Significance
Mucosal barrier tissues participate in immune defense in infections, but NADPH oxidases expressed in these epithelia are much less efficient in their oxidant output than the phagocyte oxidase. The importance of releasing low hydrogen peroxide (H2O2) concentrations as a host defense mechanism against pathogens remains unclear. Here, we demonstrate that nano to submicromolar H2O2 disrupts the tyrosine phosphorylation network in several pathogens by an oxidative dephosphorylation process; this is accomplished by irreversible chemical modification of key phosphotyrosine residues, which in turn changes protein activity without affecting bacterial viability. This process is a host-initiated antivirulence strategy, reducing the fitness of pathogens in the extracellular space.
Keywords: mucosal immunity, NADPH oxidase, bacterial tyrosine phosphorylation, DOPA, reactive oxygen species (ROS)
Abstract
Strengthening the host immune system to fully exploit its potential as antimicrobial defense is vital in countering antibiotic resistance. Chemical compounds released during bidirectional host–pathogen cross-talk, which follows a sensing-response paradigm, can serve as protective mediators. A potent, diffusible messenger is hydrogen peroxide (H2O2), but its consequences on extracellular pathogens are unknown. Here we show that H2O2, released by the host on pathogen contact, subverts the tyrosine signaling network of a number of bacteria accustomed to low-oxygen environments. This defense mechanism uses heme-containing bacterial enzymes with peroxidase-like activity to facilitate phosphotyrosine (p-Tyr) oxidation. An intrabacterial reaction converts p-Tyr to protein-bound dopa (PB-DOPA) via a tyrosinyl radical intermediate, thereby altering antioxidant defense and inactivating enzymes involved in polysaccharide biosynthesis and metabolism. Disruption of bacterial signaling by DOPA modification reveals an infection containment strategy that weakens bacterial fitness and could be a blueprint for antivirulence approaches.
Communication between the host and pathogens at the mucosal interface is governed by the chemical environment. Host-derived signals can be subverted by pathogens to enhance virulence gene expression and pathogen expansion (1), but in most circumstances the immune system prevails by coordinating defense mechanisms, thereby restricting colonization and disease. Within complex mucosal environments, pathogen virulence can be modulated in a niche-specific manner (2), which is now recognized as an important strategy that helps combating antibacterial resistance. The most promising approach to target bacterial virulence factors is through the use of compounds that affect multiple prokaryotic targets at once.
As a relatively stable, diffusible mediator host-derived hydrogen peroxide (H2O2) can modify more distant targets. H2O2 can penetrate bacterial membranes inducing transcriptional stress responses (3), but may also alter other cellular processes via oxidation. High concentrations of reactive oxygen species (ROS) and derivatives (HOCl) can induce irreversible amino acid oxidations or chlorinations, leading to loss of function by misfolding or degradation and to bacterial killing. Lower ROS levels, in particular H2O2, primarily result in oxidative modifications that are dynamic and reversible, such as thiol oxidation and disulfide bond formation in enzymes, iron-sulfur clusters, or transcriptional regulators containing redox-sensitive cysteine residues. The redox sensitivity of mammalian tyrosine kinase pathways is mainly a result of transient inactivation of protein tyrosine phosphatases or by oxidative protein kinase activation (e.g., SRC, ASK1) (4). Intestinal pathogens traversing through the mucus before attaching to or invading the epithelium can activate epithelial NADPH oxidases (NOX1, DUOX2) before recruitment of neutrophils occurs. Neutrophils engulfing bacteria produce and release large quantities of O2•− via NOX2 into the phagosome, which enables bacterial killing by the concerted action of HOCl, proteases, and antibacterial peptides (5). Intestinal epithelial cells (IEC) release low levels of H2O2 into the lumen, but the achievable concentrations are in the nano/micromolar range and inadequate for bactericidal activity. We hypothesized that, when maintained over time, IEC-derived H2O2 may overcome antioxidant defenses of pathogens and may alter redox-regulated processes in bacteria.
Bearing in mind how H2O2 acts on mammalian signaling circuits, the prime target for redox regulation in prokaryotes are tyrosine kinase pathways. Tyrosine phosphorylation coordinates a variety of key processes in bacteria, including biofilm and capsule formation, heat-shock response, DNA replication, transcription, metabolic processes, antibiotic resistance, virulence, and interspecies communication (6). Bacterial tyrosine (BY) kinases contain ATP-binding Walker motifs and a C-terminal tyrosine cluster, and exhibit no sequence or structural homology to eukaryotic kinases (7). These BY-kinases cooperate with phosphatases, various association partners, and Hanks type serine/threonine kinases in interaction networks, but mechanistic details of their regulation and often even the identity of the kinases remain poorly understood. Nevertheless, interfering with BY-kinase signaling should be beneficial because decreased virulence has been connected to interference with BY-kinase pathways (8, 9). Here we show that H2O2-mediated disruption of bacterial tyrosine signaling is a common feature in intestinal and even pulmonary pathogens, requiring low H2O2 concentrations in physiological conditions and a combination of host and bacterial features present in oxygen-restricted environments during infections. In contrast to increased phosphotyrosine (p-Tyr) signaling by phosphatase inhibition via thiol oxidation in mammalian systems, H2O2 triggered iron-associated conversion of p-Tyr to DOPA on bacterial proteins, leading to disruption of p-Tyr signaling. Proteomics data reveal that this interference with p-Tyr signaling occurs frequently in prokaryotic and eukaryotic organisms.
Results
H2O2 Disrupts Phosphotyrosine Signaling of Pathogens.
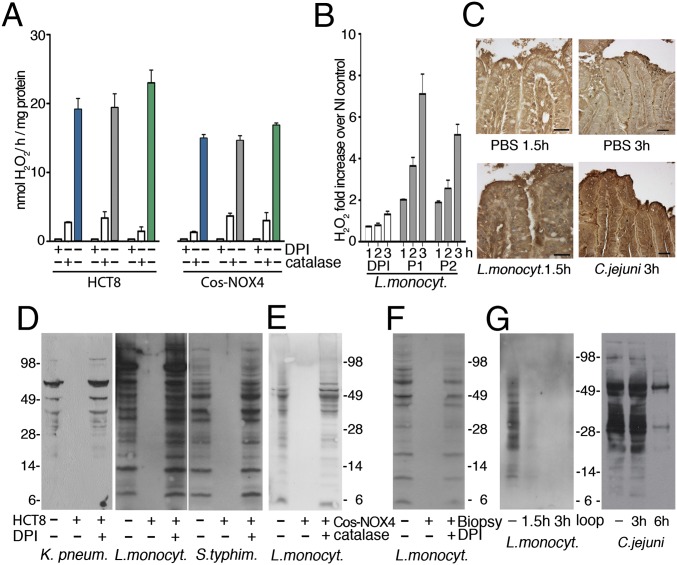
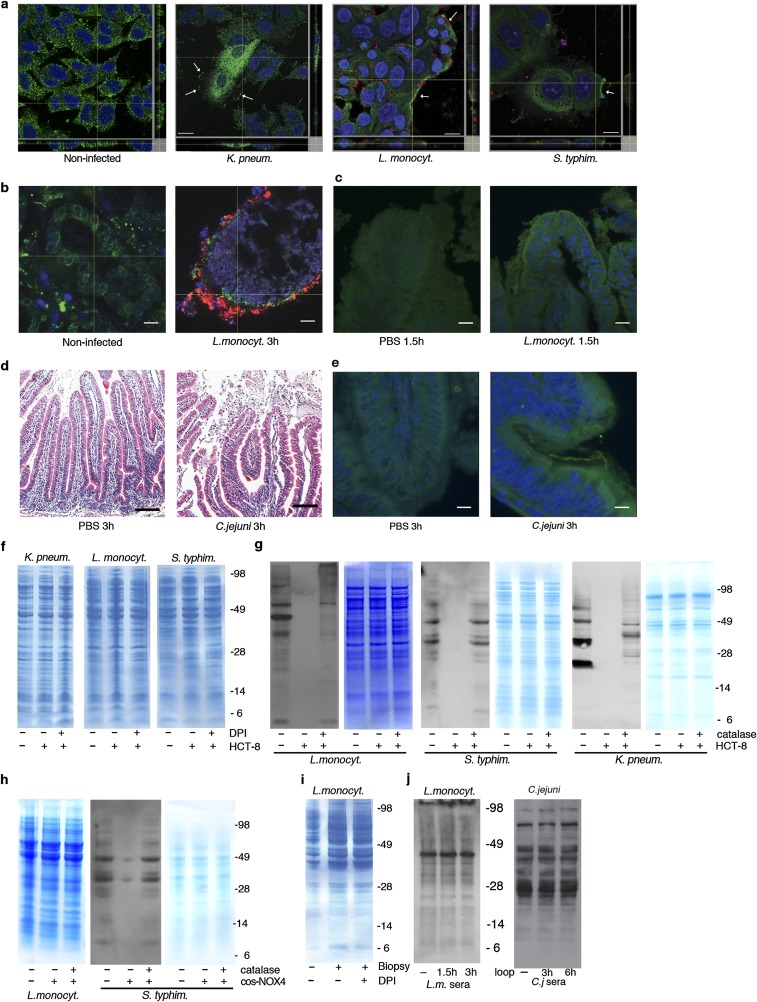
The pathogens Listeria monocytogenes, Salmonella enterica serovar Typhimurium and Klebsiella pneumoniae were chosen as representative enteric bacteria with diverse cell wall structures, motility, and antioxidant defense gene expression to determine if host epithelial H2O2 production accompanies intestinal infection as a general defense mechanism. All of these bacteria induced H2O2 release when incubated with IECs (HCT-8 cells) (Fig. 1A), an effect suppressed by the cell-permeable pan-oxidase inhibitor diphenyleneiodonium chloride (DPI) and catalase. Previously, we connected H2O2 generation upon pathogen contact in this cell type directly to NOX1, the only NADPH oxidase expressed (8). Bacterial infection triggered translocation of the NOX-p22phox complex to the plasma membrane (Fig. S1A; visualized as p22phox), inducing continuous release of low H2O2 concentrations (20 nmol H2O2/h per milligram of protein). A similar H2O2 concentration is achieved on top of a Boyden chamber filter with Cos cells expressing constitutively active NOX4 seeded on the bottom (15 nmol H2O2/h per milligram of protein) (8) (Fig. 1A). In human colon biopsies, a physiologically relevant model expressing NOX1, L. monocytogenes infection caused up-regulation and membrane recruitment of the NOX-p22phox complex concurrently with H2O2 generation (Fig. 1B and Fig. S1B). ROS generation was also detected in vivo using a ligated rabbit ileal loop infection model. Injection of L. monocytogenes or Campylobacter jejuni led to severe tissue injury in rabbits, preceded by recruitment of the NOX dimerization partner p22phox to the apical crypt surface and oxidative modification of epithelia by carbonylation (Fig. 1C and Fig. S1 C–E). These data indicate that gut epithelial H2O2 generation by NADPH oxidase is commonly observed in infection.
Fig. 1.
Mucosal H2O2 leads to tyrosine dephosphorylation in intestinal bacteria. (A) H2O2 release by IECs (HCT-8) following exposure to enteric bacteria (±DPI or catalase addition) and by Cos-NOX4 cells separated from bacteria by a filter (K. pneumoniae, blue; L. monocytogenes, gray; S. typhimurium, green). (B) H2O2 release by colonic biopsies after infection with L. monocytogenes (patients P1, P2). Control is DPI preincubation of biopsies. (C) Carbonylation of rabbit ileal loop tissues after injection with PBS, L. monocytogenes (1.5 h) or C. jejuni (3 h). (Scale bars, 25 µm.) (D and E) Anti–p-Tyr immunoblots of lysates derived from extracellular bacteria exposed to IEC (D) or Cos-NOX4 (E) for 3 h (see A). (F) Anti–p-Tyr immunoblot of extracellular L. monocytogenes after exposure to biopsies (see B) (3 h). Starting culture is used for comparison. (G) Anti–p-Tyr immunoblot of L. monocytogenes or C. jejuni recovered from the ileal loop lumen.
Fig. S1.
NOX-p22phox translocation in infected cells, biopsies, and rabbit loops and protein-expression controls. (A) Immunofluorescence images of p22phox (green) plasma membrane localization (white arrows) in HCT-8 cells after 3 h of infection with TAMRA-labeled bacteria (red). Nuclei stained with DAPI (blue). (Scale bars 20 µm.) (B) Immunofluorescence images of TAMRA-labeled L. monocytogenes (red) infection of biopsies; localization of p22phox (green) is shown (3 h). Nuclei stained with DAPI (blue). (Scale bars, 50 μm.) (C) Immunofluorescence images of p22phox localization (green) in rabbit ileal loops after infection with L. monocytogenes (red) for 1.5 h. Nuclei stained with DAPI (blue). (Scale bars, 20 µm.) (D) H&E staining of rabbit ileal loop tissues injected with either PBS or C. jejuni for 3 h. (Scale bars, 50 µm.) (E) Localization of p22phox NADPH oxidase subunit (green) in rabbit ileal loop tissues after treatment with PBS or C. jejuni for 3 h. Nuclei stained with DAPI (blue). (Scale bars, 20 µm.) (F) Coomassie blue staining of samples used for Fig. 1D (equal loading). (G) Anti–p-Tyr immunoblots of lysates derived from bacteria exposed to HCT-8 cells with and without catalase and loading control, see Fig. 1A. (H) Coomassie blue staining corresponding to Fig. 1E and anti–p-Tyr immunoblot of S. typhimurium exposed to Cos-NOX4 cells with loading control. (I) Coomassie blue staining corresponding to Fig. 1F. (J) Anti-L. monocytogenes and anti-C. jejuni sera immunoblots of bacteria retrieved from rabbit loops as loading control for Fig. 1G.
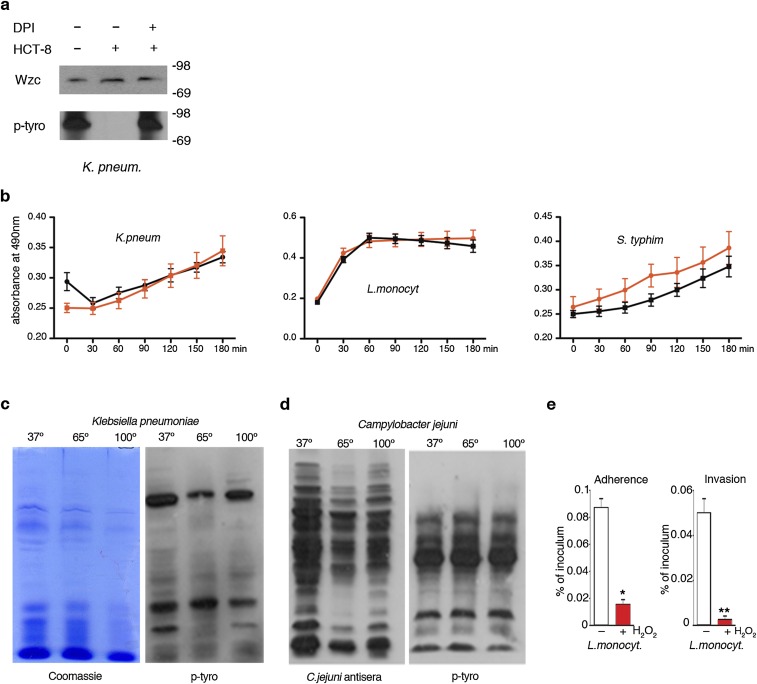
The majority of bacteria remain extracellular in the mucus layer or gut lumen at any particular time point of infection, either because only a certain fraction will invade epithelial cells (e.g., Listeria) or because particular pathogens attach to but do not invade IECs (e.g., Klebsiella). Released H2O2 is taken up by bacteria and induces a transcriptional stress response, but we speculated that H2O2 may also alter tyrosine kinase pathways. Immunoblot analysis of extracellular bacteria recovered from media of infected IECs, from the top of the filter of Cos-NOX4 cells or from biopsy media, revealed not increased but significantly decreased bacterial p-Tyr content when H2O2 was present (Fig. 1 D–F). Bacterial viability or overall bacterial protein content were not altered (Figs. S1 F–I and S2B).Tyrosine phosphorylation of Wzc, the main BY-kinase expressed in K. pneumoniae, Escherichia coli, and Acinetobacter (7), was substantially lower, but Wzc expression was not altered (Fig. S2A). These observations were further confirmed by analyzing bacteria recovered from the rabbit ileal lumen after infection. Overall tyrosine phosphorylation was significantly diminished in extracellular bacteria, whereas protein content was comparable to the starting bacterial culture (Fig. 1G and Fig. 1J).
Fig. S2.
Biochemical and functional analysis of intestinal bacteria. (A) Detection of Wzc by immunoblot using anti-E. coli Wzc antibody in K. pneumoniae lysates after treatment as in Fig. 1D. (B) Viability of K. pneumoniae, L. monocytogenes, and S. typhimurium in the absence (black) or presence (red) of exogenous 0.7 mM H2O2. Viability was assessed at indicated time points. (C) Anti–p-Tyr immunoblot and Coomassie staining of lysates prepared from K. pneumoniae treated as indicated (65 °C overnight, 100 °C 10 min). (D) Anti–p-Tyr and anti-C. jejuni antisera immunoblots of lysates prepared from C. jejuni treated as indicated (65 °C overnight, 100 °C 10 min). (E) IEC adhesion and invasion of H2O2-preexposed L. monocytogenes. Error bars represent SEM and asterisks indicate significance (**P ≤ 0.01 and *P ≤ 0.05).
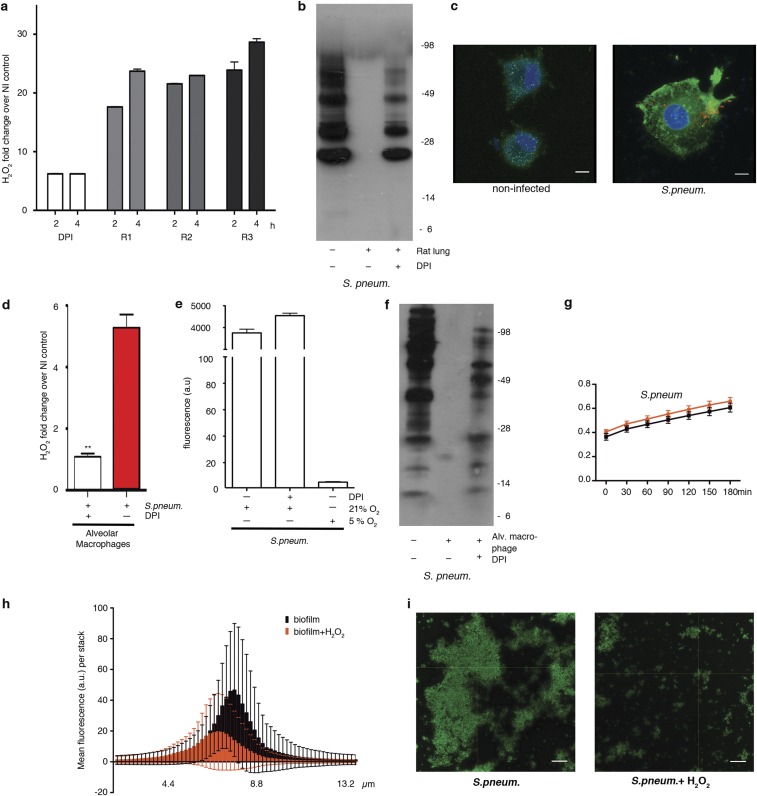
Mancini and Imlay (10) reported that a bacterial culture in iron-rich media decomposed 0.5 mM H2O2 in less than 2 h without affecting bacterial growth substantially, and an intracellular H2O2 sensor indicated an up to 500-fold gradient between outside and inside H2O2 concentrations in E. coli (11). In line with these observations, addition of 0.7 mM H2O2 to bacterial cultures mimicked tissue-based observations without reducing bacterial viability (Fig. S2B), whereas heat treatment did not alter the p-Tyr profile (Fig. S2 C and D). Bacterial tyrosine phosphorylation has been connected to pathogenicity (8, 9) and to type 3 secretion system regulation in enterohemorrhagic E. coli (12). If a similar link exists between p-Tyr signaling and L. monocytogenes virulence, it should be disrupted by H2O2. In accord, adhesion to IECs, which is a prerequisite for Listeria invasion, decreased significantly after pre-exposure of L. monocytogenes to H2O2, resulting in lower internalization of the bacterium (Fig. S2E). Oxidative tyrosine dephosphorylation may occur also at other mucosal surfaces, in particular in the lung where ROS generated by alveolar macrophages and lung epithelial cells provide host defense. We established a fetal rat lung model that permitted intraorgan incubation with bacteria and subsequent recovery of bacteria and bronchoalveolar fluid. Intratracheal injection of Streptococcus pneumoniae caused two- to threefold increased H2O2 levels above the DPI baseline with concomitant decrease of the p-Tyr content in bacteria recovered from the bronchoalveolar fluid pellet (Fig. S3 A and B). The developmental regulation of DUOX expression and the short period of pathogen exposure favored macrophages as an H2O2 source in this model. Coculture of S. pneumoniae with rat alveolar macrophages prompted increased cell adhesion and protrusions that were accompanied by NOX2 NADPH oxidase up-regulation, translocation of NOX2 to the plasma membrane, and O2•−/H2O2 production, which was inhibited by DPI (Fig. S3 C and D). S. pneumoniae itself was not affected by DPI and did not generate H2O2 in the low-oxygen conditions used throughout (Fig. S3E). Decreased p-Tyr content was detected in extracellular S. pneumoniae collected from macrophage coculture media, similar to our observations in infected rat lungs (Fig. S3F). Adding H2O2 as single bolus to growth medium induced S. pneumoniae tyrosine dephosphorylation without altering bacterial viability (Fig. S3G). Polysaccharide biosynthesis is to date the only well-characterized process regulated by bacterial tyrosine phosphorylation. Pre-exposure of S. pneumoniae to H2O2 diminished biofilm formation (Fig. S3 H and I), thus strengthening the connection between H2O2, tyrosine dephosphorylation, and pathogenic traits. These results indicate that host H2O2 production is coupled to diminished p-Tyr content in extracellular pathogens, leading to decreased pathogenicity by disrupting intrabacterial signaling.
Fig. S3.
Characterization of the pulmonary pathogen S. pneumonia. (A) H2O2 release by ex vivo cultured fetal rat lungs (rats R1, R2, R3) inoculated with S. pneumoniae. Rat lungs pretreated with DPI served as control. (B) Anti–p-Tyr immunoblot of S. pneumoniae collected from the lumen of infected rat lungs (3 h). The starting bacterial culture and DPI-pretreated rat lungs served as controls. (C) Immunofluorescence image of rat lung alveolar macrophages 30 min after exposure to TAMRA-labeled S. pneumoniae (red). Localization of NOX2 (green) is depicted. Nuclei stained with DAPI (blue). (Scale bars, 5 μm.) (D) H2O2 release by rat alveolar macrophages after 15 min of exposure to S. pneumoniae (±DPI pretreatment of cells). Error bars represent SEM and asterisks indicate significance (**P ≤ 0.01). (E) H2O2 release measured in supernatant after 24-h growth of S. pneumoniae in 21% O2 or 5% O2. Bacterial H2O2 in presence or absence of DPI is shown. (F) Anti–p-Tyr immunoblot of extracellular S. pneumoniae collected after exposure to alveolar macrophages (30 min). The starting bacterial culture and DPI served as controls. (G) Viability of S. pneumoniae at 5% O2 in the absence (black) or presence (red) of exogenous H2O2. Viability was assessed at indicated time points. (H) Three-dimensional measurement of S. pneumoniae biofilm in the presence (red) or absence (black) of H2O2. The mean fluorescent values for images in a 13.5-μm thick stack are shown. (I) Representative confocal image of S. pneumoniae biofilm (green) used for quantification in H. (Scale bars, 10 µm.)
The Chemical Microenvironment and Hemoproteins Affect Oxidative Tyrosine Dephosphorylation in Bacteria.
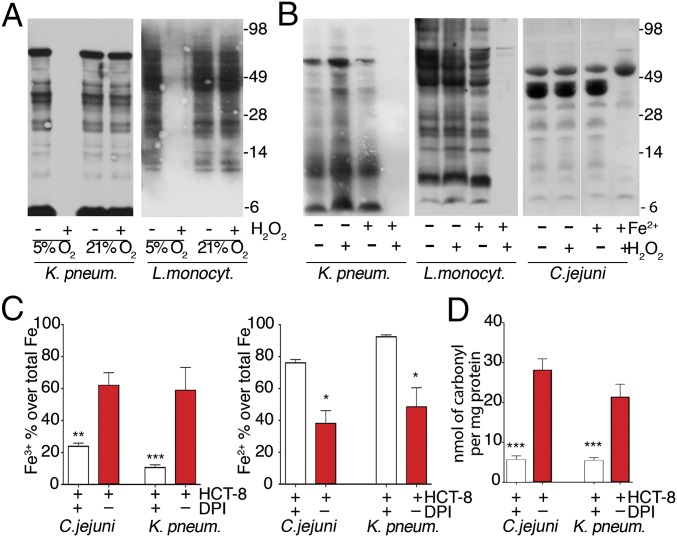
The change in bacterial p-Tyr content by oxidation suggested a distinct mechanism for phosphate group removal, as thiol-based inactivation of phosphatases would cause increased tyrosine phosphorylation. Furthermore, deletion of PTP1B, the only known phosphatase in C. jejuni, did not alter p-Tyr–dependent capsule biosynthesis. We explored the environmental conditions required for the suppression of bacterial p-Tyr by H2O2. Intestinal pathogens are typically facultative anaerobes, thriving in the low oxygen levels present in the gut (13), but many bacteria tolerate atmospheric oxygen levels. Environmental adaptation prompts usually global transcriptional responses (14), but these external stress signals may also remodel the bacterial signaling conduit. Comparison of bacteria propagated in low oxygen (5% O2) versus ambient oxygen (21% O2) before exposure to H2O2 indicated a striking difference. K. pneumoniae and L. monocytogenes grown in 21% O2 before addition of H2O2 retained their original p-Tyr profile, whereas cultures grown in 5% O2 lost the p-Tyr signal (Fig. 2A).
Fig. 2.
Oxygen and iron availability affect oxidative dephosphorylation of tyrosines. (A and B) Anti–p-Tyr immunoblots of bacteria cultured in 5% or 21% O2 (A) or in low- or high-iron conditions at 5% O2 (B) before 0.7 mM H2O2 addition (3 h). (C) Quantification of Fe3+ vs. Fe2+ in indicated bacteria cocultured with HCT-8 cells (±DPI pretreatment). (D) Quantification of carbonyl formation in C. jejuni or K. pneumoniae protein extracts after HCT-8 coculture. Error bars represent SEM and asterisks indicate significance (***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05).
We questioned whether changes in iron availability could affect the p-Tyr network, as oxidative stress in bacteria can induce iron regulators (e.g., Fur, Dps) and inactivate iron-sulfur dehydratases (3). Bacterial cultures initially grown in iron-deficient media at 5% O2 were analyzed after addition of ferrous sulfate and H2O2. The p-Tyr content was only diminished by H2O2 when extracellular iron was provided (Fig. 2B). In coculture conditions where iron is supplied by HCT-8 cells and the culture media, the Fe2+ versus Fe3+ ratio in the total pool of bacterial intracellular iron was dependent on IEC-mediated H2O2 generation (Fig. 2C), suggesting that the bacterial intracellular pool of free ferrous iron was oxidized to ferric iron. Carbonylation, a marker for site-specific, metal-catalyzed protein modification by hydroxyl radicals, was increased in bacteria exposed to HCT-8–generated H2O2 (Fig. 2D). This finding suggests that oxidative tyrosine dephosphorylation in bacteria is taking place in conditions that are present in the intestine during infections.
Cytochrome P450 or Peroxidase Activity Is Required for Oxidative Dephosphorylation.
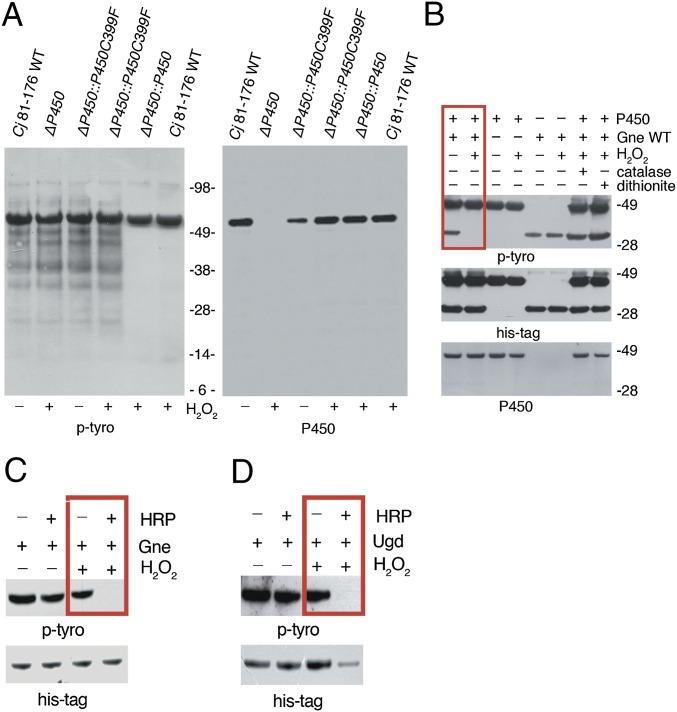
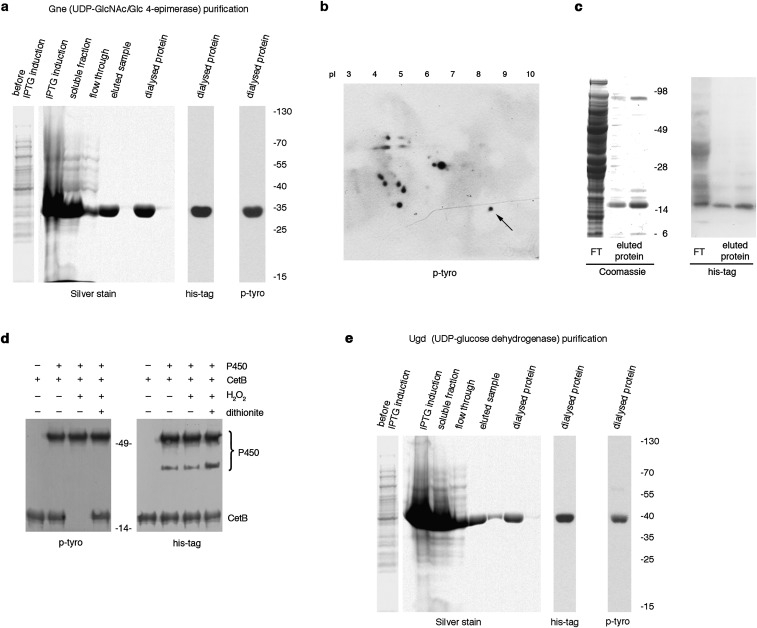
Many pathogens, including certain Pseudomonas, Mycobacterium, or Acinetobacter strains, contain cytochrome P450 monooxygenases that catalyze hydroxylation reactions via molecular oxygen reduction or by using the peroxide-shunt pathway, contributing to virulence and persistence in the host (15). Expression of an outer membrane/periplasmic cytochrome P450 (Cj1411, CjP450) in C. jejuni increased after H2O2 exposure and deletion of CjP450 decreased capsular polysaccharide production (16), raising the possibility that CjP450 participates in p-Tyr signaling. Deletion of CjP450 or mutation of a cysteine (C.j. ΔP450:P450C399F) in the heme-iron ligand signature motif of CjP450 that leads to perturbation of the heme pocket and loss of catalytic activity in P450 enzymes, rendered the C. jejuni p-Tyr network insensitive to H2O2 (Fig. 3A). To verify direct participation of CjP450 in oxidative tyrosine dephosphorylation, both CjP450 and Gne, a UDP-GlcNAc/Glc 4-epimerase phosphorylated on the active site Tyr146, were expressed and purified from E. coli (Fig. S4A) (16). Dephosphorylation of Gne was dependent on addition of H2O2 and CjP450, and not observed in the presence of catalase and sodium dithionite, respectively (Fig. 3B). When H2O2 is available, cytochrome P450s can exhibit peroxidase-like activity (15, 17). We asked if other hemoproteins, such as HRP, could catalyze this reaction in a similar fashion. Replacing CjP450 with HRP during exposure of Gne to H2O2 removed p-Tyr equally efficiently (Fig. 3C).
Fig. 3.
Cytochrome P450 or peroxidases are required for oxidative phosphate removal. (A) Anti–p-Tyr immunoblot of C. jejuni strains with or without exposure to H2O2. C. jejuni 81-176 (WT) was compared with C. jejuni ∆cjj81176_1410 (∆P450), to the P450 reconstituted strain (∆P450::P450), or to C. jejuni ∆P450 reconstituted with P450 active site cysteine mutant (∆P450::P450 C399F). Blots were reprobed with anti-P450 antibody. (B) Tyrosine dephosphorylation of C. jejuni Gne by C. jejuni cytochrome P450 in the presence or absence of 1 mM H2O2. Catalase and dithionite served as controls for H2O2 and P450 activity, respectively. Anti–p-Tyr, anti-His tag, and anti-P450 immunoblots are shown. (C) Tyrosine dephosphorylation of Gne by HRP in the presence or absence of 1 mM H2O2. (D) Tyrosine dephosphorylation of E. coli Ugd by HRP in the presence or absence of 1 mM H2O2.
Fig. S4.
Production of recombinant Gne and Ugd, and identification of CetB as tyrosine kinase substrate. (A and E) Overexpression and purification of recombinant Gne and Ugd. Silver staining, anti–p-Tyr, and anti-His immunoblotting of affinity purified (A) His-Gne or (E) His-Ugd expressed in E. coli. Both Gne and Ugd are tyrosine phosphorylated by E. coli BY-kinases in these conditions. (B) Anti–p-Tyr immunoblot of 2D gel electrophoresis of C. jejuni outer membrane proteins. The arrow indicates the spot identified as CetB (CJJ81176_1204) by MS-MS. (C) Coomassie-stained gel and anti-His immunoblot of His-tagged CetB, expressed and affinity purified from E. coli. FT denotes flow through. (D) Anti–p-Tyr immunoblot depicting CetB dephosphorylation by recombinant C. jejuni cytochrome P450 in the presence of H2O2. Dithionite served as inhibitor of cytochrome P450 activity. Anti-His blot served as loading control.
To date, Gne is the only characterized BY-kinase substrate in C. jejuni, impeding analysis of other p-Tyr–containing proteins in this bacterium. Using 2D gel electrophoresis of C. jejuni outer membrane fractions followed by p-Tyr detection and MS, we identified CetB (Cj1189c), a protein involved in energy taxis and motility (18), as a tyrosine kinase substrate (Fig. S4B). CetB, cloned from C. jejuni 81-176, was expressed and purified using E. coli (Fig. S4C). As with Gne, CetB was also tyrosine phosphorylated by E. coli BY-kinases, reflecting the relaxed substrate specificity of bacterial tyrosine kinases (19). CetB was dephosphorylated on tyrosine only when H2O2 and cytochrome P450 were present (Fig. S4D). Many bacteria, including E. coli, do not express cytochrome P450 enzymes, but they contain heme peroxidases. A comparable decrease in p-Tyr was observed when purified recombinant UDP-glucose dehydrogenase (Ugd), a BY-kinase substrate expressed in K. pneumoniae and other bacteria, was treated with HRP in the presence of H2O2 (Fig. 3D and Fig. S4E). Ugd, which catalyzes the synthesis of UDP-glucuronic acid, is activated by phosphorylation of Tyr71, and regulates capsule biosynthesis and colanic acid production (20). This result emphasizes the connection between H2O2-induced tyrosine dephosphorylation and attenuation of pathogenicity determinants.
H2O2-Induced Conversion of Phosphotyrosine to Protein-Bound DOPA.
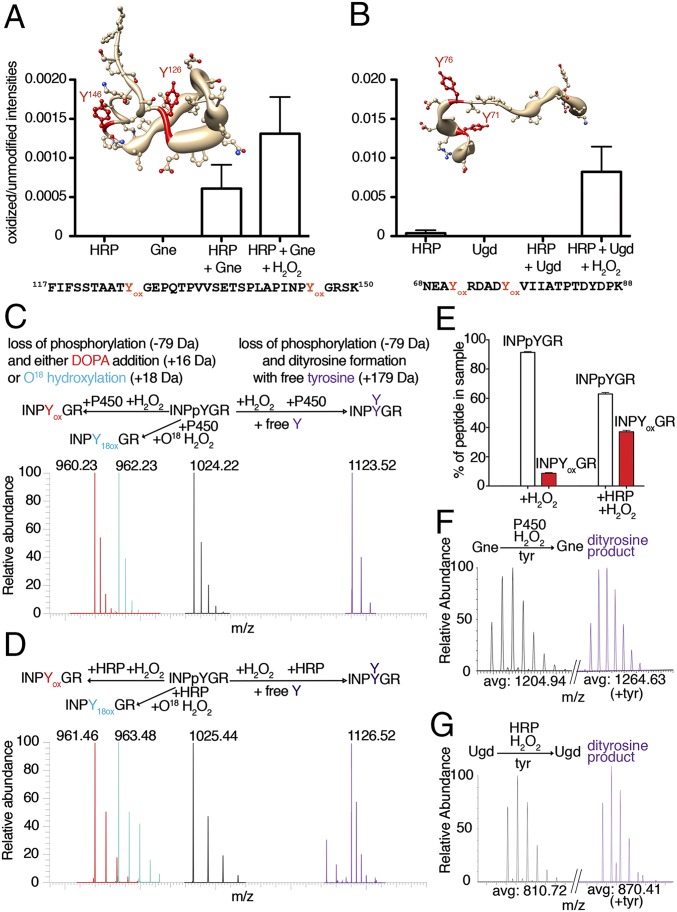
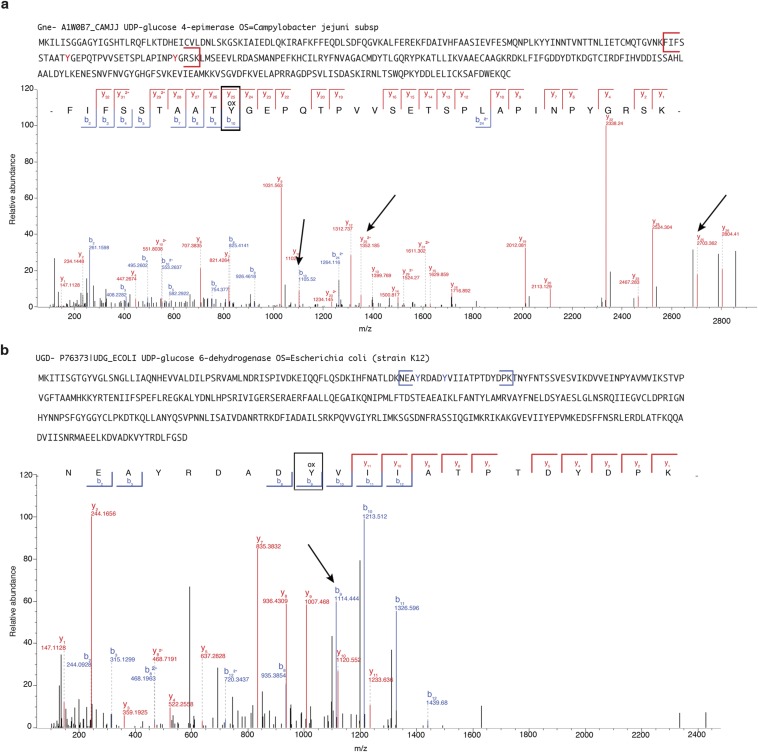
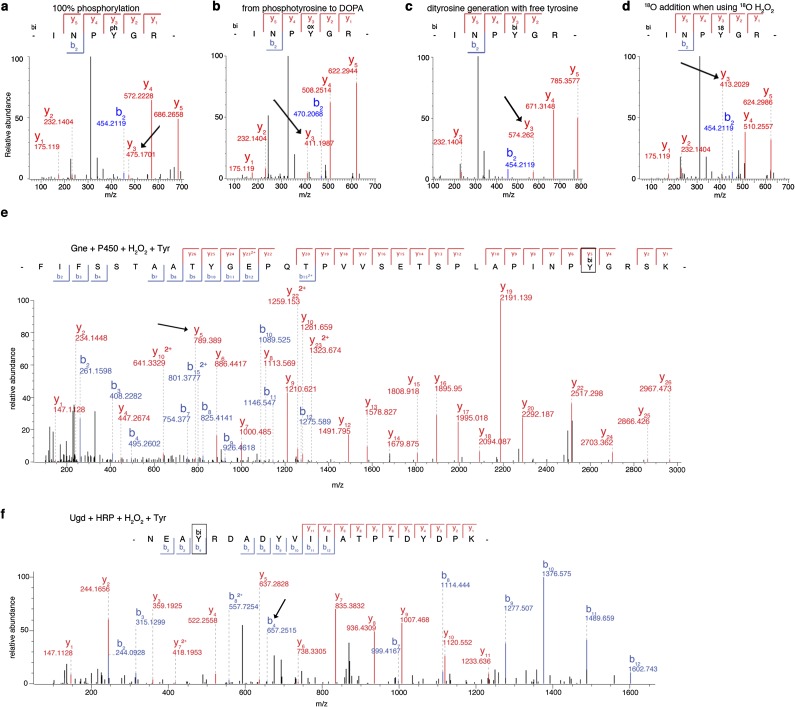
To understand how tyrosine dephosphorylation occurs, we sought to identify the final product of this reaction by MS analysis of peptides derived from recombinant Gne or Ugd after exposure to H2O2 and HRP. In particular, both bacterial proteins contain phosphorylated key tyrosyl residues in their active site (Gne Tyr146) or as cofactor affinity enhancer (Ugd Tyr71) (20, 21), which are important for polysaccharide biosynthesis. Peptides containing these tyrosines were found to be oxidized by exogenous H2O2, leading to •OH-induced DOPA formation (Fig. 4 A and B and Fig. S5). To ensure that the loss of tyrosine phosphorylation did not arise from variations in turnover of the limited pool of p-Tyr proteins, we determined concomitant loss of the p-Tyr signal and DOPA addition by performing analogous experiments using CjP450 (or HRP) together with a synthetic phosphopeptide (100% p-Tyr), comprising the sequence surrounding Gne Tyr146 in the presence of H2O2. The MS/MS spectra confirmed substantial loss of the phosphate group on tyrosine and matching DOPA modification, resulting in a net mass change of 63 Da on modified tyrosines (Fig. 4 C and D and Fig. S6 A–D). Quantification revealed that the increase in DOPA signal in the peptide was accompanied by a decrease in the phosphorylated peptide (Fig. 4E).
Fig. 4.
DOPA modification displaces phosphate via tyrosinyl radical formation. (A and B) Oxidized over unmodified peptide intensities of (A) the Gne peptide (amino acids 117–150) containing Tyr146 and of (B) the Ugd peptide (amino acids 68–88) containing Tyr71 in the MS spectra. Yox indicates DOPA-modified tyrosine. Localization of oxidized tyrosine residues is shown in worm-style with width proportional to residue accessibility and in the peptide sequence. (C and D) MS spectra of a synthetic phosphotyrosine peptide (biotin-INPpYGR) after treatment with 1 mM H2O2 and either (C) CjP450 or (D) HRP. The starting phosphorylated peptide is in black, DOPA in red, dityrosine formation in purple, and 18O oxidation in cyan. The reactions leading to each modification are indicated. (E) Quantification of pY vs. Yox in the synthetic INPpYGR peptide upon exposure to 1 mM H2O2 ± HRP using MS/MS. (F and G) Isotopic peak MS spectra indicating dityrosine formation (purple) for tryptic peptides derived from Gne (F) or Ugd (G) following treatment as indicated.
Fig. S5.
MS/MS analysis of oxidized Gne and Ugd. (A) MS/MS data representative of the C. jejuni Gne (A1W0B7_CAMJJ) peptide (amino acids 117–150). The full sequence of Gne with the peptide containing the active site is also shown in brackets. The peaks corresponding to the DOPA formation are indicated with arrows. (B) MS/MS data representative of the E. coli Ugd peptide (amino acids 68–88). The full sequence of Ugd with the peptide containing the phosphotyrosines that modulate Ugd function is in brackets. The peak corresponding to DOPA formation is indicated with an arrow.
Fig. S6.
MS/MS data of the phosphopeptide and dityrosine formation on Gne or Ugd in the presence of free tyrosine. (A–D) MS/MS data from peptide experiments representative of chromatograms in Fig. 4 C and D. Tyrosyl residue indicated with an arrow. (A) Starting peptide with 100% phosphorylation on tyrosine. (B) DOPA formation on the p-Tyr position in the presence of CjP450/HRP and 1 mM H2O2. Expected net mass shift of −63 Da. (C) Dityrosine formation in the presence of CjP450/HRP, 1 mM H2O2 and free tyrosine in excess. Expected net mass shift +100 Da. (D) 18O addition to tyrosine in the presence of CjP450/HRP and 1 mM 18O H2O2. Expected net mass shift of −61 Da from the starting material or +2 Da from 16O oxidation. (E and F) Representative MS/MS spectra showing mass fingerprint sequencing from protein experiments in Fig. 4 F and G. The position for dityrosine addition is indicated (arrows) on (E) Gne (amino acids 117–150) and (F) Ugd (amino acids 68–88).
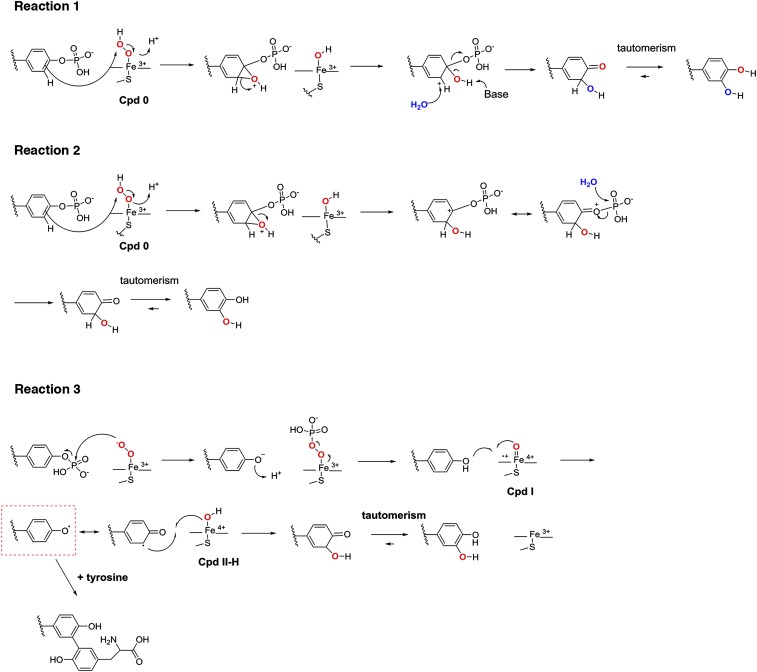
A number of potential chemical mechanisms can be invoked to explain the conversion of p-Tyr to DOPA (Fig. S7). The source for hydroxylation could be water (reaction 1) or H2O2 (reactions 2 and 3). Because H218O2 labeled DOPA (Fig. 4 C and D and Fig. S6D), we ruled out reaction 1. Reaction 2 would proceed via a 2-hydroxy-3,5-cyclohexadienone intermediate, whereas reaction 3 would predict generation of a tyrosinyl radical intermediate (Fig. S7, red box) (22, 23). The addition of free tyrosine to the oxidation reaction will discriminate between these two possibilities, because only a tyrosinyl radical intermediate will permit dityrosine formation. Nano LC-MS/MS analysis of the synthetic phosphopeptide, or of Gne and Ugd treated with H2O2 in the presence of l-tyrosine and CjP450 (or HRP), indicated dityrosine formation at active-site tyrosines with a net mass change of 100 Da (Fig. 4 C, D, F. and G and Fig. S6 C, E, and F). These results support reaction 3 as the mechanism leading to oxidative phosphate removal and DOPA addition on tyrosine. Analysis of the solvent accessibility surface area of modified tyrosines did not provide a clear link between surface exposure, tyrosinyl radical formation, and subsequent DOPA modification (Fig. S8), suggesting that the microenvironment surrounding certain tyrosines leads to specific, nonrandom oxidation events.
Fig. S7.
Putative chemical mechanisms underlying the loss of tyrosine phosphorylation and emergence of DOPA modification in the H2O2-mediated reaction of cytochrome P450 with Gne.
Fig. S8.
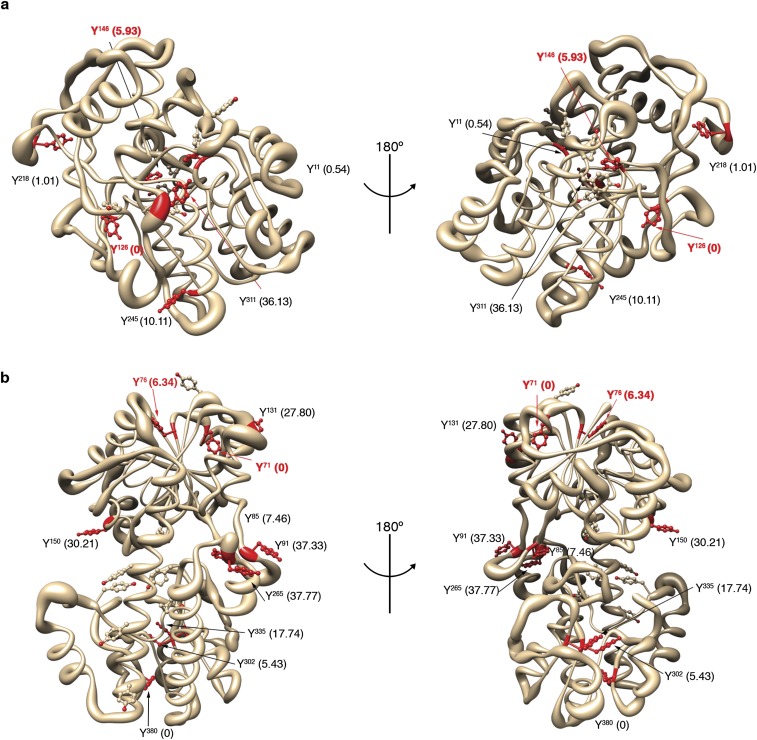
Gne and Ugd 3D models highlighting tyrosyl residues with dityrosine modifications detected in this study. Cartoon showing identified Gne (A) and Ugd (B) tyrosyl residues found to undergo a dityrosine modification in red. Multiple dityrosine formations are possible, although not all sites lead to DOPA modification. Numbers in parenthesis correspond to the solvent accessibility surface area for each dityrosine residue.
K. pneumoniae DOPA Proteomics and Computational Chemistry Approach.
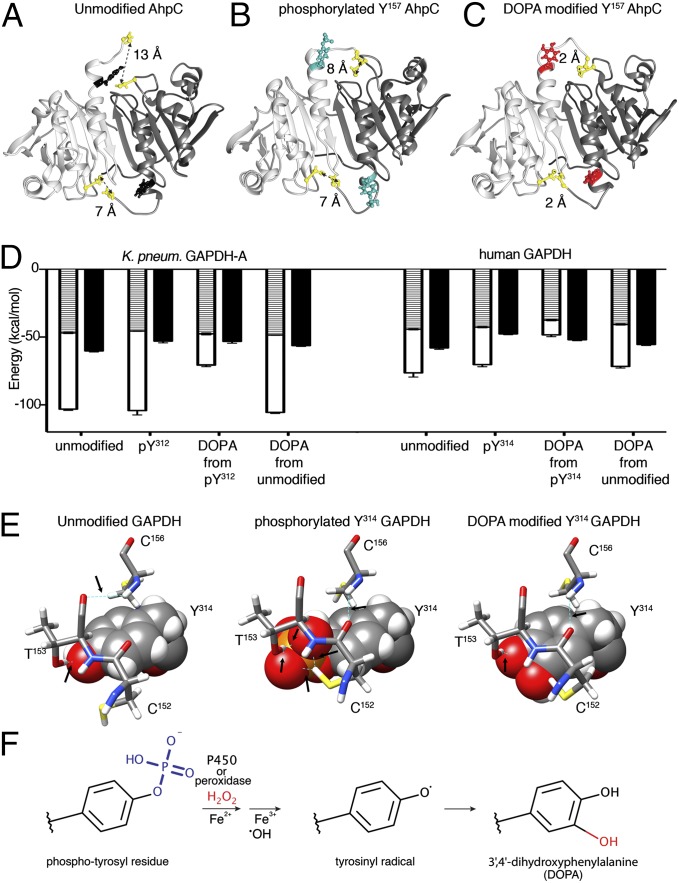
To assess bacterial protein-bound (PB) DOPA modifications globally, K. pneumoniae grown at 5% O2 was exposed to a bolus of H2O2 or, separated by a filter, to 15–20 nmol H2O2/h per milligram of protein continuously released by Cos-NOX4 cells, mimicking a rate of H2O2 release similar to stimulated IECs (24). Without using any enrichment strategy, MS/MS analysis revealed up-regulation of the ROS scavengers superoxide dismutase (SOD, UniProtKB-M7QF58, twofold) and alkyl hydroperoxide reductase [AhpC 1.3-fold, AhpD 180-fold (by H2O2), AhpF twofold (by NOX4)], but not catalase (KatG). The relative abundance of PB-DOPA after treatment was increased (25–35%) over untreated conditions or when exposed to NOX4-deficient Cos-22phox cells. Analysis of DOPA sites (Table S1) revealed that AhpC itself, a thiol-based peroxidase and the primary scavenger of low H2O2 concentrations in E. coli (25), was modified on Tyr157, a residue previously identified as an AhpC phosphorylation site in K. pneumoniae and E. coli (9, 12). Tyr157 is located in the vicinity of the resolving Cys166 that reacts with Cys47 (26). We used molecular dynamics simulation of AhpC in different states to analyze disulfide bond connectivity, which revealed that the backbone regions move considerably closer together in the DOPA-modified AhpC dimer (Fig. 5 A–C), which may affect turnover. Thus, H2O2 cannot only alter tyrosines in the catalytic center of enzymes involved in metabolic and virulence-associated pathways, but also hydroxylates AhpC, the initial antioxidant defense enzyme.
Table S1.
Bacterial peptides with corresponding DOPA and p-Tyr modifications
| Protein | Peptide* | H2O2 | NOX4 | DOPA28 | pTyr9,10 | |
| AhpC | Hydroperoxide reductase subunit C | 154AAQYox VASHPGEVCPAK169 | ☑ | + | + | ☑ |
| gapA | Glyceraldehyde-3-phosphate dehydrogenase | 308LVSWYox DNETGYSNK321 | ☑ | ☑ | ☑ | ☑ |
| Pal | Peptidoglycan-associated outer membrane lipoprotein | 154PAVLGHDEAAYox AK166 | ☑ | ☑ | + | + |
| leuS | Leucine tRNA ligase | 93NNTAPAPWTYDNIAYox MK109 | ☑ | ☑ | + | + |
| fusA | Elongation factor G | 665QSGGRGQYGHVVIDMYox PLEPGSNPK689 | ☑ | + | + | + |
| Lysine decarboxylase | 477NIDNEHMYox LDPIK490 | ☑ | + | + | + | |
| Omp | Outer membrane protein A | 25DNTWYox AGGK33 | ☑ | + | + | + |
| AdhE | Aldehyde-alcohol dehydrogenase | 858QILLDTYYox GREFVEGEAAAK875 | ☑ | ☑ | + | + |
| secA | Protein translocase subunit SecA | 523DMADLVYox MTEAEK534 | ☑ | + | + | + |
| proS | Proline–tRNA ligase | 1MRTSQYox LLSTLK12 | + | ☑ | + | + |
| rfaD | ADP-l-glycero-d-manno-heptose-6-epimerase | 39FVNLVDLNIADYox MDK53 | ☑ | ☑ | + | + |
| pntB | NAD(P) transhydrogenase subunit beta | 425RSMNTGYox AGVQNPLFFK441 | + | ☑ | + | + |
| rplU | 50S Ribosomal protein L21 | 1MYox AVFQSGGK10 | ☑ | ☑ | + | + |
| rplP | 50S Ribosomal protein L16 | 87GNVEYox WVALIQPGK100 | ☑ | ☑ | + | + |
| rplM | 50S Ribosomal protein L13 | 13RDWYox VVDATGK23 | ☑ | ☑ | + | + |
| rpmG | 50S Ribosomal protein L33 | 11LVSSAGTGHFYox TTTK26 | + | + | ☑ | ☑ |
| Uncharacterized protein (KP700603_09168) | 1MYox QHHNWQGALLDYPVSK18 | + | ☑ | + | + | |
| fbaA | Fructose-bisphosphate aldolase class II | 277DSVSYox GVVK285 | + | + | ☑ | ☑ |
| fbaA | Fructose-bisphosphate aldolase class II | 314ANEAYox LQGQLGNPKGEDQPNKK327 | + | + | ☑ | ☑ |
| fumA | Fumarase A = fumarate hydratase class I; aerobic isozyme | 427TPEGYox ASGSLGPTTAGR443 | + | + | ☑ | ☑ |
| groEL | Cpn60 chaperonin GroEL, large subunit of GroESL | 351QQIEEATSDYox DREK364 | + | + | ☑ | ☑ |
| icd | Isocitrate dehydrogenase specific for NADP+ | 388TVTYox DFER395 | + | + | ☑ | ☑ |
| pgk | Phosphoglycerate kinase | 232SLYox EADLVDEAKR244 | + | + | ☑ | ☑ |
| ppa | Inorganic pyrophosphatase | 133AQIAHFFEHYox KDLEK147 | + | + | ☑ | ☑ |
| pykF | Pyruvate kinase | 33LNFSHGDYox AEHGQR46 | + | + | ☑ | ☑ |
| talB | Transaldolase B | 262KLSYox TGEVK270 | + | + | ☑ | ☑ |
| tnaA | Tryptophanase | 84SYox YALAESVK93 | + | + | ☑ | ☑ |
| Tryptophanase/l-cysteine desulfhydrase, PLP-dependent | 89NIFGYox QYTIPTHQGR103 | + | + | ☑ | ☑ | |
| Tryptophanase/l-cysteine desulfhydrase, PLP-dependent | 231FAENAYox FIK239 | + | + | ☑ | ☑ | |
| Tryptophanase/l-cysteine desulfhydrase, PLP-dependent | 256ETYKYox ADMLAMSAK269 | + | + | ☑ | ☑ | |
| yfiD | Stress-induced alternate pyruvate formate-lyase subunit | 36AGYox AEDEVVAVSK48 | + | + | ☑ | ☑ |
Fig. 5.
DOPA modification on oxidative stress-associated K. pneumoniae proteins. (A–C) AhpC 3D model depicting Tyr157 in relation to Cys47 and Cys166 in unmodified (A), p-Tyr157 (B), and DOPA-Tyr157 (C) conformation. Cys–Cys distances indicated in Angströms. (D) Binding energy for NAD+ to GAPDH (K. pneumoniae Tyr312, human Tyr314) in unmodified, phosphorylated, p-Tyr to DOPA, or unmodified to DOPA form. Van der Waals energy (hatched), electrostatic energy (white), and desolvation energy (black). (E) Comparison of unmodified, p-Tyr314, and DOPA-Tyr314 human GAPDH. Tyr314 is shown space filling, side chains are sticks, hydrogen bonds are dashed lines in cyan, indicated with arrows. (F) Schematic representation of the key reactions leading to oxidative dephosphorylation of bacterial tyrosyl residues and conversion to DOPA.
Proteomics data compiled in Table S1 indicate that DOPA addition on particular tyrosine residues, identified by us in K. pneumoniae or reported in E. coli K12 grown at 21% oxygen (27), match p-Tyr modifications (9, 12). A frequently modified enzyme is GAPDH-A (gapA), which is susceptible to DOPA modification on Tyr312, a residue identified also as a phosphorylation site in E. coli (12). GAPDH, a glycolytic enzyme and H2O2 sensor with peroxidatic activity, contributes to colonization and invasion (28). Tyr312 in K. pneumoniae GAPDH (Tyr314 in human GAPDH) represents in unmodified form a critical residue in a proton-shuttle mechanism essential for the H2O2 sensitivity of the enzyme (29). Molecular dynamics simulations indicated that NAD+ binding to phosphorylated Tyr312 lowered the desolvation energy (∼10%), whereas subsequent DOPA modification decreased the electrostatic energy by ∼40% and NAD+ binding by 50% (Fig. 5D). The situation for human GAPDH is similar, although the NADPH/NADP+ ratio for K. pneumoniae GAPDH would be higher. The direct conversion from unmodified Tyr312 to DOPA-Tyr312, a process unlikely to occur in physiological conditions, would cause less structural change than a DOPA conversion from a phosphorylated residue (Fig. 5D). In contrast to the increased resistance of a human GAPDH Y314F mutant to oxidative inactivation (29), conversion of p-Tyr314 to DOPA will be comparable to exposure of wild-type GAPDH to 200 µM H2O2 (29) and will not alter adaptation to oxidative stress. A subset of p-Tyr314 modified GAPDH might be present at any given time in mammalian tissues (30). Phosphorylated GAPDH will not require the proton relay for H2O2 sensitivity because the space for H2O2 interaction with Cys152 will be occupied by the phosphate group in p-Tyr314 and the creation of two hydrogen bonds between Cys152 and p-Tyr314 (Fig. 5E). Oxidative dephosphorylation and DOPA addition on Tyr314 will then alter NAD+ affinity. These examples demonstrate that oxidative dephosphorylation and subsequent DOPA addition act as regulator of enzyme function, and that the outcome will differ depending on the structural and functional context. Improved techniques for detecting and stabilizing tyrosine modifications will permit discovering many more candidates for p-Tyr conversion, particularly in oxidative stress conditions.
Discussion
Phosphorylation-driven signaling networks are crucial for maintaining functional responses in all organisms. Similarly conserved is the release of diffusible mediators, in particular H2O2, for protection and adaptation. In mammalian systems thiol oxidation is a potent pathway modifier, often accelerating or causing disease when deregulated. In bacteria exposure to H2O2 concentrations in the nano to submicromolar range has been associated with oxidation of reactive cysteinyl residues and [4Fe-4S]2+ clusters. Damaging irreversible modifications, such as methionine oxidation and 3-chlorotyrosine formation, were connected to the myeloperoxidase–H2O2–chloride axis and bacterial killing in the phagosome of neutrophils (5). Using a range of conditions, including host–pathogen interaction models, constant exposure of bacteria to nanomolar H2O2 that mimics host epithelial H2O2 release during infection, or to a single bolus of H2O2, we report here disruption of p-Tyr signaling by oxidative p-Tyr conversion to DOPA (Fig. 5F).
Tyrosines in the vicinity of lysines, in particular in YXX(XX)K or KXXX(X)Y motifs, seem prone to hydroxylation or chlorination (27, 31), and are also preferred phosphorylation sites in E. coli proteins (12). These motifs are present in the DOPA-modified bacterial proteins Gne, Ugd, AhpC, and GAPDH-A featured in this study. The nearby positive charge may favor the deprotonated form of tyrosine, facilitating phosphorylation and oxidative electron transfer. The presence of DOPA on proteins involved in ROS conversion [AhpC, SOD (27)] or in redox-sensitive processes (GAPDH-A), and the predicted structural change by DOPA incorporation pose the question of how their activity will be altered by Tyr hydroxylation. Redox-active PB-DOPA can generate radicals leading to enhanced oxidative damage, but has also been linked to peroxyl radical scavenging and antioxidant defense. The majority of bacterial DOPA-modified proteins identified to date are involved in carbohydrate metabolism (present study and ref. 27), which constitutes also a preferred target for BY-kinase–mediated Tyr phosphorylation and a prerequisite for virulence determinants such as capsule and biofilm formation.
Besides H2O2, the availability of intrabacterial iron, peroxidase activity (e.g., via compound I) and a low oxygen environment were required for p-Tyr conversion to DOPA. The intestinal niche is largely devoid of oxygen except for a 70-μm oxygenation zone maintained by capillary diffusion (13). Oxygen measurements by electron paramagnetic resonance or phosphorescence oximetry detected luminal PO2 levels of 1–40 mmHg (≤5% O2), depending on the section of the intestinal tract (32). Albeit lung PO2 levels are much higher, in pulmonary infection—and in particular in cystic fibrosis—anaerobic bacteria are frequently present, indicating a low oxygen environment. Propagation of bacteria in 21% oxygen does not reflect the in vivo situation for many mucosal pathogens. During oxidative stress modification of tyrosine residues to DOPA seems to occur frequently. PB-DOPA modifications were prominently associated with mitochondrial proteins in heart and brain tissues (33). The presence of H2O2 and transition metals in mitochondria creates an ideal environment for DOPA additions, and modified proteins included SOD2, cytochrome c, and ATPases (27, 33). Other DOPA-modified proteins were connected to oxidative stress pathways (NRF2, HSP90), the cytoskeletal regulatory network, or are multifunctional adapters (14-3-3 family) (33). Many of the modified Tyr residues in these proteins are predicted phosphorylation sites. Our study connects oxidative stress to interference with p-Tyr signaling by directly converting tyrosine residues that are associated with the catalytic activity of enzymes. Oxidative stress may likely trigger additional modifications throughout the protein, for example methionine oxidations. Tyrosine phosphorylation promotes not only signaling responses, but also affects protein localization and protein–protein interactions, and it is expected that many DOPA modifications caused by oxidative stress or mitochondrial dysfunction will disrupt protein networks, thereby altering numerous biological processes.
Materials and Methods
In vitro organ culture of human colon biopsies was performed as described previously (9). For infection studies and fetal lung explant studies, Sprague–Dawley rats and ligated rabbit ileal loops from Chinchilla rabbits were used. Ethical approval by the respective overseeing bodies was obtained. Fully informed consent was obtained from parents and, where appropriate, children. Ethical permission was obtained from the Ethics Committee of Our Lady’s Childrens’ Hospital Crumlin, Ireland. All animal procedures were performed after ethical approval from the Royal College of Surgeons in Ireland and licensed by the Department of Health and Children, Ireland or under the supervision of the Romanian National Sanitary Veterinary Agency (Law 471/2002, Government ordinance 37/2002) with approval of the Ethics Committee of Banat’s University of Agricultural Sciences and Veterinary Medicine, King Michael I of Romania, Timisoara. Mass spectrometry, structural analysis, and graphical representations are described in SI Materials and Methods. Three-dimensional models of K. pneumoniae GAPDH-A (B5XS72_KLEP3) and K. pneumoniae AhpC (M7PUV9_KLEPN) were generated using the homology modeling program Modeler 9v14; molecular dynamics simulation and docking were performed with HADDOCK and evaluated in University of California, San Francisco Chimera. Detailed procedures and biochemical/microbiological methods are described in SI Materials and Methods.
SI Materials and Methods
Bacteria, Cell Lines, and Culture Conditions.
The following bacterial strains were used: Campylobacter jejuni 81-176 was cultured on Mueller–Hinton Agar, then subcultured in biphasic media containing RPMI 1640 supplemented with 3% (vol/vol) FBS for infection studies. Listeria monocytogenes EGDe (gift from P. Cossart, Department of Cell Biology and Infection, INSERM U604, Paris) was cultured on Brain Heart Infusion media, Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, and Klebsiella pneumoniae subsp. pneumoniae ATCC 700603 was cultured on Luria-Bertani (LB) media; if not indicated otherwise, all bacterial strains were grown at 37 °C in microaerophilic conditions (5% CO2, 5% O2, 90% N2), approximately 40 mmHg. Streptococcus pneumoniae ATCC 6305 was grown on Todd Hewitt media at 37 °C overnight, then subcultured 1:10 (vol/vol) in fresh media and grown until OD600 = 0.2 to ensure bacteria were in the exponential phase to prevent endogenous H2O2 production. HCT-8 cells (ATCC CCL-244) were maintained in RPMI 1640 with 10% (vol/vol) FBS. A rat alveolar macrophage cell line ATCC CRL-2192, kindly provided by J. Baugh, Conway Institute, University College Dublin, Dublin, was grown in Ham’s F-12K media supplemented with 15% (vol/vol) FBS. Cells were grown at 21% O2, 5% CO2, and transferred to microaerophilic conditions for infection 3 h before the experiment. All treatments were performed at microaerophilic conditions if not stated otherwise.
Microbiological Methods.
Invasion and adhesion assays were performed as previously reported (8). Bacterial cell viability was assessed using the XTT [2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide] assay (Life Technologies). For all in vitro experiments, bacteria were exposed at time 0 once to 0.7 mM H2O2 in media, except for C. jejuni (5 mM H2O2), and analyzed after the indicated time periods (maximum up to 3 h, except for C. jejuni up to 8 h). A bolus of 0.5 mM H2O2 to Escherichia coli in LB medium will result in an initial concentration of less than 3 μM H2O2 inside E. coli with a complete extracellular H2O2 clearance in 1.5–2 h (10, 11).
Molecular Biology.
Gene deletion and reconstitution for cytochrome P450 encoded by cjj81176_1410 (C. jejuni 81–176, National Center for Biotechnology Information: YP_001001066.1) has been described previously (16). Mutants were generated by site-directed mutagenesis using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). The PRY107 plasmid (KanR) was used as template followed by natural transformation of C. jejuni 81-178 ΔP450 strain to give C. jejuni 81-176 ΔP450::P450C339F strain. The oligonucleotides used were C339FF (gtaggggaaagaatttgtatagga) and C339FR (catcccctttcttaaacatatcct). Mutants were generated by site-directed mutagenesis using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). Gne epimerase (cjj81176_1148) was amplified by PCR with primers GneRhaF (catcatcaccaccatcacaaaattcttattagcggtggt) and GneRhaR (gtggcggccgctctattaacactgtttttcccaatcaaaacg) using Velocity DNA polymerase (MyBio). CetB (cjj81176_1204) was amplified using primers CetBRhaF (catcatcaccaccatcacatgtcaagagaaatttttttacaa) and CetBRhaR (gtggcggccgctctattatttagcttcttgaagaga). Chloramphenicol (20 µg/mL) and kanamycin (50 µg/mL) were used for selection. For expression of His-tagged CetB, the Expresso Rhamnose Cloning and Protein Expression Kit (Lucigen) was used following the manufacturer’s instructions. His-tagged Gne and Ugd (Ugd kindly provided by C. Whitfield, Department of Molecular Cellular Biology, University of Guelph, Guelph, Ontario, Canada) were cloned into a pet27b+ vector and transformed into BL21Star(DE3) CE1535 strain (kindly provided by M. Baldus, Bijvoet Center for Biomolecular Research, Utrecht University, Utrecht, The Netherlands) (34).
Protein Purification.
E. coli cultures were grown in LB broth containing 30 μg/mL kanamycin at 36 °C to OD600 = 0.5 before harvesting. Either l-rhamnose or isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture in a final concentration of 0.2% (wt/vol) and E. coli was grown for additional 6 or 18 h at 36 °C. E. coli lysates containing 50 mM Hepes pH 7.5, 0 or 25 mM imidazole, and EDTA-free protease inhibitors mixture were loaded onto a HisTrap FF column or complete His-Tag column, washed with 0 or 25 mM imidazole and eluted with 250–500 mM imidazole on an AKTApurifier. Collected samples were desalted and concentrated on Centricon 10 kDa membranes. C. jejuni cytochrome P450 purification was described elsewhere (16). Protein identity and purity were confirmed by Coomassie blue staining and immunoblotting. Bacterial proteins expressed and isolated from E. coli were always purified in tyrosine phosphorylated form, albeit the extent of p-Tyr was not quantified.
Coculture of Bacteria with Cells in Normal or Iron-Modified Conditions.
Bacteria in RPMI 1640 containing 3% (vol/vol) FBS (OD600 = 0.4; 107 bacteria, 1 mL) were incubated with HCT-8 cells at 37 °C in microaerophilic conditions with a multiplicity of infection of 50. HCT-8 cells were either pretreated with DPI for 20 min (25 µM) followed by washout or catalase was added. Bacteria were not exposed to DPI except when indicated. Nonadherent, extracellular bacteria were removed by centrifugation of media (3,300 × g, 5 min), and were used for immunoblotting. Viable counts were performed for inocula to ensure that comparable numbers of live bacteria were present for each bacterial strain. Exposure of bacteria to H2O2 released by Cos cells stably expressing the constitutively active NOX4-p22phox complex or as negative control Cos cells expressing only p22phox was performed using Boyden chambers. Cells in DMEM, 10% (vol/vol) FBS medium were seeded into the bottom chamber 24 h before start of the experiment and then moved to microaerophilic conditions for 3 h in fresh DMEM, 3% (vol/vol) FBS. Bacteria grown in microaerophilic conditions were resuspended in 5% O2 conditioned DMEM with 3% (vol/vol) FBS, placed on top of the filter (3-µm pore size) and incubated for 3 h. Bacteria were harvested from the filter for analysis.
Quantification of Protein Phosphotyrosine Levels in Modified Iron Conditions.
C. jejuni 81-176, L. monocytogenes EGDe and K. pneumoniae were grown microaerophilic in LB medium, diluted into iron-free minimal essential media 1:20, and grown to midlog phase (OD600 = 0.2–0.3). Bacteria were collected by centrifugation (3000 × g, 10 min) and diluted to OD600 = 0.2 in MEM. Iron (II) sulfate (40 μM) was added as indicated and cultures were grown microaerophilic until OD600 = 0.5 was reached. To C. jejuni 81–176 cultures, 5 mM H2O2 was added and the growth continued microaerophilic for 8 h. L. monocytogenes and K. pneumoniae cultures were exposed to 0.7 mM H2O2 for 3 h in microaerophilic conditions. Bacteria were then diluted to OD600 = 0.2, collected by centrifugation, washed twice with 25 mM Tris⋅HCl, resuspended in 30 μL Laemmli buffer (Sigma), and heated for 5 min at 95 °C. Boiled samples were loaded on 10% SDS/PAGE gels, separated by electrophoresis, and immunoblotted. The control samples followed an identical protocol without supplemental iron or added H2O2. Equal protein loading of bacterial lysates and similar protein expression patterns were assessed for every treatment modality by Coomassie blue staining.
Ex Vivo Analysis.
Polarized in vitro organ culture.
Polarized in vitro organ culture (pIVOC) experiments of colon biopsies were performed as described previously (8). Bacterial infections were conducted using microaerophilic preconditioned media or buffers. L. monocytogenes was added at a final OD600 = 0.2 to the apical side of biopsies and H2O2 release was measured at different time points as described in Biochemical Methods, below. Controls were either noninfected biopsies or biopsies pretreated with 20 µM DPI for 20 min with washout before addition of C. jejuni or L. monocytogenes. Fully informed consent was obtained from parents and, where appropriate, children. Ethical permission was obtained from the Ethics Committee of Our Lady’s Childrens’ Hospital Crumlin, Ireland.
Rat lung cultures.
Sprague–Dawley rats (Harlan Laboratories) were kept in specific pathogen-free conditions. Timed mating was performed and fetuses were delivered via caesarean section and killed by decapitation on embryonic day 21.5. Whole fetal lungs were dissected under sterile conditions. The harvested lungs were kept in ice-cold DMEM/F12 media for maximal 1 h. The lungs were then washed twice in HBSS Ca2+ Mg2+ before being transferred to a transwell insert containing warm DMEM/F12 with 3% (vol/vol) FBS, followed by incubation in 5% CO2, 21% O2 at 37 °C overnight. Next morning bacteria grown at 5% O2 were introduced into the lungs under a dissecting microscope by using a Teflon tip G25 cannula inserted into the intact trachea. After 2 and 4 h of incubation, the bronchoalveolar fluid was recovered and centrifuged. The supernatant was used for determination of H2O2. After H2O2 measurement recovered bacteria were lysed and analyzed by immunoblotting. All animal procedures were performed after ethical approval from the Royal College of Surgeons in Ireland and licensed by the Department of Health and Children, Ireland.
In Vivo Analysis.
Ligated rabbit ileal loops.
Female rabbits (Chinchilla breed) were starved for 2 h before infection. Anesthesia was performed intravenously with ketamine (35 mg/kg) and xylazine (5 mg/kg). The incision line was injected subcutaneously with 2 mL xylazine 1%. After laparotomy four ileal loops (5 cm in length) were isolated and ligated. The loops were injected with either PBS, C. jejuni 81-176, or L. monocytogenes EDGe using 1 mL of culture at OD600 = 0.3 in PBS (pH 7.4). For each microorganism, three rabbits were used. After closure of the abdomen rabbits were placed in cages for 180–360 min (C. jejuni) or 90–180 min (L. monocytogenes). Rabbits were killed by intravenous injection of sodium pentobarbital (120 mg/kg). Fluid accumulated in each loop was collected separately and spun at 120 × g for 5 min to remove debris, followed by centrifugation at 3000 × g to collect bacteria. Subsequently, the segments were fixed in Carnoy reagent [60% ethanol/30% chloroform/10% glacial acetic acid (vol/vol/vol)] for 24 h at 4 °C. The experiments were performed under the supervision of the Romanian National Sanitary Veterinary Agency (Law 471/2002, Government ordinance 37/2002) with approval of the Ethics Committee of Banat’s University of Agricultural Sciences and Veterinary Medicine, King Michael I of Romania, Timisoara, Romania.
General Materials.
The following antibodies were used: antiphosphotyrosine (sc-7020, Santa Cruz); anti-His antibody (sc-53073, Santa Cruz); anti-Campylobacter antibody (sc-58100, Santa Cruz) and anti-Campylobacter serum (8); anti-p22phox (mAb 449, gift from D. Roos, Blood Cell Research, Sanquin Research, Amsterdam); anti-gp91phox (NOX2; BD Biosciences); anti-Campylobacter P450 (16); anti-wzc antibody (gift from C. Grangeasse, Molecular Microbiology and Structural Biochemistry, CNRS-UCBL-UMR 5086, Lyon, France); Difco Listeria O Antisera types 1, 4, and Poly (BD Biosciences); DPI (15–25 µM, 15–30 min preincubation followed by PBS wash), bovine liver catalase (300 U/mL); HRP type II (P8250 Sigma; 400 U/mL); sodium dithionite (used in excess); l-tyrosine (47 µM for Gne and 174 µM for Ugd experiments according to Tyr content).
Biochemical Methods.
H2O2 production was measured using Amplex UltraRed/HRP (Life Sciences) without sodium azide addition. Accumulation of fluorescent product (RFU) was measured at indicated time points, H2O2 was determined via a standard curve obtained in the same conditions, and values were adjusted to the protein content of the sample (IEC, biopsies). Values are expressed as nanomole of H2O2 per hour per milligram of protein. Catalase and DPI were used as controls for H2O2 generation and flavoenzyme involvement, respectively. Cos-NOX4 cells were used as continuous physiological H2O2 generating system that will not necessitate contact between bacteria and cells (8). For Cos-NOX4 cells H2O2 was measured on top of the Boyden chamber filter (location of bacteria). For the pIVOC system the Amplex UltraRed/HRP solution was mixed at every time point 1:1 with media (50 μL of 500 μL total) collected at the apical side of the pIVOC followed by fluorescence readings (λex = 550 nm; λem = 580 nm) on a Synergy Mx fluorimeter with measurements performed 60, 120, and 180 min after the addition of bacteria. Noninfected control tissues were set as baseline and fluorescence was plotted as percent change over baseline.
Immunoblotting, 2D electrophoresis, and the corresponding MS/MS analysis were performed as described previously (8). For in vitro assays, recombinant tyrosine phosphorylated proteins were treated with 1 mM H2O2 for 1 h at 37 °C in Tris pH7 buffer in a 1:20 enzyme to substrate ratio (HRP/cjP450:Ugd/Gne) before EndoLysC digestion. A biotinylated, 100% phosphotyrosine containing synthetic peptide (biotin-INPpYGR; Biomatik) at 95% purity was used for MS/MS analysis. 0.5 mM peptide was bound to agarose streptavidin beads (Millipore) before exposure to 1 mM H2O2 and 5 U/mL of HRP, or 3 µL of purified cjP450 in a 60-µL reaction for 1 h at 37°. For the dityrosine experiment with free tyrosine in P450 reactions the peptide was not bound to streptavidin beads. A commercial iron determination assay (ABCAM ab83366) was used to quantify the intracellular ratio of Fe2+ vs. Fe3+ in bacteria. For carbonyl detection in bacterial protein extracts an assay kit (ABCAM ab126287) was used.
MS, Structural Analysis, and Graphic Representations.
MS analysis was performed as described in ref. 35. Protein samples were digested with EndoLysC, and synthetic peptides were separated from streptavidin beads and directly analyzed in MS. Proteolytic or synthesized peptides were analyzed on a Q-Exactive mass spectrometer connected to an Ultimate Ultra3000 chromatography system incorporating an autosampler (both Thermo Scientific). Proteolytic or synthesized peptides for each sample (5 μL) were applied to a homemade column (100-mm length, 75-μm inside diameter) (packed with 1.9 μm ReprosilAQ C18 by Dr. Maisch) and separated using either a 40-min (for purified proteins and peptides) or 240-min (for whole-proteome samples) reverse-phase acetonitrile gradient [3–32% (vol/vol) acetonitrile] with a 250-nL/min flow rate. The mass spectrometer was operated in positive ion mode with a capillary temperature of 220 °C and a 2000 V potential applied to the column. The intensity ratios of the modified peptides over the base peptides was used for normalization and expressed as fold increase over the untreated samples.
C. jejuni 81-176 Gne UDP-glucose 4-epimerase (A1W0B7) and E. coli Ugd glucose 6-dehydrogenase (P76373) were modeled using SWISS-MODEL workspace with UDP-galactose-4-epimerase from Aspergillus nidulans (PDB ID code 4lis.1) and UDP-glucose dehydrogenase from K. pneumoniae (PDB ID code 3pjg.1) as templates. University of California, San Francisco (UCSF) Chimera was used for protein visualization. The MS/MS package in UCSF Chimera was used for the solvent-accessibility surface area calculations and the render by attribute is in Worm style (https://www.cgl.ucsf.edu/chimera/docs/ContributedSoftware/render/render.html#worms). Chemical reactions were drawn with the sketch module in Marvin Beans v15.7.13.0, (https://www.chemaxon.com). For Fig. 4 C and D, chromatograms of the two charged peptides were used for the indicated experiments and combined in a single graph. For easier calculation of mass changes, the peaks were labeled with the one charged equivalent mass in the figure.
Molecular Dynamics Simulation and Docking.
Three-dimensional models of K. pneumoniae GAPDH-A (B5XS72_KLEP3) were generated using the homology modeling program Modeler 9v14 (www.salilab.org/modeller/) with structures of human or E. coli GAPDH serving as initial template (PDB ID codes 1U8F and 1S7C, respectively). An AhpC structure from E. coli (PDB ID code 4QL7) was used for K. pneumoniae AhpC (M7PUV9_KLEPN). Putative structural changes caused by phosphorylation and subsequent DOPA modification on Tyr157 in AhpC (K. pneumoniae) or on Tyr312 in GAPDH-A (K. pneumoniae) and on Tyr314 in human GAPDH, respectively, were simulated using a NAMD 2.11 program (36) (www.ks.uiuc.edu/Research/namd/) plug-in in VMD v1.9.2 (www.ks.uiuc.edu/Research/vmd/). For AhpC simulation the dimer was used as a starting unit, all disulfide bridges were removed, all atoms were used, and all movements were followed for 20 ps. In the AhpC dimer both Tyr157 residues were either unmodified, phosphorylated, or DOPA-modified. In each GAPDH simulation the monomer was used as a starting unit containing cofactor (NAD+) and water molecules during the calculations, and all movements were followed for 200 ps. To investigate the influence of GAPDH p-Tyr312 or DOPA-Tyr312 modification on enzymatic activity via NAD+ binding affinity, the docking runs were performed with HADDOCK (37, 38). Docking was performed with default parameters using the Web server version of HADDOCK with a refinement interface. To gain the Van der Waals, electrostatic, and desolvation energy for each enzyme, HADDOCK automatically performed the molecular dynamics before and after each docking trial by including water into the calculation. All structures were superimposed and differences were evaluated in UCSF Chimera.
Immunofluorescence and Immunohistochemistry.
Immunofluorescence of cells or tissues was performed with a Zeiss LSM 700 confocal microscope with a Plan-Apochromat 63×/1.40 Oil DIC M27 objective as described previously (8), using either TAMRA-labeled bacteria or bacteria-specific antibodies. To facilitate 3D data visualization, Fiji was used to create a maximum-intensity projection from the z-stacks. The first view from the 36 angles in projection is shown. Fixed rabbit tissue samples were processed, embedded in paraffin, and cut into 5-µm sections by microtome. Sections were deparaffinized with xylene (10 min) and hydrated using an ethanol gradient [100% 6 min, 95% 6 min, 70% 3 min (vol/vol)] to distilled water (3 min). Samples were stained with hematoxylin (7 min) and eosin (3 min). The stained slides were dehydrated [70% Industrial Methylated Spirit (IMS) 1 min, 95% IMS 2 min, 100% IMS 2 min (vol/vol)], cleared in xylene, and mounted in DPX (Distrene 80, dibutyl phthalate, xylene). Slides were analyzed using a Leica DFC300× camera and the IM50 imaging software. For detection of tissue protein carbonylation after infection, the OxyIHC Oxidative Stress Detection Kit (S7450 Millipore) was used.
Biofilm Assay.
Biofilm was analyzed using SYTO9 (Life Sciences) as described by Oh and Jeon (39). Cultured bacteria were grown in microaerophilic conditions overnight. The bacterial culture was then adjusted to OD600 = 0.1 and 1 mL of this solution was added to an optical grade plastic bottom 24-well microplate. Bacteria were incubated for an additional 72 h in microaerophilic conditions. Bacteria were washed gently with autoclaved water to remove planktonic cells and fixed with 3% (vol/vol) paraformaldehyde in PBS for 1 h. Images were then analyzed for total intensity of SYTO9 fluorescence over the imaged volumes using Fiji. Briefly, after staining with SYTO9, 50 independent 13.2-µm stacks of 60 images at a 0.22-µm Z resolution were imaged for all conditions. The mean fluorescence of the different slices in the stacks was measured and a SD was computed from the aggregated data per slice. The mean fluorescence with the corresponding SDs per slice was then plotted as a function of depth with the start of the stack set to zero.
Statistical Analysis.
Data are expressed as mean ± SEM, n = 3, or as indicated. Statistical differences between means were determined by a two-tailed unpaired Student’s t test. In the figures, ***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05.
Acknowledgments
We thank N. Corcionivoschi for his contributions; K. O’Neill and C. King for technical assistance; C. Whitfield, P. Cossart, M. Baldus, C. Grangeasse, and J. Baugh for providing reagents; P. Nagy for discussions; and the staff and patients at the National Referral Center for Pediatric Gastroenterology, Our Lady’s Children’s Hospital Crumlin, Dublin, Ireland. The work was supported by the National Children Research Centre K/12/1 (to B.B. and U.G.K.); Science Foundation Ireland 10/IN.1/B2988 (to U.G.K.); and the COST Action BM1203 (to U.G.K. and L.A.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605443113/-/DCSupplemental.
References
- 1.Cameron EA, Sperandio V. Frenemies: Signaling and nutritional integration in pathogen-microbiota-host interactions. Cell Host Microbe. 2015;18(3):275–284. doi: 10.1016/j.chom.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamada N, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(6086):1325–1329. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat Rev Microbiol. 2013;11(7):443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 5.Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2013;18(6):642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 6.Wright CJ, et al. Characterization of a bacterial tyrosine kinase in Porphyromonas gingivalis involved in polymicrobial synergy. MicrobiologyOpen. 2014;3(3):383–394. doi: 10.1002/mbo3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao JD, Wong D, Av-Gay Y. Microbial protein-tyrosine kinases. J Biol Chem. 2014;289(14):9463–9472. doi: 10.1074/jbc.R113.520015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcionivoschi N, et al. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe. 2012;12(1):47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin MH, et al. Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol Cell Proteomics. 2009;8(12):2613–2623. doi: 10.1074/mcp.M900276-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancini S, Imlay JA. The induction of two biosynthetic enzymes helps Escherichia coli sustain heme synthesis and activate catalase during hydrogen peroxide stress. Mol Microbiol. 2015;96(4):744–763. doi: 10.1111/mmi.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilan DS, et al. HyPer-3: A genetically encoded H(2)O(2) probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem Biol. 2013;8(3):535–542. doi: 10.1021/cb300625g. [DOI] [PubMed] [Google Scholar]
- 12.Hansen AM, et al. The Escherichia coli phosphotyrosine proteome relates to core pathways and virulence. PLoS Pathog. 2013;9(6):e1003403. doi: 10.1371/journal.ppat.1003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marteyn B, Scorza FB, Sansonetti PJ, Tang C. Breathing life into pathogens: The influence of oxygen on bacterial virulence and host responses in the gastrointestinal tract. Cell Microbiol. 2011;13(2):171–176. doi: 10.1111/j.1462-5822.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 14.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14(6):683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Guengerich FP, Munro AW. Unusual cytochrome p450 enzymes and reactions. J Biol Chem. 2013;288(24):17065–17073. doi: 10.1074/jbc.R113.462275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez LA, et al. Cj1411c encodes for a cytochrome P450 involved in Campylobacter jejuni 81-176 pathogenicity. PLoS One. 2013;8(9):e75534. doi: 10.1371/journal.pone.0075534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad B, Mah DJ, Lewis AR, Plettner E. Water oxidation by a cytochrome p450: Mechanism and function of the reaction. PLoS One. 2013;8(4):e61897. doi: 10.1371/journal.pone.0061897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuter M, van Vliet AH. Signal balancing by the CetABC and CetZ chemoreceptors controls energy taxis in Campylobacter jejuni. PLoS One. 2013;8(1):e54390. doi: 10.1371/journal.pone.0054390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, et al. Evolution of bacterial protein-tyrosine kinases and their relaxed specificity toward substrates. Genome Biol Evol. 2014;6(4):800–817. doi: 10.1093/gbe/evu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacour S, Bechet E, Cozzone AJ, Mijakovic I, Grangeasse C. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS One. 2008;3(8):e3053. doi: 10.1371/journal.pone.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YY, Ko TP, Lin CH, Chen WH, Wang AH. Conformational change upon product binding to Klebsiella pneumoniae UDP-glucose dehydrogenase: A possible inhibition mechanism for the key enzyme in polymyxin resistance. J Struct Biol. 2011;175(3):300–310. doi: 10.1016/j.jsb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Houée-Lévin C, et al. Exploring oxidative modifications of tyrosine: An update on mechanisms of formation, advances in analysis and biological consequences. Free Radic Res. 2015;49(4):347–373. doi: 10.3109/10715762.2015.1007968. [DOI] [PubMed] [Google Scholar]
- 23.Nagy P, Lechte TP, Das AB, Winterbourn CC. Conjugation of glutathione to oxidized tyrosine residues in peptides and proteins. J Biol Chem. 2012;287(31):26068–26076. doi: 10.1074/jbc.M112.371690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S, Imlay J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch Biochem Biophys. 2012;525(2):145–160. doi: 10.1016/j.abb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsonage D, Karplus PA, Poole LB. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci USA. 2008;105(24):8209–8214. doi: 10.1073/pnas.0708308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, et al. The first global screening of protein substrates bearing protein-bound 3,4-Dihydroxyphenylalanine in Escherichia coli and human mitochondria. J Proteome Res. 2010;9(11):5705–5714. doi: 10.1021/pr1005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pancholi V, Chhatwal GS. Housekeeping enzymes as virulence factors for pathogens. Int J Med Microbiol. 2003;293(6):391–401. doi: 10.1078/1438-4221-00283. [DOI] [PubMed] [Google Scholar]
- 29.Peralta D, et al. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol. 2015;11(2):156–163. doi: 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 30.Guo A, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci USA. 2008;105(2):692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergt C, Fu X, Huq NP, Kao J, Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2004;279(9):7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 32.Albenberg L, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, et al. Endogenous 3,4-dihydroxyphenylalanine and dopaquinone modifications on protein tyrosine: Links to mitochondrially derived oxidative stress via hydroxyl radical. Mol Cell Proteomics. 2010;9(6):1199–1208. doi: 10.1074/mcp.M900321-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renault M, et al. Cellular solid-state nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 2012;109(13):4863–4868. doi: 10.1073/pnas.1116478109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turriziani B, et al. On-beads digestion in conjunction with data-dependent mass spectrometry: A shortcut to quantitative and dynamic interaction proteomics. Biology (Basel) 2014;3(2):320–332. doi: 10.3390/biology3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5(5):883–897. doi: 10.1038/nprot.2010.32. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk AD, Bonvin AM. Solvated docking: Introducing water into the modelling of biomolecular complexes. Bioinformatics. 2006;22(19):2340–2347. doi: 10.1093/bioinformatics/btl395. [DOI] [PubMed] [Google Scholar]
- 39.Oh E, Jeon B. Role of alkyl hydroperoxide reductase (AhpC) in the biofilm formation of Campylobacter jejuni. PLoS One. 2014;9(1):e87312. doi: 10.1371/journal.pone.0087312. [DOI] [PMC free article] [PubMed] [Google Scholar]