity was moderate, with no small study effects or publication bias. Lastly, a rankogram of insulin, sulfonylureas, metformin and TZDs for chemoprevention of HCC provides a formal rank order for suggested treatment strategies for diabetic patients.
Conclusions
In summary, our network meta-analysis provides a complete picture of the HCC-modifying effects of different antidiabetic medications by using Bayesian analytical approach. Specifically, metformin and TZDs have beneficial effects on HCC incidence, while insulin or sulphonylureas therapy may be associated with a higher risk of HCC. In light of potential confounding factors in observational studies, there remains a need for more well-designed randomized controlled trials, together with pathophysiological studies, to elucidate the potential role and the clinical efficacy of metformin and TZDs anticancer agents and to describe the details of their biological mechanism of action. Our analysis may contribute clinical decision making regarding appropriate antidiabetic treatment for patients with DM with a high risk of HCC.
Material and Methods
Literature Search
The protocol for this systematic review was performed in agreement with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement46. RCT and observational studies relevant to this meta-analysis were searched through PubMed, the Cochrane database and the EMBASE.com database, using the keywords ‘hypoglycemic agents, metformin, sulfonylurea compounds, TZDs, insulin, and cancer’ up to 31 October 2015. No language, publication date, or publication status restrictions were imposed. The most updated data for a given study or database were selected. Reference lists from cited articles were also manually searched for additional eligible trials.
Selection criteria
Criteria for inclusion of an article in this meta-analysis were as follows: (1) RCTs and epidemiologic studies, including cohort study or case-control study; (2) the objective was designed to evaluate the relationship between hypoglycemic agents and HCC incidence; (3) patients with no prior diagnosis of primary liver cancer or other cancers most likely to metastasize to the liver; (4) risk estimates should be provided or could be estimated with sufficient information. Duplicated or overlap reports were eliminated according to the same title, author list or publication date to avoid giving double weight to estimates derived from the same research or population. We then reviewed the full articles passing the first screening of titles/abstracts.
Data extraction
Two investigators (Zhou YY, Zhu GQ) independently reviewed the full manuscripts of eligible studies and extracted information into an electronic database: the first author, year of publication, country or area, patients’ characteristics, study design, type of hypoglycemic agents, study time or follow-up time, adjusted confounders, number of HCC cases and controls. Any discrepancies regarding the extraction of data were resolved by consensus and arbitration by an additional investigator (Zheng MH). When relevant information was unclear, or when some needed data was unavailable directly from the study, the original authors were sought for eligible data by email.
Quality Assessment
To assess the risk of bias in the observational studies, we used the Newcastle–Ottawa Quality Assessment Scale with some modifications to match the needs of this study, which included the following items: patient selection, comparability of antidiabetic medication and observation group, and assessment of outcome. In terms of RCT, we used the Cochrane risk of bias tool to determine the quality. The quality of the methodology was independently assessed by two investigators (Zhou YY, Zhu GQ).
Data analysis
Initially, we performed standard pairwise meta-analysis with a random-effects model by using STATA 12.0 (Stata Corporation, College Station, Texas, USA). For indirect and mixed comparisons, a Bayesian network meta-analysis followed, with a random-effect hierarchical model by means of Markov chain Monte Carlo methods with Gibbs sampling from 1,000 iterations obtained after a 5,000 iteration training phase. We performed random-effects pairwise and network meta-analyses to obtain estimates for the incidence of HCC. According to Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, the incidence of HCC was presented as RR with 95% CIs. Clinical heterogeneity was assessed with the I2 statistics. A formal confirmation of heterogeneity was then obtained by referring to the I2 statistic, judging values of less than 25% to be minimal, 25% to 49% to be moderate, and 50% or greater to be substantial. Nevertheless, the node splitting method was also utilized to estimate inconsistency of the model, which separated evidence into direct and indirect and compared them respectively. Small study effects were explored by inspecting comparison-adjusted funnel plots. Publication bias was assessed by Begg’s rank correlation test and Egger’s test among comparisons of different antidiabetic therapies. Egger’s test and Begg’s test was then performed to evaluate publication bias by comparison of P values, which if <0.10 indicating significant publication bias.
To rank the treatments for an outcome, we estimated the relative ranking probability of each treatment and obtained the treatment hierarchy of competing interventions using rankograms, surface under the cumulative ranking probabilities. To check for the presence of inconsistency, we used the previously described node-splitting method, which separates evidence for a particular comparison into direct and indirect47. Specifically, the results from the traditional pairwise meta-analysis were referred to as direct evidence, while indirect evidence meant results from network meta-analysis. Then we assessed the agreement between the direct and indirect evidence and reported its Bayesian P value.
Additional Information
How to cite this article: Zhou, Y.-Y. et al. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci. Rep. 6, 33743; doi: 10.1038/srep33743 (2016).
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81500665) and Project of New Century 551 Talent Nurturing in Wenzhou.
Footnotes
Author Contributions Y.-Y.Z., G.-Q.Z., S.-W.F. and M.-H.Z. designed the study. Y.-Y.Z., T.L. and J.-N.Z. screened studies and extracted data. G.-Q.Z. and Z.C. did the statistical analyses. T.-T.Z. prepared figures. Y.-Y.Z., G.-Q.Z., M.B., S.-W.F. and M.-H.Z. reviewed the results, interpreted data, and wrote the manuscript. All authors saw and approved the final version of the paper.
References
- El-Serag H. B. Hepatocellular carcinoma. The New England journal of medicine 365, 1118–1127, doi: 10.1056/NEJMra1001683 (2011). [DOI] [PubMed] [Google Scholar]
- Davila J. A., Morgan R. O., Shaib Y., McGlynn K. A. & El-Serag H. B. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 54, 533–539, doi: 10.1136/gut.2004.052167 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag H. B., Tran T. & Everhart J. E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 126, 460–468 (2004). [DOI] [PubMed] [Google Scholar]
- Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta diabetologica 47, 87–95, doi: 10.1007/s00592-010-0187-3 (2010). [DOI] [PubMed] [Google Scholar]
- Oberaigner W. et al. Increased cancer incidence risk in type 2 diabetes mellitus: results from a cohort study in Tyrol/Austria. BMC public health 14, 1058, doi: 10.1186/1471-2458-14-1058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. S., Calle E. E., Teras L. R., Petrelli J. & Thun M. J. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American journal of epidemiology 159, 1160–1167, doi: 10.1093/aje/kwh161 (2004). [DOI] [PubMed] [Google Scholar]
- Inoue M. et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Archives of internal medicine 166, 1871–1877, doi: 10.1001/archinte.166.17.1871 (2006). [DOI] [PubMed] [Google Scholar]
- El-Serag H. B., Hampel H. & Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 4, 369–380, doi: 10.1016/j.cgh.2005.12.007 (2006). [DOI] [PubMed] [Google Scholar]
- Donadon V., Balbi M., Casarin P., Vario A. & Alberti A. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: potential role of insulin. World journal of gastroenterology 14, 5695–5700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadon V. et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World journal of gastroenterology 15, 2506–2511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. W. et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. The American journal of gastroenterology 107, 46–52, doi: 10.1038/ajg.2011.384 (2012). [DOI] [PubMed] [Google Scholar]
- Bosetti C. et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. The oncologist 18, 148–156, doi: 10.1634/theoncologist.2012-0302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker S. L., Majumdar S. R., Veugelers P. & Johnson J. A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes care 29, 254–258 (2006). [DOI] [PubMed] [Google Scholar]
- Chen H. P. et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 62, 606–615, doi: 10.1136/gutjnl-2011-301708 (2013). [DOI] [PubMed] [Google Scholar]
- Donadon V., Balbi M., Mas M. D., Casarin P. & Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver international: official journal of the International Association for the Study of the Liver 30, 750–758, doi: 10.1111/j.1478-3231.2010.02223.x (2010). [DOI] [PubMed] [Google Scholar]
- Chang C. H. et al. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 55, 1462–1472, doi: 10.1002/hep.25509 (2012). [DOI] [PubMed] [Google Scholar]
- Home P. D. et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53, 1838–1845, doi: 10.1007/s00125-010-1804-y (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. C., Tong M. J., Govindarajan S. & Henderson B. E. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. Journal of the National Cancer Institute 83, 1820–1826 (1991). [DOI] [PubMed] [Google Scholar]
- Oliveria S. A., Koro C. E., Yood M. U. & Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2, 47–57 (2008). [Google Scholar]
- Hassan M. M. et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 116, 1938–1946, doi: 10.1002/cncr.24982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T. et al. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver international: official journal of the International Association for the Study of the Liver 30, 479–486, doi: 10.1111/j.1478-3231.2009.02191.x (2010). [DOI] [PubMed] [Google Scholar]
- Nkontchou G. et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. The Journal of clinical endocrinology and metabolism 96, 2601–2608, doi: 10.1210/jc.2010-2415 (2011). [DOI] [PubMed] [Google Scholar]
- Ruiter R. et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes care 35, 119–124, doi: 10.2337/dc11-0857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S. et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 24, 2449–2455, doi: 10.1093/annonc/mdt204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg K. W., McGlynn K. A., Sahasrabuddhe V. V. & Jick S. Anti-diabetic medications and risk of primary liver cancer in persons with type II diabetes. British journal of cancer 111, 1710–1717, doi: 10.1038/bjc.2014.447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti C. et al. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: a nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiology and drug safety 24, 771–778, doi: 10.1002/pds.3801 (2015). [DOI] [PubMed] [Google Scholar]
- Miele L. et al. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterology research and practice 2015, 570356, doi: 10.1155/2015/570356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Lin J. W., Wu L. C., Lai M. S. & Chuang L. M. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism 97, E1170–E1175, doi: 10.1210/jc.2012-1162 (2012). [DOI] [PubMed] [Google Scholar]
- Singh S., Singh P. P., Singh A. G., Murad M. H. & Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. The American journal of gastroenterology 108, 881–891, quiz 892, doi: 10.1038/ajg.2013.5 (2013). [DOI] [PubMed] [Google Scholar]
- Decensi A. et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer prevention research 3, 1451–1461, doi: 10.1158/1940-6207.CAPR-10-0157 (2010). [DOI] [PubMed] [Google Scholar]
- Harris K. & Smith L. Safety and efficacy of metformin in patients with type 2 diabetes mellitus and chronic hepatitis C. The Annals of pharmacotherapy 47, 1348–1352, doi: 10.1177/1060028013503108 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H., Gao C., Fang L., Zhao H. C. & Yao S. K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scandinavian journal of gastroenterology 48, 78–87, doi: 10.3109/00365521.2012.719926 (2013). [DOI] [PubMed] [Google Scholar]
- Franciosi M. et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PloS one 8, e71583, doi: 10.1371/journal.pone.0071583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman G. W. et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes care 33, 322–326, doi: 10.2337/dc09-1380 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasello G., Urso L., Conte P. & Favaretto A. Effects of sulfonylureas on tumor growth: a review of the literature. The oncologist 18, 1118–1125, doi: 10.1634/theoncologist.2013-0177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A., Frasca F., Pandini G., Sciacca L. & Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine reviews 30, 586–623, doi: 10.1210/er.2008-0047 (2009). [DOI] [PubMed] [Google Scholar]
- Pollak M. N., Schernhammer E. S. & Hankinson S. E. Insulin-like growth factors and neoplasia. Nature reviews. Cancer 4, 505–518, doi: 10.1038/nrc1387 (2004). [DOI] [PubMed] [Google Scholar]
- Moore M. A., Park C. B. & Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation 7, 89–107 (1998). [PubMed] [Google Scholar]
- Jalving M. et al. Metformin: taking away the candy for cancer? European journal of cancer 46, 2369–2380, doi: 10.1016/j.ejca.2010.06.012 (2010). [DOI] [PubMed] [Google Scholar]
- Gallagher E. J. & LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Annals of the New York Academy of Sciences 1243, 54–68, doi: 10.1111/j.1749-6632.2011.06285.x (2011). [DOI] [PubMed] [Google Scholar]
- Bailey C. J. & Turner R. C. Metformin. The New England journal of medicine 334, 574–579, doi: 10.1056/NEJM199602293340906 (1996). [DOI] [PubMed] [Google Scholar]
- Dowling R. J., Goodwin P. J. & Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC medicine 9, 33, doi: 10.1186/1741-7015-9-33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation 108, 1167–1174, doi: 10.1172/JCI13505 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. Journal of gastroenterology 45, 1097–1102, doi: 10.1007/s00535-010-0310-9 (2010). [DOI] [PubMed] [Google Scholar]
- Wu C. W., Farrell G. C. & Yu J. Functional role of peroxisome-proliferator-activated receptor γ in hepatocellular carcinoma. Journal of gastroenterology and hepatology 27, 1665–1669, doi: 10.1111/j.1440-1746.2012.07213.x (2012). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology 62, 1006–1012, doi: 10.1016/j.jclinepi.2009.06.005 (2009). [DOI] [PubMed] [Google Scholar]
- Lumley T. Network meta-analysis for indirect treatment comparisons. Statistics in medicine 21, 2313–2324, doi: 10.1002/sim.1201 (2002). [DOI] [PubMed] [Google Scholar]