Significance
The photoreceptor phototropin mediates various blue light-induced responses, including phototropism, chloroplast movement, stomatal opening, and leaf flattening. Two BTB/POZ proteins, NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) and ROOT PHOTOTROPISM 2 (RPT2), were identified as early signaling components in phototropin-mediated phototropism and leaf flattening, and a phototropin substrate, BLUE LIGHT SIGNALING1 kinase, specifically mediates the phototropin-mediated stomatal opening. However, early signaling components in the chloroplast movement remain to be determined. We found that RPT2 and the NPH3/RPT2-like (NRL) protein NRL PROTEIN FOR CHLOROPLAST MOVEMENT 1 (NCH1) redundantly mediate the chloroplast accumulation response but not the avoidance response. Our findings indicate that phototropin-mediated phototropism, leaf flattening, and the chloroplast accumulation response, but not the chloroplast avoidance response and stomatal opening, are mediated by NRL proteins.
Keywords: Arabidopsis, blue light, chloroplast movement, Marchantia, phototropin
Abstract
In green plants, the blue light receptor kinase phototropin mediates various photomovements and developmental responses, such as phototropism, chloroplast photorelocation movements (accumulation and avoidance), stomatal opening, and leaf flattening, which facilitate photosynthesis. In Arabidopsis, two phototropins (phot1 and phot2) redundantly mediate these responses. Two phototropin-interacting proteins, NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) and ROOT PHOTOTROPISM 2 (RPT2), which belong to the NPH3/RPT2-like (NRL) family of BTB (broad complex, tramtrack, and bric à brac) domain proteins, mediate phototropism and leaf flattening. However, the roles of NRL proteins in chloroplast photorelocation movement remain to be determined. Here, we show that another phototropin-interacting NRL protein, NRL PROTEIN FOR CHLOROPLAST MOVEMENT 1 (NCH1), and RPT2 redundantly mediate the chloroplast accumulation response but not the avoidance response. NPH3, RPT2, and NCH1 are not involved in the chloroplast avoidance response or stomatal opening. In the liverwort Marchantia polymorpha, the NCH1 ortholog, MpNCH1, is essential for the chloroplast accumulation response but not the avoidance response, indicating that the regulation of the phototropin-mediated chloroplast accumulation response by RPT2/NCH1 is conserved in land plants. Thus, the NRL protein combination could determine the specificity of diverse phototropin-mediated responses.
To adapt to a fluctuating light environment, plants have evolved various light responses and photoreceptor systems. Phototropins (phot1 and phot2 in Arabidopsis thaliana) are blue light receptor kinases (1) belonging to AGC kinase VIII family (named after the cAMP-dependent protein kinase A, cGMP-dependent protein kinase G, and phospholipid-dependent protein kinase C) (2). phot1 and phot2 redundantly mediate hypocotyl phototropism, the chloroplast accumulation response, stomatal opening, leaf positioning, and leaf flattening, although functional differences between phot1 and phot2 exist (3–6). For example, phot2 is the main photoreceptor for the chloroplast avoidance response (7, 8) and palisade cell development (9). However, how phot1 and phot2 regulate these responses was still undetermined.
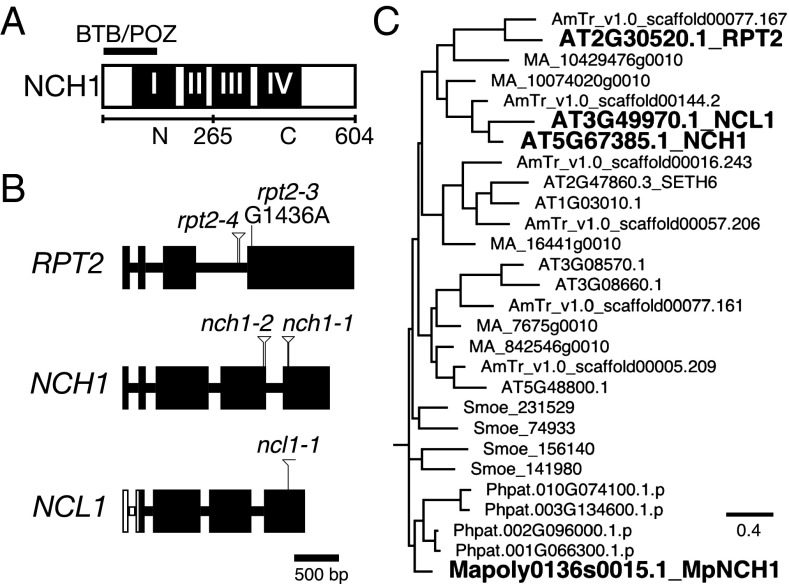
NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) and ROOT PHOTOTROPISM 2 (RPT2) were identified through molecular genetic analyses of phototropism-deficient mutants (10, 11). NPH3 and RPT2 are founding members of the NPH3/RPT2-like (NRL) family, which consists of ∼30 proteins in Arabidopsis (12). NRL proteins have an N-terminal BTB (broad complex, tramtrack, and bric à brac) domain and four conserved regions, I–IV (10), with region I partially overlapping the BTB domain (Fig. 1A and Fig. S1). NPH3 and RPT2 are localized on the plasma membrane, like phototropins, and interact with them (10, 13). The nph3 mutant is completely defective in hypocotyl and root phototropism (10, 11). The rpt2 mutant lacks root phototropism but retains partial hypocotyl phototropism under a low blue light fluence rate (11, 13). The blue light-induced dephosphorylation of NPH3, which is dependent on phot1, is thought to be an early event in the phototropic response (10, 14, 15). rpt2 mutants exhibit excessive levels of blue light-induced NPH3 dephosphorylation and have an enhanced rate of NPH3 internalization, indicating that RPT2 mediates phototropism via the regulation of the phosphorylation status and localization of NPH3 (16). Both nph3 and rpt2 mutants are also impaired in leaf flattening and positioning (6, 17–19). However, nph3 and rpt2 exhibit normal chloroplast photorelocation movements and stomatal opening (6, 17, 20). Thus, NPH3 and RPT2 likely mediate auxin-dependent processes among phototropin-mediated responses. Indeed, phot1 and NPH3 mediate auxin transport during blue light-induced root phototropism via the regulation of the subcellular localization of an auxin efflux transporter PIN-FORMED 2 (21). Another phototropin-interacting protein family, PHYTOCHROME KINASE SUBSTRATE, is also necessary for leaf positioning and flattening, as well as phototropism, but not for chloroplast photorelocation movements and stomatal opening (17, 22, 23). Thus, signaling pathways for chloroplast photorelocation movements and stomatal opening may be very different from those for phototropin-mediated auxin responses.
Fig. 1.
Identification of NCH1 as a phototropin-signaling component. (A) Protein structure of NCH1. Four conserved regions I to IV (black boxes) and a BTB/POZ domain (black bar) are indicated. N- and C-terminal regions that are used in yeast two-hybrid assay are indicated below. (B) Gene structure and mutation sites of RPT2, NCH1, and NCL1 genes. The rectangles and the intervening bars indicate exons and introns, respectively. The white rectangles and bars in the NCL1 gene indicate the predicted exon and intron sequences, respectively, which are similar to those of NCH1 but were deduced to be noncoding. The positions of the T-DNA insertion sites and the point mutation are indicated. (C) A RPT2/NCH1 clade in the NRL protein phylogenetic tree. The full NRL protein phylogenetic tree is presented in Fig. S2. Bar indicates 0.5 substitutions per site. AT, Arabidopsis thaliana; AmTr, Amborella trichopoda; MA, Picea abies; Smoe, Selaginella moellendorfii; Phpat, Physcomitrella patens; and Mapoly, Marchantia polymorpha.
Fig. S1.
NRL protein sequence alignment. (A) The protein sequence alignment was performed using the MUSCLE program (www.ebi.ac.uk/Tools/msa/muscle/). Regions colored red, blue, orange, and green indicate the four conserved sequence domains (I–IV, respectively) defined by Motchoulski and Liscum (10). Upper red lines indicate the position of the BTB/POZ domain. A lower black line indicates the deletion in the conserved N-terminal region specific to Arabidopsis NCL1. NCH1, At5g67385; and NCL1, At3g49970; NPH3, At5g64330; RPT2, At2g30520. (B) Alignment of Arabidopsis NCH1 and NCL1 gene sequences. The start codons are colored orange. The 5′ region of the start codon of the Arabidopsis NCL1 gene is highly similar to NCH1 and Brassicaceae NCL1 exon1 and exon2 sequences but a thymine insertion (red) produces a premature stop codon (red bold). AlNCL1, Arabidopsis lyrata NCL1, XM_002866659; BrNCL1, Brassica rapa NCL1, XM_009117436; CrNCL1, Capsella rubella NCL1, XM_006290276; and EsNCL1, Eutrema salsugineum NCL1, XM_006404018.
Chloroplasts move toward weak light (the accumulation response) and escape from strong light (the avoidance response). In land plants from liverwort to flowering plants, phototropin is the blue light receptor for chloroplast photorelocation movement (24). Similar to Arabidopsis, the functional divergence between phototropins is found also in the fern Adiantum capillus-veneris and the moss Physcomitrella patens (25, 26). Importantly, in the liverwort Marchantia polymorpha, which belongs to the most basal land plant lineage, a single phototropin, Mpphot, mediates both the accumulation and avoidance responses (27). Thus, although the functional divergence of phototropins occurred during land plant evolution, phototropins intrinsically have the ability to mediate both the accumulation and avoidance responses.
How one type of photoreceptor (i.e., phototropin) mediates different physiological responses is an important theme in understanding complex light-signaling pathways in plants. Here, we report that two phototropin-interacting NRL proteins, NRL PROTEIN FOR CHLOROPLAST MOVEMENT 1 (NCH1) and RPT2, are essential for the phototropin-mediated chloroplast accumulation response. Furthermore, NPH3, RPT2, and NCH1 are not involved in the chloroplast avoidance response or stomatal opening. Thus, the combination of NRL proteins may determine the functional specificities among phototropin-mediated responses.
Results
Identification of NCH1 as a Phototropin Signaling Factor.
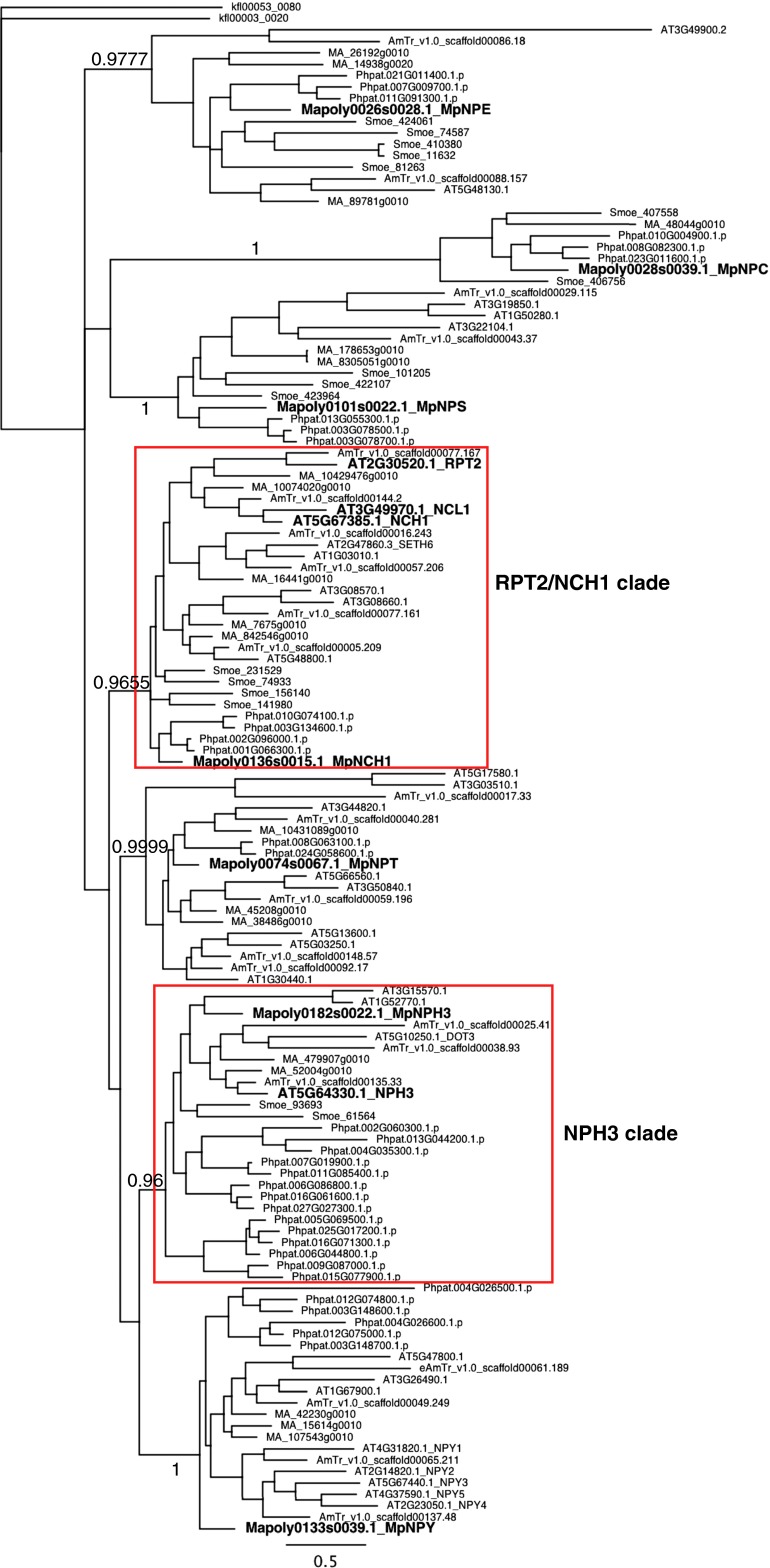
Because NPH3 and RPT2 are early signaling components in phototropin-mediated responses, we hypothesized that NRL proteins, other than NPH3 and RPT2, are involved in chloroplast photorelocation movements. While analyzing the transfer DNA (T-DNA) insertion mutants of some NRL genes, we found that the T-DNA lines of the NRL gene At5g67385 were deficient in chloroplast photorelocation movements (see below in detail). Here, we named At5g67385 as NCH1, and the two T-DNA lines as nch1-1 (SALK_064178) and nch1-2 (GABI_326_A01) (Fig. 1B). Previously, NCH1 was identified as a plant-immunity regulator gene, AtSR1 interaction protein 1 (SR1IP1) (28), and also named as NPH3/RPT2-Like 31 (NRL31), but hereafter we call At5g67385 as NCH1. NCH1 showed a higher similarity to RPT2 than to NPH3 (Fig. S1). A phylogenetic analysis indicated that NRL proteins are classified into seven major clades and that NCH1 belonged to the same clade as RPT2 but a distinct clade from NPH3 (Fig. 1C and Fig. S2). At3g49970 (named as NCH1-LIKE 1, NCL1) was the closest paralog of NCH1 (Fig. 1 B and C and Figs. S1 and S2) (12, 29). However, there is no evidence of its in vivo expression and we did not detect any defects of the ncl1 mutant at least in chloroplast photorelocation movement and stomatal opening (see below), suggesting that the NCL1 in A. thaliana, if functional, has a role other than chloroplast photorelocation movement and stomatal opening (see below, SI Text, and Fig. S1).
Fig. S2.
The Bayesian phylogenetic tree of NRL proteins. The parameters used were as follows: preset amino acid model program, mixed (LG, blosum, and wag); length of set rates, inverse-gamma; number of gamma categories, 4; Markov-chain Monte Carlo start tree, random; number of generation, 1,000,000; number of runs, 2; number of chains, 4; heated chain temperature, 0.2; sample frequency, 200; and burn-in fraction, 0.25. klfl00053_0080 were used as the outgroup. The numbers at the nodes indicate posterior probabilities. Bars indicate substitution values per site. Both RPT2/NCH1 and NPH3 clades are indicated (red box). Seven Marchantia and four Arabidopsis NRL proteins (NCH1, RPT2, NCL1, and NPH3) are bold-faced. AT, Arabidopsis thaliana; AmTr, Amborella trichopoda; MA, Picea abies; Smoe, Selaginella moellendorfii; Phpat, Physcomitrella patens; Mapoly, Marchantia polymorpha; and kfl, Klebsormidium flaccidum.
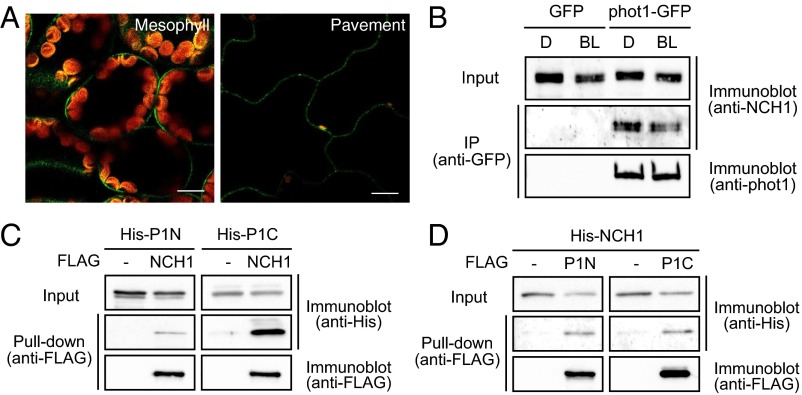
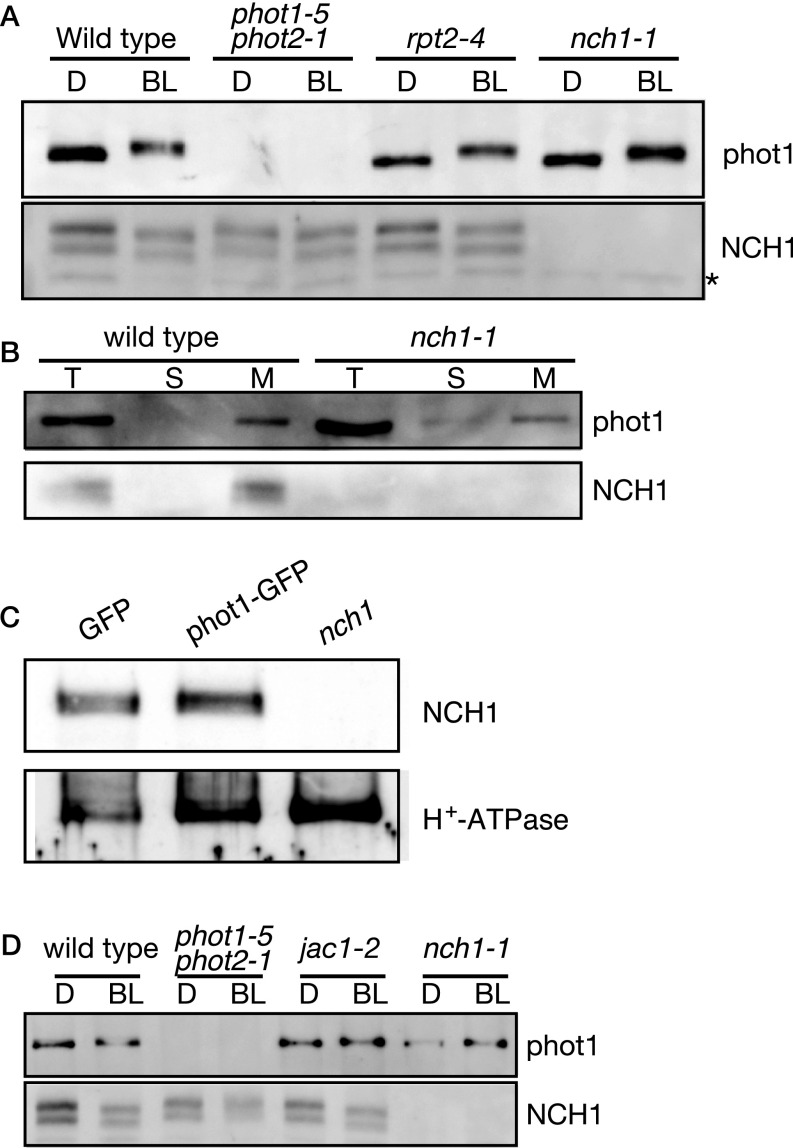
To examine the subcellular localization of NCH1, we constructed transgenic nch1-1 plants expressing the NCH1–GFP fusion gene under the control of the native NCH1 promoter (NCH1pro:NCH1–GFP). NCH1–GFP fluorescence was detected in both the pavement and mesophyll cells. In both cell types, NCH1–GFP was localized on the plasma membrane (Fig. 2A). An immunoblot analysis using an NCH1 antiserum revealed smeared bands around ∼60 kDa (frequently as prominent two bands) in wild type, phot1phot2, and rpt2, but not in nch1 mutant plants (Fig. S3A). The bands were detected in the microsomal fraction, similar to phot1 (Fig. S3 A and B). In an immunoprecipitation analysis of the microsomal fraction with an anti-GFP antibody, endogenous NCH1 was coimmunoprecipitated with phot1–GFP but not with GFP alone in a blue light-independent manner (Fig. 2B and Fig. S3C). In vitro pull-down assay revealed that NCH1 directly interacted with both N- and C-terminal domains of phot1 (Fig. 2 C and D). Similar to nph3 and rpt2 mutant plants (10, 11), the blue light-induced autophosphorylation of phot1 was normal in nch1 mutant plants (Fig. S3 A and D), indicating that NCH1 is downstream of the phototropins. Consistent with the interaction between NPH3 and RPT2 (13), yeast two-hybrid analysis revealed the interactions between N- and C-terminal domains of NPH3, RPT2, and NCH1 (Fig. 1A), although the interaction between RPT2 and NCH1 was much weaker (Fig. S4). Collectively, these data show that NCH1 is a phototropin-interactive protein similar to NPH3 and RPT2.
Fig. 2.
(A) Plasma-membrane localization of NCH1–GFP. Images are false colored to show GFP (green) and chlorophyll (red) fluorescence. (Scale bars, 10 µm.) (B) Coimmunoprecipitation of NCH1 with phot1. Microsomal fraction of the proteins from 10-d-old light-grown seedlings of transgenic GFP and phot1–GFP lines probed with anti-NCH1 or anti-phot1 antibodies. (C and D) Pull-down assay of phot1 and NCH1. FLAG- or His-tagged phot1 or NCH1 proteins were synthesized by in vitro transcription/translation reactions. The proteins bound to anti-FLAG beads were detected using anti-FLAG and anti-His.
Fig. S3.
An immunoblot analysis of NCH1 proteins. (A) Immunoblot analysis of phot1 and NCH1 proteins. Microsomal fraction of the proteins (corresponding to 50-µg total proteins) were used for an immunoblotting analysis with phot1 and NCH1 antibodies. The 13-d-old seedlings were dark-adapted for 24 h (D) and then irradiated with 6.4 µmol⋅m–2⋅s–1 of blue light for 1 h (BL). An asterisk indicates the position of a nonspecific protein band. (B) Total protein extracts (T) were fractionated into soluble (S) and microsomal (M) fractions by ultracentrifugation (100,000 × g, 1 h, 4 °C). Immunoblotting was performed using phot1 and anti-NCH1 antibodies. (C) Microsomal fraction of the proteins from 10-d-old light-grown seedlings of transgenic GFP, phot1–GFP, and nch1-1 mutant plants probed with anti-NCH1 or anti–H+-ATPase antibodies. H+-ATPase is a marker for microsomal proteins. (D) Immunoblot analysis of phot1 and NCH1 proteins in jac1 mutant plants. Details are same as A.
Fig. S4.
Yeast two-hybrid analysis. Yeast cells expressing the indicated bait and prey vectors were cultured on SD agar media supplemented with a mixture of the appropriate amino acids without Leu and Trp (-LT), His, Leu, and Trp (-HLT), or Ala, His, Leu, and Trp (-AHLT). Yeast cells were spotted with serial dilution (1, 1/10, and 1/100 from Left). Yeast cells expressing BD-RPT2 C/AD-NPH3 N, BD-RPT2 C/AD-RPT2 N, or BD-NCH1 C/AD-NPH3 N could grow well on both -HLT and -AHLT media, indicating that RPT2 C-NPH3 N, RPT2 C-RPT2 N, or NCH1 C-NPH3 N interactions is strong. Conversely, yeast cells expressing BD-NCH1 C/AD-NCH1 N could grow well on both -HLT but very weakly on -AHLT media. Yeast cells expressing BD-RPT2 C/AD-NCH1 N could grow weakly on -HLT media. Thus, RPT2 C-NCH1 N or NCH1 C-NCH1 N interaction is weak. Yeast cells containing empty BD or AD vectors could not grow on both -HLT and -AHLT media, indicating that the interactions detected here are specific.
NCH1 Is Not Involved in the Hypocotyl Phototropic Response and Leaf Flattening.
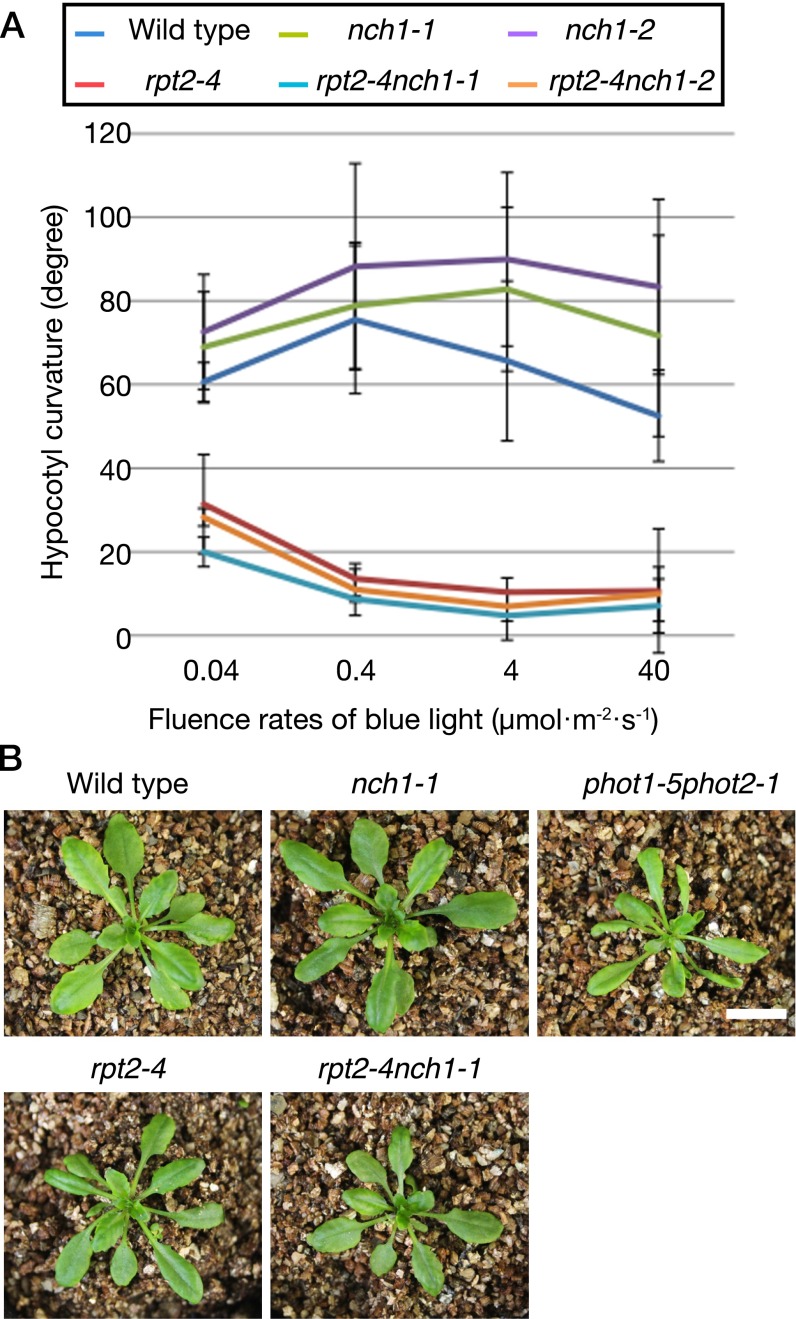
We analyzed hypocotyl phototropic responses in wild type, nch1 (nch1-1 and nch1-2), rpt2-4 (SAIL_140_D03) (Fig. 1A), and rpt2nch1 (rpt2-4nch1-1 and rpt2-4nch1-2). Similar to other rpt2 mutant plants (11, 13, 20), rpt2-4 is defective in the phototropic response, especially at higher fluence rates (Fig. S5A). The nch1 mutant plants showed a slightly strong phototropic response compared with wild type, although the only differences between wild type and nch1-2 in phototropic response at 4 and 40 µmol⋅m–2⋅s–1 were statistically significant (Fig. S5A) (Student’s t test, 0.01 < P < 0.05 for wild type vs. nch1-2 at 4 and 40 µmol⋅m–2⋅s–1). rpt2nch1 mutant plants were similar to rpt2-4 (Fig. S5A) (Student’s t test, P > 0.1 for rpt2-4 vs. rpt2-4nch1-1 or rpt2-4nch1-2 at all fluence rates), indicating that NCH1 is not a positive regulator of phototropin-mediated phototropic responses.
Fig. S5.
Hypocotyl phototropic response and leaf flattening in rpt2 and nch1 mutant backgrounds. (A) The phototropic response of the hypocotyls in etiolated seedlings. The hypocotyl curvature was induced by unilateral irradiation with blue light at the indicated fluence rates in the 3-d-old etiolated seedlings for 12 h. Data are presented as means of three independent experiments, and the error bars indicate SEs. (B) Leaf-flattening response. After seeds were sown on vermiculite soil, plants were grown under continuous white light conditions (∼70 µmol⋅m–2⋅s–1) for 25 d at 22 °C. (Scale bar, 1 cm.)
When grown under the continuous white light, nch1-1 mutants exhibited normal leaf flattening, like wild type (Fig. S5B). Under our light conditions, the leaves of rpt2 mutant plants were slightly curled but leaf curling was not as severe as in the phot1phot2 double mutants (Fig. S5B). The leaf curling of rpt2nch1 double mutants was similar to that of rpt2 (Fig. S5B). Thus, NCH1 is not involved in leaf flattening.
RPT2, NCH1, and NPH3 Are Not Involved in Stomatal Opening.
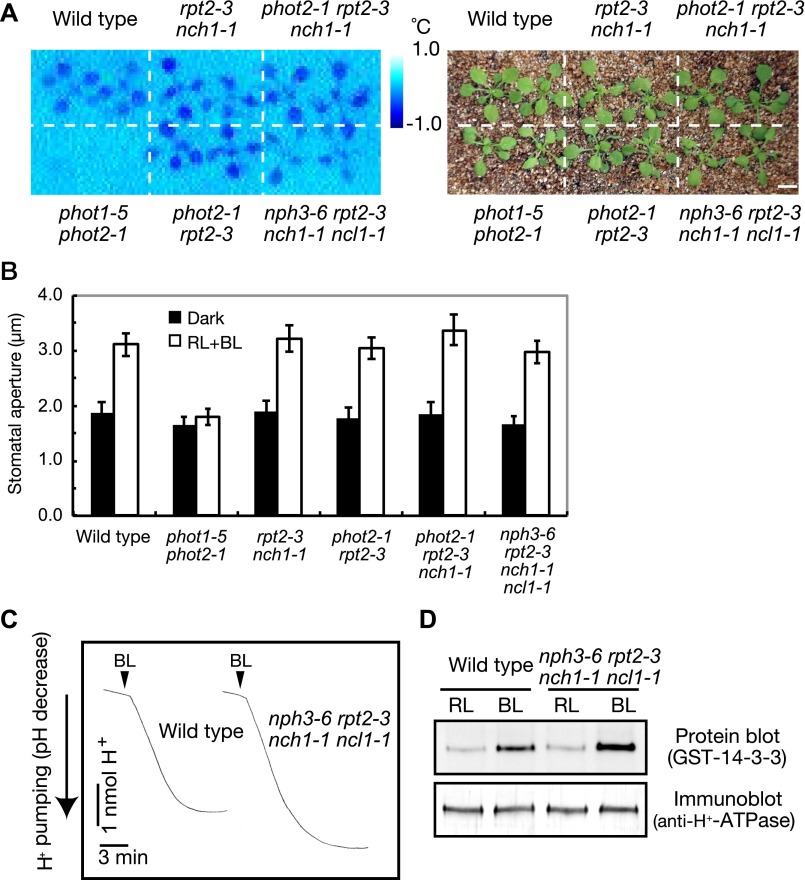
Recently, it was reported that the rpt2 mutant and phot2rpt2 double mutant exhibit normal blue light-induced stomatal opening (20), in contrast to a previous study (13). We examined a possible redundancy among four NRL genes in stomatal opening using rpt2nch1, phot2rpt2, phot2rpt2nch1, and nph3rpt2nch1ncl1 mutant plants (Fig. S6). When the blue light-induced stomatal opening in intact leaves was measured using an infrared thermograph, a blue light-induced decrease in leaf temperature caused by stomatal opening was found in wild type but not phot1phot2 double mutants, as described previously (Fig. S6A) (30). Consistently, blue light-induced stomatal opening in isolated epidermal tissues was found in wild type but not in phot1phot2 (Fig. S6B). In both assays, normal blue light-induced stomatal opening was detected in rpt2nch1, phot2rpt2, and phot2rpt2nch1 (Fig. S6 A and B), indicating that RPT2 and NCH1 are dispensable for phototropin-mediated stomatal opening. Furthermore, nph3rpt2nch1ncl1 quadruple-mutant plants exhibited normal blue light-induced stomatal opening (Fig. S6 A and B). Blue light-induced proton pumping (Fig. S6C) and H+-ATPase phosphorylation (Fig. S6D), which are essential for phototropin-mediated stomatal opening (30), were also normal in the quadruple-mutant plants (Fig. S6 C and D). Thus, at least four NRL genes are not involved in phototropin-mediated stomatal opening.
Fig. S6.
The blue light-induced stomatal opening in nph3rpt2nch1ncl1 quadruple mutants. (A) Thermal image of a blue light-dependent leaf temperature decrease. Arabidopsis wild-type and mutant (Right) plants were illuminated with red light (RL: 80 µmol⋅m–2⋅s–1) for 50 min and then a weak blue light (BL: 5 µmol⋅m–2⋅s–1) was superimposed. Subtractive images (Left) were obtained by subtracting an initial thermal image (before blue light exposure) from an image taken 15 min after blue light exposure. (Scale bar, 1 cm.) (B) Light-dependent stomatal opening in the epidermis. Epidermal strips from dark-adapted plants were illuminated with red light (RL: 50 µmol⋅m–2⋅s–1) and blue light (BL: 10 µmol⋅m–2⋅s–1) for 2 h. Bars represent means ± SDs (n = 75, pooled from triplicate experiments). (C) Blue light-dependent H+ pumping in guard cell protoplasts from wild-type and nph3rpt2nch1ncl1 quadruple-mutant plants. Guard cell protoplasts were illuminated by red light (600 µmol⋅m–2⋅s–1) for 2 h, and a pulse of blue light (100 µmol⋅m–2⋅s–1, 30 s) was applied as indicated. (D) Blue light-dependent phosphorylation of the H+-ATPase in guard cell protoplasts. The phosphorylation of the H+-ATPase was determined by protein blotting using a GST-14-3-3 protein. The total H+-ATPase proteins were detected by immunoblotting using an anti–H+-ATPase antibody.
RPT2 and NCH1 Are Essential for the Chloroplast Accumulation Response but Not the Avoidance Response.
The chloroplast photorelocation movement was analyzed through the leaf transmittance of red light (31). In wild type (black in Fig. 3 and Fig. S7), weak blue light (3 µmol⋅m–2⋅s–1) induces a decrease in leaf transmittance caused by the chloroplast accumulation response, whereas strong blue light (20 and 50 µmol⋅m–2⋅s–1) induces an increase in the leaf transmittance as a result of the avoidance response. After the strong blue light was turned off, a rapid decrease in leaf transmittance occurred (called the “dark recovery response”). In the nch1 mutant plants (indigo in Fig. 3 and Fig. S7), the chloroplast accumulation response was slightly weaker compared with that of the wild type, although the difference was statistically insignificant in many experiments (Fig. 3 A and B). However, the avoidance response was strongly enhanced in the nch1 mutants (Fig. 3 A and B). Not only the speed, but also the amplitude of the avoidance response was strongly enhanced in nch1 mutants, especially at 20 µmol⋅m–2⋅s–1 (Fig. 3 A and B) (Student’s t test, P < 0.005 for wild type vs. nch1). Furthermore, the dark recovery response was impaired in the nch1 mutants (Fig. 3 A and B) (Student’s t test, P < 0.01 for wild type vs. nch1). The nch1ncl1 mutant showed a phenotype similar to that of nch1 single mutants (cyan in Fig. S7 G and H) (Student’s t test, P > 0.2 for nch1-1 vs. nch1-1ncl1-1 at all fluence rates). However, in rpt2nch1 (orange in Fig. 3 and Fig. S7), the increase—but not the decrease—in leaf transmittance occurred even under the weak blue light condition (Fig. 3 A and B and Fig. S7 A and B), indicating that rpt2nch1 is completely defective in the accumulation response. The enhanced avoidance response at 20 and 50 µmol⋅m–2⋅s–1 observed in nch1 was suppressed in rpt2nch1. At 50 µmol⋅m–2⋅s–1, the increase in the leaf transmittance in rpt2nch1 was much lower than that in wild type. The avoidance response may have been saturated by the preceding light condition (20 µmol⋅m−2⋅s−1).
Fig. 3.
Chloroplast photorelocation movements in rpt2 and nch1 mutant plants. (A–D) Analysis of chloroplast photorelocation movements through the measurement of leaf transmittance changes. (A and C) After a dark treatment for 10 min, the samples were sequentially irradiated with continuous blue light at 3, 20, and 50 µmol⋅m–2⋅s–1 for 60, 40, and 40 min, indicated by white, light blue, and blue arrows, respectively. The light was turned off after 150 min (black arrow). Data are presented as means of three independent experiments, and the error bars indicate SEs. (B and D) Changes in leaf transmittance rates during the 2–6 min after changes in the light fluence rates (3, 20, and 50 µmol⋅m–2⋅s–1 or dark) are indicated as averages of transmittance changes for 1 min. Data are presented as means of three independent experiments and the error bars indicate SEs.
Fig. S7.
Chloroplast photorelocation movements in rpt2 and nch1 mutant backgrounds. Analysis of chloroplast photorelocation movements through the measurement of leaf transmittance changes in the indicated lines. (A, C, E, G, I, and K) After dark treatment for 10 min, the samples were sequentially irradiated with continuous blue light at 3, 20, and 50 µmol⋅m–2⋅s–1 for 60, 40, and 40 min, as indicated by white, light blue, and blue arrows, respectively. The light was turned off at 150 min (black arrow). Data are presented as means of three independent experiments, and the error bars indicate SEs. (B, D, F, H, J, and L) Changes in leaf transmittance rates over 2–6 min after changes in the light fluence rate (3, 20, and 50 µmol⋅m–2⋅s–1 or dark) are indicated as percentages of transmittance changes for 1 min. Data are presented as means of three independent experiments, and the error bars indicate SEs. (A and B) Analysis in two rpt2 alleles. (C and D) The NCH1pro:NCH1–GFP transgene (CCG1-2) rescued the impaired chloroplast accumulation response in phot2-1rpt2-3nch1-1. (E and F) Partial rescue of the nch1 mutant phenotype in NCH1pro:NCH1–GFP transgenic lines (CCG1-2, CCG2-8, and CCG5-5). (G and H) No detectable involvement of four NRL proteins in the chloroplast avoidance response. (I and J) Genetic interactions between PHOT1 and RPT2/NCH1. (K and L) Comparison of phenotypes between rpt2nch1 and jac1 mutants. Data are presented as means of three independent experiments (five experiments in A and B, and four experiments in I and J) and the error bars indicate SEs.
Contrary to a previous study that detected no defects in chloroplast photorelocation movements in the rpt2 mutant plants (13), we found that the rpt2 mutant plants, rpt2-3 (20) and rpt2-4, exhibited a weak accumulation response (red in Fig. 3 A and B and Fig. S7 A and B) (Student’s t test, P < 0.01 for wild type vs. rpt2 at 3 µmol⋅m–2⋅s–1) but the avoidance response and the dark recovery response were normal (Fig. 3 A and B and Fig. S7 A and B) (Student’s t test, for wild type vs. rpt2, P > 0.05 in the avoidance response and P > 0.2 in the dark recovery response). Four rpt2nch1 (rpt2-3nch1-1, rpt2-3nch1-2, rpt2-4nch1-1, and rpt2-4nch1-2) mutants displayed the same defect in the accumulation response (Fig. 3 A and B and Fig. S7 A and B). Furthermore, the expression of NCH1–GFP driven by the NCH1 native promoter (NCH1pro:NCH1–GFP, abbreviated as CCG) (cyan in Fig. S7 C and D) rescued the accumulation rate in phot2rpt2nch1 (Fig. S7 C and D) (Student’s t test, P < 0.0005 for phot2-1rpt2-4nch1-1 vs. CCG in phot2-1rpt2-4nch1-1 at 3 µmol⋅m–2⋅s–1). NCH1pro:NCH1–GFP partially rescued nch1 defects in the dark recovery response (Fig. S7 E and F) (Student’s t test, P < 0.05 for nch1-1 vs. CCG lines). However, the defects in the accumulation and avoidance responses were only subtly rescued although statistically insignificant. This partial rescue might be partly caused by lesser accumulation of NCH1–GFP in CCG lines (Fig. S8). Thus, NCH1 and RPT2 are necessary specifically for the accumulation response.
Fig. S8.
An immunoblot analysis of NCH1–GFP proteins. The 50-µg total proteins extracted from 15-d-old seedlings were used for the immunoblotting analyses with NCH1 and phot1 antibodies. A star indicates full-length NCH1 in wild type. Black and white circles indicate full-length NCH1–GFP and the degradation products, respectively.
Similar to rpt2nch1, the nph3rpt2nch1ncl1 quadruple-mutant plants (orange in Fig. S7 G and H) retained the avoidance response (Fig. S7 G and H), indicating that NPH3, RPT2, NCH1, and NCL1 are not involved in the avoidance response.
phot2rpt2nch1 Showed no Chloroplast Photorelocation Movement Like phot1phot2.
To reveal the relationships of RPT2 and NCH1 with phototropins, the chloroplast movement in double- and triple-mutant plants between nch1, rpt2, phot1, and phot2 was analyzed. phot1rpt2 (green in Fig. S7 I and J) exhibited a weak accumulation response similar to the phot1 (cyan in Fig. S7 I and J) and rpt2 single mutants (Fig. S7 I and J) (Student’s t test, P > 0.3 for phot1-5rpt2-4 vs. phot1-5 or rpt2-4 at 3 µmol⋅m–2⋅s–1). The phot1nch1 (yellow green in Fig. S7 I and J) displayed an enhanced avoidance response and an impaired dark recovery response similar to nch1 but showed a weak accumulation response similar to phot1 (Fig. S7 I and J) (Student’s t test, P < 0.05 for nch1-1 vs. phot1-5nch1-1 at 3 µmol⋅m–2⋅s–1). Both chloroplast accumulation and the avoidance responses in phot1rpt2nch1 (magenta in Fig. S7 I and J) were similar to those of rpt2nch1 (Fig. S7 I and J) (Student’s t test, P > 0.5 for rpt2-4nch1-1 vs. phot1-5rpt2-4nch1-1 at all fluence rates). phot2rpt2 (green in Fig. 3 C and D) and phot2nch1 (yellow green in Fig. 3 C and D) exhibited a severe impairment of the avoidance response, similar to that of the phot2 mutant (cyan in Fig. 3 C and D), although they showed weaker accumulation responses than phot2, and similar to those of rpt2 and nch1 (Fig. 3 C and D) (Student’s t test, P < 0.01 for phot2-1 vs. phot2-1rpt2-4 or phot2-1nch1-1 at 3 µmol⋅m–2⋅s–1). Prominently, the phot2rpt2nch1 (magenta in Fig. 3C and D and Fig. S7 C and E) completely lacked both the accumulation and avoidance responses (Fig. 3 C and D), like the phot1phot2 double mutant. These results indicated that both phot1 and phot2 mediated the accumulation response through RPT2 and NCH1.
The RPT2/NCH1 Homolog Mediates the Chloroplast Accumulation Response in the Liverwort M. polymorpha.
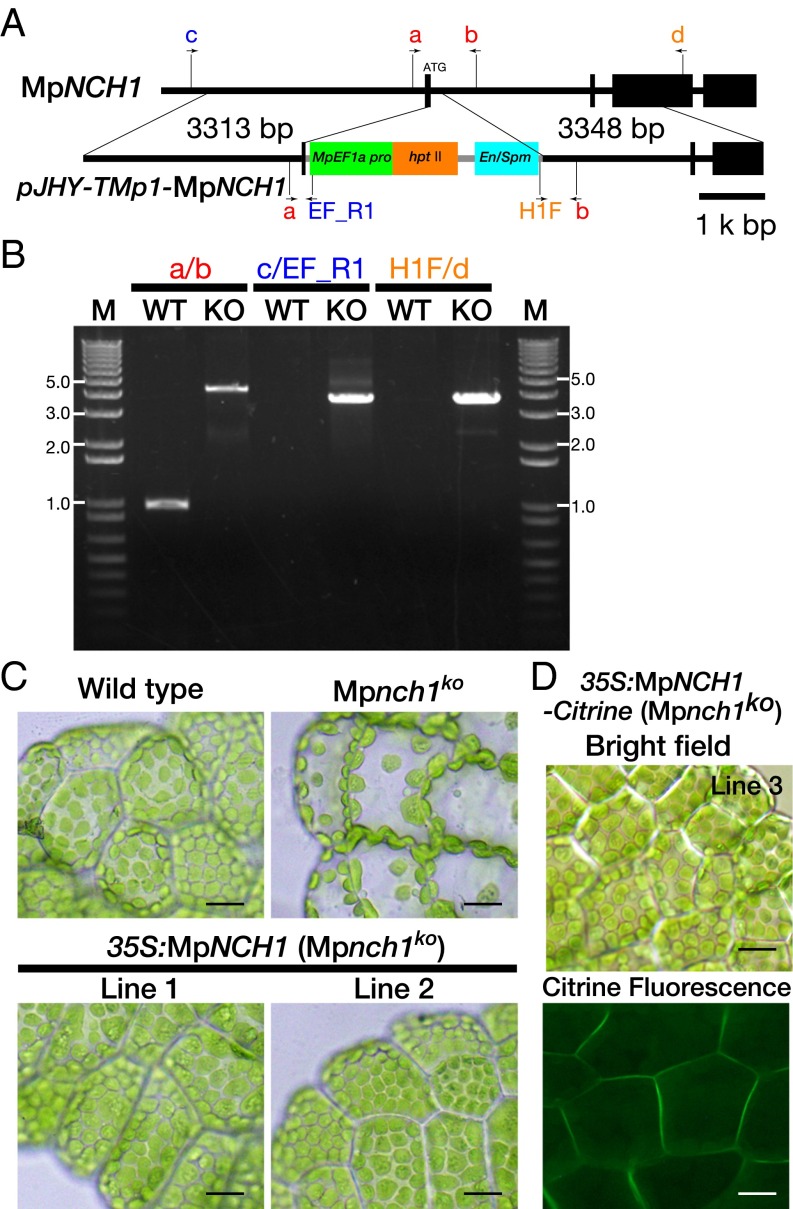
To investigate the conserved role of NRL proteins in phototropin-mediated chloroplast movements in land plants, we searched and characterized RPT2/NCH1 homologous genes in the liverwort M. polymorpha. M. polymorpha has seven NRL genes and each one NRL gene in seven major clades (Fig. S2). MpNCH1 (Mapoly0136s0015) belongs to a clade that includes the Arabidopsis RPT2 and NCH1 genes (Fig. 1B and Fig. S2), and has the same intron position as Arabidopsis NCH1 (the third intron of MpNCH1 corresponds to the fourth intron of NCH1) (Fig. 1A and Fig. 4A). The amino acid sequence of MpNCH1 is similar to those of RPT2 and NCH1 (Fig. S9). We generated an MpNCH1 knockout line, Mpnch1ko, using the homologous recombination-based gene-targeting method (32) (Fig. 4B). In the rim region of wild-type gemmalings (the early stage of thalli developing from gemmae) that were cultured under the white light conditions for 3 d, chloroplasts covered the upper surface of the cells as a result of the chloroplast accumulation response (Fig. 4C). However, in Mpnch1ko only a few chloroplasts were situated on the upper cell surface under the same conditions (Fig. 4C), and the distribution of chloroplasts was similar to that in the strong-light–irradiated wild-type cells where the chloroplast avoidance response was induced (27). When the chloroplasts that were localized on the anticlinal walls in Mpnch1ko were irradiated with a strong blue light using a confocal laser scanning microscope, chloroplasts escaped from the irradiated area (Movie S1), indicating that Mpnch1ko is deficient in the accumulation response but not in the avoidance response. The defects of Mpnch1ko in the chloroplast distribution were completely rescued by the expression of wild-type MpNCH1 cDNA or the NCH1–Citrine fusion gene driven under the control of the cauliflower mosaic virus 35S promoter (Fig. 4 C and D). The functional NCH1–Citrine was predominantly localized on the plasma membrane (Fig. 4D). Thus, NCH1 is necessary for the phototropin-mediated accumulation response in both Arabidopsis and M. polymorpha, and phototropin-NCH1 signaling on the plasma membrane is conserved in land plants.
Fig. 4.
MpNCH1 mediates the chloroplast accumulation response in M. polymorpha. (A) Targeting of the MpNCH1 gene by homologous recombination. Rectangles indicate exons and the intervening bars indicate introns. A 210-bp region, including a part of the first exon, was replaced with the MpEF1a:HPTII cassette of pJHY-TMp1-MpNCH1. The positions and directions of PCR primers used in B are indicated by lines and arrows, respectively. (B) Genotyping of the Mpnch1ko line by PCR. The positions of the PCR primers used are shown in A. KO, Mpnch1ko; M, DNA molecular size marker; WT, wild type. The size of the DNA marker is indicated at both sides of the gel. (C) Chloroplast distribution under the weak continuous white-light conditions (∼50 µmol⋅m–2⋅s–1). Gemmalings of wild type, Mpnch1ko, and two complemented Mpnch1ko lines were cultured under continuous white light for 3 d. (D) Plasma-membrane localization of MpNCH1–Citrine. Gemmalings of a complemented 35S:MpNCH1–Citrine Mpnch1ko line (line 3) were cultured under continuous white light for 3 d. Images are false-colored to show Citrine (green). Plant samples in the bright field and epi-fluorescent images are different. (Scale bars, 20 µm.)
Fig. S9.
The sequence alignment of M. polymorpha and Arabidopsis RPT2/NCH1 subfamily proteins. The protein sequence alignment was performed using the MUSCLE program (www.ebi.ac.uk/Tools/msa/muscle/). Regions colored red, blue, orange, and green indicate the four conserved sequence domains (I to IV, respectively) defined by Motchoulski and Liscum (10). Upper red lines indicate the positions of the BTB/POZ domains. A bottom black line indicates the deletion in the conserved N-terminal region specific to Arabidopsis NCL1. RPT2, At2g30520; NCH1, At5g67385; NCL1, At3g49970; and MpNCH1, Mapoly0136s0015.
SI Text
NCL1 Is Likely Nonfunctional.
The chromosomal regions containing NCH1 and NCL1 are in an intragenome syntenic relationship (Plant Genome Duplication Database: chibba.agtec.uga.edu/duplication/). NCH1/NCL1 gene duplication might have occurred in Brassicaceae, whereas the RPT2/NCH1 gene duplication seems to have occurred before the separation of Amborella and other angiosperm (Fig. 1C). However, among the NCL1 genes from some Brassicaceae species whose genome sequences are available, only the Arabidopsis thaliana NCL1 harbors a thymine insertion in the annotated second exon (Fig. S1B). This insertion produces the premature stop codon (specific to A. thaliana NCL1) if the start codon conserved in the Brassicaceae NCH1/NCL1 genes is used. Furthermore, there is no cDNA or expressed sequence tag (EST) data for NCL1 in the The Arabidopsis Information Resource (TAIR) database, whereas there are several cDNA and EST clones for NCH1. Additionally, an ncl1 T-DNA line (Fig. 1B) showed the wild-type phenotype (cyan in Fig. S7 G and H) (Student’s t test, P > 0.1 for wild type vs. ncl1-1 at all fluence rates) and the nch1ncl1 double mutant resembled the nch1 single mutant (cyan in Fig. S7 G and H) (Student’s t test, P > 0.2 for nch1-1 vs. nch1-1ncl1-1 at all fluence rates). Furthermore, nph3rpt2nch1ncl1 quadruple-mutant plants exhibited normal blue light-induced stomatal opening (Fig. S6). Thus, NCL1 might regulate other responses if functional.
The Phenotypes of rpt2nch1 and jac1 Mutants Are Similar but Distinct.
The jac1 mutants (41) share common phenotypes, including the defective accumulation response, the enhanced avoidance response (the magnitude), and the impaired dark recovery response (42) with rpt2nch1, suggesting that RPT2 and NCH1 mediate the accumulation response through the regulation of JAC1. However, a pair-wise mutant analysis indicated that the jac1phenotypes (cyan in Fig. S7 K and L) were somewhat different from those of rpt2nch1. Although the magnitude of the avoidance response increased in jac1 and rpt2nch1, the avoidance response was much stronger at 20 µmol⋅m–2⋅s–1 in rpt2nch1 compared with jac1 (Fig. S7 K and L) (Student’s t test, P < 0.01 for rpt2-4nch1-1 vs. jac1-1 at 20 µmol⋅m–2⋅s–1). At 20 and 50 µmol⋅m–2⋅s–1, the avoidance response was saturated in rpt2nch1 but not in jac1 (Fig. S7 K and L). Furthermore, rpt2nch1jac1 (red in Fig. S7 K and L) exhibited a weaker avoidance response than rpt2nch1 (Fig. S7 K and L) (Student’s t test, P < 0.001 for rpt2-4nch1-1 vs. rpt2-4nch1-1jac1-1 at 20 µmol⋅m–2⋅s–1), consistent with jac1 being defective in the regulation of actin filaments under the high-light condition (47). We have never detected the interaction between NCH1/RPT2 and JAC1 in the yeast two-hybrid assay. Our findings suggest that JAC1 has some functions that are independent of RPT2 and NCH1.
Discussion
NRL proteins, such as NPH3 and NAKED PINS IN YUC MUTANTS 1 (NPY1), may mediate auxin-mediated responses via auxin transport and auxin signaling, together with AGC kinase VIIIs, such as phototropin and PINOID (33). The phototropin–NPH3 module regulates phototropic responses and leaf flattening (6, 10, 17), and the PINOID–NPY1 module regulates auxin-mediated organogenesis and root gravitropic responses (29, 34–36). Phototropin–NPH3 and PINOID–NPY1 regulate the localization of auxin efflux carrier PIN-FORMED (PIN) proteins (21, 37) and result in the activation of AUXIN RESPONSE FACTOR transcription factors (38, 39). In this study, we found that two phototropin-interacting NRL proteins, RPT2 and NCH1, mediate the chloroplast accumulation response. Because chloroplast photorelocation movement is cell autonomous and occurs even in enucleated cells (40), RPT2 and NCH1 mediate the chloroplast accumulation response independently of auxin transport and auxin-mediated transcription. Thus, the AGC kinase–NRL module can regulate other molecular mechanisms, in addition to the auxin-related mechanism, via PIN regulation.
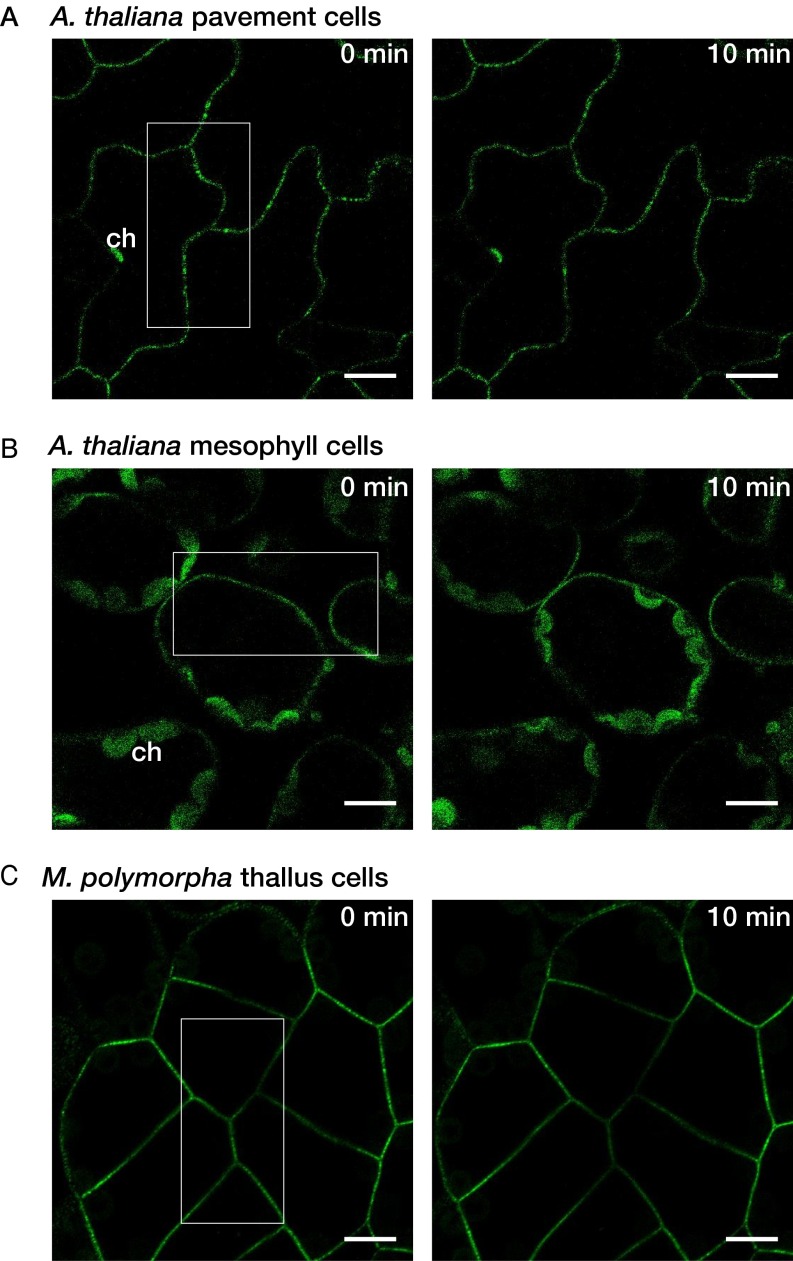
NCH1 is specific to the chloroplast accumulation response and NPH3 is specific to phototropism and leaf flattening, but RPT2 mediates both responses. Because NCH1 and NPH3 can activate phototropin signaling pathways in the absence of RPT2 (i.e., rpt2 mutants), RPT2 could not simply function as a molecular hub for the phototropin signaling pathways. Although RPT2 is a modulator of NPH3, which is essential for phototropism (16), the relationship between RPT2 and NCH1 in the chloroplast accumulation response is redundant. Although blue light induces the changes in NPH3 localization pattern (i.e., the internalization from the plasma membrane to cytosol) (16), we could not detect any clear changes of localization pattern of NCH1 at least during 10 min of blue light irradiation in both A. thaliana and M. polymorpha (Fig. S10). Thus, blue light-activated phototropins might mediate chloroplast accumulation response through the mechanism other than the regulation of NCH1 localization.
Fig. S10.
No apparent light-induced changes in NCH1 localization pattern. A part (indicated by white rectangles) of pavement (A) and mesophyll cells (B) of A. thaliana NCH1pro:NCH1–GFP line or cells of M. polymorpha 35S:MpNCH1-Citrine line (C) were irradiated with blue laser (488 nm, approximately 1,000 µmol⋅m–2⋅s–1) for 10 min. ch, chloroplasts. (Scale bars, 10 µm.)
Previously, we identified J-domain protein required for chloroplast accumulation response 1 (jac1) mutants (41) that were defective in the accumulation response and the enhanced avoidance response, like rpt2nch1 (42), suggesting that JAC1 is a regulator for the accumulation response, together with RPT2/NCH1. However, the phenotypes of both jac1 and rpt2nch1jac1 mutant plants were considerably different from that of rpt2nch1 (SI Text and Fig. S7 K and L), and the amount of NCH1 was normal in jac1 (Fig. S3D), suggesting that JAC1 has slightly different functions from RPT2 and NCH1. Nevertheless, it is important to elucidate the relationship between JAC1 and RPT2/NCH1.
An RPT2/NCH1 ortholog, MpNCH1, mediates the chloroplast accumulation response and localizes on the plasma membrane in M. polymorpha, indicating that the signaling mechanism for the accumulation response is conserved in land plants. Phototropins and components for the motility system of chloroplast photorelocation movements, such as CHLOROPLAST UNUSUAL POSITIONING 1 (CHUP1) and KINESIN-LIKE PROTEIN FOR ACTIN-BASED CHLOROPLAST MOVEMENT (KAC), are highly conserved from liverwort to seed plants (25–27, 43–46). However, M. polymorpha and other nonseed plant genome database searches identified RPT2/NCH1, CHUP1 and KAC orthologs—but not those of JAC1 or some other genes—in nonseed plant genome sequences (24). Therefore, although the photoreceptor (phototropin), phototropin-interacting protein (RPT2/NCH1), and motility system (CHUP1 and KAC) are conserved, the signal transduction pathway might have developed during land plant evolution.
In conclusion, the specificity of some phototropin-mediated signaling pathways was determined by the members and the combinations of phototropin-interacting NRL proteins. NPH3 is specific to phototropism and leaf flattening, and NCH1 is specific to chloroplast accumulation response. The involvement of NRL proteins in the chloroplast avoidance response is presently unknown. The phototropin-interacting kinase BLUE LIGHT SIGNALING1 mediates stomatal opening and is the direct substrate of phototropin (30). However, NRL proteins are not direct substrates of phototropin. In addition to NPH3 and RPT2, we should analyze the phototropin-dependent signaling pathway taking into account NCH1.
We identified one NPH3 ortholog, MpNPH3 (Mapoly0182s0022), in the M. polymorpha genome (Fig. S2), implying that the divergence of NRL proteins in phototropin-dependent signaling occurred early in land plant evolution and that AGC kinase–NRL modules are an early innovation during land plant evolution.
Materials and Methods
For the plant materials, the analysis of phototropin-mediated responses, confocal laser scanning microscopy, immunoblotting, and other methods, please see SI Materials and Methods.
SI Materials and Methods
Arabidopsis Lines and the Growth Condition.
The wild-type and mutant lines have a Columbia gl1 background. Arabidopsis cultures were performed as described previously (41). Briefly, seeds were sown on 0.8% agar medium containing one-thirds strength Murashige & Skoog inorganic salt and 1% sucrose, and cultured under white light at ∼100 μmol⋅m–2⋅s–1 (16 h)/dark (8 h) cycle at 23 °C in an incubator. SALK and SAIL T-DNA knockout lines, SALK_064178 (nch1-1), SALK_021412 (ncl1-1), and SAIL_140_D03 (rpt2-4), were provided by the Arabidopsis Biological Resource Center. nch1-2 is a GABI-Kat line, 326_A01 (48). phot1-5 (49), phot2-1 (7), nph3-6 (10), rpt2-3 (20), and jac1-1 (41) were described previously. Double-, triple-, and quadruple-mutants were generated by genetic crossing. Transgenic GFP and phot1–GFP lines were described previously (30).
Phylogenetic Analyses of RPT2 and NCH1.
Amino acid sequences of the full-length NRL proteins were aligned using the MUSCLE program (50) implemented in Geneious software (v6.1.8, Biomatters; www.geneious.com/) with default parameters. Amino acid sequences for the conserved BTB/POZ domain and DVI regions were extracted and combined. Unaligned gaps were removed from the resulting alignment using Gblocks (molevol.cmima.csic.es/castresana/Gblocks_server.html). The Bayesian phylogenetic tree construction was performed using MrBayes 2.0.9 (51).
Generation of Transgenic Arabidopsis Plants.
To construct a vector for NCH1pro:NCH1–GFP, the NCH1 gene fragment, including a 2,568-bp 5′ sequence (before the start codon) and the gene body region that contained the ORF but lacked the stop codon, was cloned into the PstI and KpnI restriction enzyme sites of the pPZP221/35S:GFP-nosT binary vector (52). The latter was constructed by cloning the GFP cDNA into the pPZP221/35S-nosT binary vector (53). The nch1-1 mutants were transformed with pPZP221/NCH1pro:NCH1–GFP-nosT by the floral-dipping method using Agrobacterium tumefaciens (GV3101::pMP90).
Confocal Laser Scanning Microscopy.
To observe the subcellular localization of NCH1–GFP in A. thaliana and MpNCH1-Citrine in Marchantia polymorpha, a confocal microscope (C2+; Nikon) was used. Fluorescence was observed at 499–529 nm for fluorescent proteins and 553–618 nm for chlorophyll autofluorescence.
Immunoblot Analysis.
An NCH1 rabbit polyclonal antibody was raised against a purified His-tag-NCH1 C-terminal domain (328–604) fusion protein (Eurofins Genomics). The phot1 antibody was described previously (46). Seedlings were homogenized in extraction buffer [50 mM Mops-KOH (pH 7.5), 100 mM NaCl, 2.5 mM EDTA, 1 mM DTT, 1 mM PMSF, 10 µM leupeptin, and phosphatase inhibitor mixture (Nacalai tesque)]. After centrifuging three times at 12,000 × g, the supernatants (total protein fraction) were quantified using the Bio-Rad Protein Assay (Bio-Rad). Total protein homogenates were ultracentrifuged at 100,000 × g for 1 h at 4 °C. The resultant supernatants and pellets were used as soluble and microsomal protein fractions, respectively. An immunoprecipitation analysis with anti-GFP was performed as previously described (30).
In Vitro Pull-Down Assay.
In vitro pull-down assay was performed as previously described (30). FLAG- and His-tagged protein pair were synthesized using an in vitro transcription/translation system (BioSieg) and kept on ice for 10 min in a binding buffer containing 20 mM Tris⋅HCl (pH 7.4), 140 mM NaCl and 0.1% Triton X-100. After brief centrifugation, the supernatants were incubated with anti-FLAG Ms agarose (Sigma-Aldrich) for 1 h. The bound proteins were used for immunoblotting analysis using anti-FLAG (Sigma-Aldrich) and anti-His antibodies (GeneScript).
Yeast Two-Hybrid Assay.
The binding domain (bait) or the activation domain (prey) plasmids containing the genes for NCH1, RPT2, and NPH3 were constructed by subcloning the cDNAs into the pGBKT7 or pGADT7 vectors, respectively (Matchmaker Yeast Two-Hybrid System; Takara Bio). The bait and prey plasmids were transformed into the yeast strains AH109 and Y187, respectively. The mated transformants were selected on SD agar media without Leu and Trp and spotted on to the indicated media.
Analysis of Hypocotyl Phototropic Responses.
The phototropic responses were analyzed as previously described with some modifications (54). The sterilized seeds were sown on one-third strength Murashige & Skoog agar plates without sucrose. After the cold treatment at 4 °C in the darkness for 2 d, the seeds were irradiated with white light (∼80 µmol⋅m–2⋅s–1) for 3 h and then grown vertically in the dark for 54 h at 23 °C. The etiolated seedlings were irradiated for 12 h with unilateral blue light (at 0.04, 0.4, 4, or 40 µmol⋅m–2⋅s–1) using blue light-emitting diodes. The hypocotyl curvature was measured using the ImageJ program (National Institutes of Health; https://imagej.nih.gov/ij/).
Measurement of Stomatal Responses to Blue Light.
Thermal imaging was carried out using TVS-8500 (NEC Avio Infrared Technologies) (30). Subtraction images were prepared using PE Professional software (NEC Avio Infrared Technologies). The stomatal aperture in the abaxial epidermis was measured as described previously (5). Briefly, epidermal strips were incubated in 5 mM Mes-Bis-Tris propane (pH 6.5), 50 mM KCl and 0.1 mM CaCl2 under red light (50 µmol⋅m–2⋅s–1) superimposed by blue light (10 µmol⋅m–2⋅s–1) for 2 h at 24 °C. Blue light-dependent H+ pumping from guard cell protoplasts was determined with a glass pH electrode (5). The reaction mixture (0.8 mL) contains 0.125 mM Mes-NaOH (pH 6.0), 1 mM CaCl2, 0.4 M mannitol, 10 mM KCl, and guard cell protoplasts (50-µg proteins). Guard cell protoplasts were prepared enzymatically from leaves (55, 56). The phosphorylation of a penultimate Thr residue of the H+-ATPase was determined by protein blotting using GST-14-3-3 (57). An immunoblot analysis was carried out using anti–H+-ATPase antibodies (57).
Analyses of Chloroplast Photorelocation Movements.
To detect chloroplast photorelocation movements in A. thaliana, the measurement of leaf transmittance changes was performed as described previously (31). Detached third leaves from 16-d-old seedlings were placed on 1% (wt/vol) gellan gum in a 96-well plate. Leaves were dark-adapted at least for 1 h before transmittance measurement. In M. polymorpha, chloroplast distribution and Citrine fluorescence were observed using a fluorescent microscopy (Axiophot; Zeiss). For the observation of the chloroplast avoidance response in Mpnch1ko, a confocal microscope (SP5; Leica Microsystems) was used as described previously (58).
Growth Conditions of M. polymorpha.
The wild-type male accession of M. polymorpha, Takaragaike (Tak)-1, was used. Plants were cultured on one-half-strength Gamborg’s B5 agar medium at 22 °C under continuous white light (∼50 µmol⋅m–2⋅s–1).
Targeted Gene Knockout and Genetic Complementation of MpNCH1.
The 5′ and 3′ homology arms [–3,313∼+57 (3,370 bp) and +268∼+3,615 (3,348 bp) from the start codon, respectively] of the MpNCH1 gene were cloned into the PacI and AscI sites of pJHY-TMp1 (32), respectively. Tak-1 × Tak-2 F1 spores were used for Agrobacterium-mediated transformation, as described previously (32). The selection and PCR screening of MpNCH1 knockout plants were performed as described previously (32). Briefly, hygromycin-resistant plants were screened by PCR with the primer pair 5′-AAGGAGGAGGAACGAACCAT-3′ (i) and 5′-CGAATCTATCCCCCACGAAT-3′ (ii). Putative Mpnch1ko lines were confirmed using PCR with primers outside of the 5′- or 3′-homologous arms [(iii) 5′-CCCACTCACCTTTTCCACAT-3′ or (iv) 5′-GTGTTCTTCTCAGGCGGAAG-3′, respectively] and inside the MpEFpro::hptII cassette (EF_R1, 5′-GAAGGCTTCTGATTGAAGTTTCCTTTTCTG-3′ for the 5′-arm and H1F, 5′-GTATAATGTATGCTATACGAAGTTATGTTT-3′ for the 3′-arm).
To generate the complementation construct, MpNCH1 cDNA PCR fragments with or without a stop codon were cloned into pENTR/d-TOPO (Life Technologies). The resulting MpNCH1 cassette was subcloned into a binary vector pMpGWB302 or pMpGWB306 (59) using LR clonase II (Life Technologies), resulting in the cauliflower mosaic virus pro35S:MpNCH1 or pro35S:MpNCH1-Citrine binary vector, respectively. These binary vectors were introduced into regenerating thalli of Mpnch1ko, as described previously (60).
Supplementary Material
Acknowledgments
We thank Kimitsune Ishizaki for the database search of the MpNCH1 gene; Yoriko Matsuda for the generation of Marchantia polymorpha Mpnch1ko; Yasuomi Tada for in vitro transcription/translation system; and Mineko Shimizu, Atsuko Tsutsumi, and Mika Niwaki for assistance in plant culturing. The SALK T-DNA insertion lines were obtained from the Arabidopsis Biological Resource Center. This work was supported by the Grant-in-Aid for Scientific Research Grants 26840097 and 15KK0254 (to N.S.), 23120521, 25120719, 26711019, and 15K14552 (to A.T.), 25440140 (to S.-G.K.), 21227001 (to K.S.), 23120516, 25120716, and 25113009 (to T.K.), and 20227001, 23120523, 25120721, and 25251033 (to M.W.) from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Bank of Japan database (accession nos. LC125899–LC125906).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602151113/-/DCSupplemental.
References
- 1.Christie JM. Phototropin blue-light receptors. Annu Rev Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 2.Bögre L, Okrész L, Henriques R, Anthony RG. Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 2003;8(9):424–431. doi: 10.1016/S1360-1385(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 3.Suetsugu N, Wada M. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: Phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 2013;54(1):8–23. doi: 10.1093/pcp/pcs165. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, et al. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98(12):6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinoshita T, et al. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414(6864):656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 6.Inoue S, Kinoshita T, Takemiya A, Doi M, Shimazaki K. Leaf positioning of Arabidopsis in response to blue light. Mol Plant. 2008;1(1):15–26. doi: 10.1093/mp/ssm001. [DOI] [PubMed] [Google Scholar]
- 7.Kagawa T, et al. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291(5511):2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 8.Jarillo JA, et al. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410(6831):952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 9.Kozuka T, Kong SG, Doi M, Shimazaki K, Nagatani A. Tissue-autonomous promotion of palisade cell development by phototropin 2 in Arabidopsis. Plant Cell. 2011;23(10):3684–3695. doi: 10.1105/tpc.111.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motchoulski A, Liscum E. Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286(5441):961–964. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Wada T, Ishiguro S, Okada K. RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell. 2000;12(2):225–236. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingerich DJ, Hanada K, Shiu SH, Vierstra RD. Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell. 2007;19(8):2329–2348. doi: 10.1105/tpc.107.051300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell. 2004;16(4):887–896. doi: 10.1105/tpc.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedmale UV, Liscum E. Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J Biol Chem. 2007;282(27):19992–20001. doi: 10.1074/jbc.M702551200. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchida-Mayama T, et al. Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci. 2008;174(6):626–633. [Google Scholar]
- 16.Haga K, Tsuchida-Mayama T, Yamada M, Sakai T. Arabidopsis ROOT PHOTOTROPISM2 contributes to the adaptation to high-intensity light in phototropic responses. Plant Cell. 2015;27(4):1098–1112. doi: 10.1105/tpc.15.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carbonnel M, et al. The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 2010;152(3):1391–1405. doi: 10.1104/pp.109.150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada A, Takemiya A, Inoue S, Sakai T, Shimazaki K. Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol. 2013;54(1):36–47. doi: 10.1093/pcp/pcs094. [DOI] [PubMed] [Google Scholar]
- 19.Kozuka T, Suetsugu N, Wada M, Nagatani A. Antagonistic regulation of leaf flattening by phytochrome B and phototropin in Arabidopsis thaliana. Plant Cell Physiol. 2013;54(1):69–79. doi: 10.1093/pcp/pcs134. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi T, Takemiya A, Harada A, Shimazaki K. Disruption of ROOT PHOTOTROPISM2 gene does not affect phototropin-mediated stomatal opening. Plant Sci. 2013;201-202:93–97. doi: 10.1016/j.plantsci.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Wan Y, et al. The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell. 2012;24(2):551–565. doi: 10.1105/tpc.111.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lariguet P, et al. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA. 2006;103(26):10134–10139. doi: 10.1073/pnas.0603799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demarsy E, et al. Phytochrome kinase substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 2012;31(16):3457–3467. doi: 10.1038/emboj.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suetsugu N, Wada M. Evolution of the cp-actin-based motility system of chloroplasts in green plants. Front Plant Sci. 2016;7:561. doi: 10.3389/fpls.2016.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M. Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol. 2004;45(4):416–426. doi: 10.1093/pcp/pch045. [DOI] [PubMed] [Google Scholar]
- 26.Kasahara M, Kagawa T, Sato Y, Kiyosue T, Wada M. Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 2004;135(3):1388–1397. doi: 10.1104/pp.104.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu A, et al. Phototropin encoded by a single-copy gene mediates chloroplast photorelocation movements in the liverwort Marchantia polymorpha. Plant Physiol. 2014;166(1):411–427. doi: 10.1104/pp.114.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Du L, Shen C, Yang Y, Poovaiah BW. Regulation of plant immunity through ubiquitin-mediated modulation of Ca2+-calmodulin-AtSR1/CAMTA3 signaling. Plant J. 2014;78(2):269–281. doi: 10.1111/tpj.12473. [DOI] [PubMed] [Google Scholar]
- 29.Furutani M, et al. The gene MACCHI-BOU 4/ENHANCER OF PINOID encodes a NPH3-like protein and reveals similarities between organogenesis and phototropism at the molecular level. Development. 2007;134(21):3849–3859. doi: 10.1242/dev.009654. [DOI] [PubMed] [Google Scholar]
- 30.Takemiya A, et al. Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun. 2013;4:2094. doi: 10.1038/ncomms3094. [DOI] [PubMed] [Google Scholar]
- 31.Wada M, Kong SG. Analysis of chloroplast movement and relocation in Arabidopsis. Methods Mol Biol. 2011;774:87–102. doi: 10.1007/978-1-61779-234-2_6. [DOI] [PubMed] [Google Scholar]
- 32.Ishizaki K, Johzuka-Hisatomi Y, Ishida S, Iida S, Kohchi T. Homologous recombination-mediated gene targeting in the liverwort Marchantia polymorpha L. Sci Rep. 2013;3:1532. doi: 10.1038/srep01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbosa ICR, Schwechheimer C. Dynamic control of auxin transport-dependent growth by AGCVIII protein kinases. Curr Opin Plant Biol. 2014;22:108–115. doi: 10.1016/j.pbi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Qin G, Dai X, Zhao Y. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(47):18825–18829. doi: 10.1073/pnas.0708506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Qin G, Dai X, Zhao Y. NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc Natl Acad Sci USA. 2008;105(52):21017–21022. doi: 10.1073/pnas.0809761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Dai X, Cheng Y, Zhao Y. NPY genes play an essential role in root gravitropic responses in Arabidopsis. Mol Plant. 2011;4(1):171–179. doi: 10.1093/mp/ssq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furutani M, Nakano Y, Tasaka M. MAB4-induced auxin sink generates local auxin gradients in Arabidopsis organ formation. Proc Natl Acad Sci USA. 2014;111(3):1198–1203. doi: 10.1073/pnas.1316109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper RM, et al. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell. 2000;12(5):757–770. doi: 10.1105/tpc.12.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aida M, Vernoux T, Furutani M, Traas J, Tasaka M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 2002;129(17):3965–3974. doi: 10.1242/dev.129.17.3965. [DOI] [PubMed] [Google Scholar]
- 40.Wada M. Chloroplast photoorientation in enucleated fern protonemata. Plant Cell Physiol. 1988;29(7):1227–1232. [Google Scholar]
- 41.Suetsugu N, Kagawa T, Wada M. An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 2005;139(1):151–162. doi: 10.1104/pp.105.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodama Y, Suetsugu N, Kong SG, Wada M. Two interacting coiled-coil proteins, WEB1 and PMI2, maintain the chloroplast photorelocation movement velocity in Arabidopsis. Proc Natl Acad Sci USA. 2010;107(45):19591–19596. doi: 10.1073/pnas.1007836107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usami H, et al. CHUP1 mediates actin-based light-induced chloroplast avoidance movement in the moss Physcomitrella patens. Planta. 2012;236(6):1889–1897. doi: 10.1007/s00425-012-1735-6. [DOI] [PubMed] [Google Scholar]
- 44.Suetsugu N, et al. The KAC family of kinesin-like proteins is essential for the association of chloroplasts with the plasma membrane in land plants. Plant Cell Physiol. 2012;53(11):1854–1865. doi: 10.1093/pcp/pcs133. [DOI] [PubMed] [Google Scholar]
- 45.Oikawa K, et al. Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell. 2003;15(12):2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suetsugu N, et al. Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2010;107(19):8860–8865. doi: 10.1073/pnas.0912773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichikawa S, Yamada N, Suetsugu N, Wada M, Kadota A. Red light, phot1 and JAC1 modulate phot2-dependent reorganization of chloroplast actin filaments and chloroplast avoidance movement. Plant Cell Physiol. 2011;52(8):1422–1432. doi: 10.1093/pcp/pcr087. [DOI] [PubMed] [Google Scholar]
- 48.Rosso MG, et al. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol. 2003;53(1-2):247–259. doi: 10.1023/B:PLAN.0000009297.37235.4a. [DOI] [PubMed] [Google Scholar]
- 49.Huala E, et al. Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science. 1997;278(5346):2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 50.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 52.Suetsugu N, Higa T, Kong SG, Wada M. PLASTID MOVEMENT IMPAIRED1 and PLASTID MOVEMENT IMPAIRED1-RELATED 1 mediate photorelocation movements of both chloroplasts and nuclei. Plant Physiol. 2015;169(2):1155–1167. doi: 10.1104/pp.15.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25(6):989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- 54.Suetsugu N, Kong SG, Kasahara M, Wada M. Both LOV1 and LOV2 domains of phototropin2 function as the photosensory domain for hypocotyl phototropic responses in Arabidopsis thaliana (Brassicaceae) Am J Bot. 2013;100(1):60–69. doi: 10.3732/ajb.1200308. [DOI] [PubMed] [Google Scholar]
- 55.Ueno K, Kinoshita T, Inoue S, Emi T, Shimazaki K. Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 2005;46(6):955–963. doi: 10.1093/pcp/pci104. [DOI] [PubMed] [Google Scholar]
- 56.Takemiya A, Yamauchi S, Yano T, Ariyoshi C, Shimazaki K. Identification of a regulatory subunit of protein phosphatase 1 which mediates blue light signaling for stomatal opening. Plant Cell Physiol. 2013;54(1):24–35. doi: 10.1093/pcp/pcs073. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita T, Shimazaki Ki. Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999;18(20):5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong SG, Arai Y, Suetsugu N, Yanagida T, Wada M. Rapid severing and motility of chloroplast-actin filaments are required for the chloroplast avoidance response in Arabidopsis. Plant Cell. 2013;25(2):572–590. doi: 10.1105/tpc.113.109694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishizaki K, et al. Development of gateway binary vector series with four different selection markers for the liverwort Marchantia polymorpha. PLoS One. 2015;10(9):e0138876. doi: 10.1371/journal.pone.0138876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubota A, Ishizaki K, Hosaka M, Kohchi T. Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci Biotechnol Biochem. 2013;77(1):167–172. doi: 10.1271/bbb.120700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.