Significance
Endoplasmic reticulum (ER)-associated degradation (ERAD) mediated by the E3 ubiquitin ligase Hrd1 controls ER stress through clearance of misfolded proteins. However, the physiological roles—in particular, the cell- and organ-specific functions of Hrd1 ERAD—remain undefined. Here we demonstrate that Hrd1 ERAD governs a critical checkpoint of B-cell immunity via controlling the activation-induced apoptosis of B cells. At the molecular level, Hrd1 functions as an ubiquitin ligase for the death receptor CD95/Fas. Given the fact of the ubiquitous roles of Fas in cell death and the ubiquitous expression of the antiapoptotic ubiquitin ligase Hrd1, Hrd1-mediated Fas degradation is likely a common biological mechanism in regulating Fas-induced cell death.
Keywords: Hrd1, Fas, B cells, ubiquitination
Abstract
Humoral immunity involves multiple checkpoints during B-cell development, maturation, and activation. The cell death receptor CD95/Fas-mediated apoptosis plays a critical role in eliminating the unwanted activation of B cells by self-reactive antigens and in maintaining B-cell homeostasis through activation-induced B-cell death (AICD). The molecular mechanisms controlling AICD remain largely undefined. Herein, we show that the E3 ubiquitin ligase Hrd1 protected B cells from activation-induced cell death by degrading the death receptor Fas. Hrd1-null B cells exhibited high Fas expression during activation and rapidly underwent Fas-mediated apoptosis, which could be largely inhibited by FasL neutralization. Fas mutation in Hrd1 KO mice abrogated the increase in B-cell AICD. We identified Hrd1 as the first E3 ubiquitin ligase of the death receptor Fas and Hrd1-mediated Fas destruction as a molecular mechanism in regulating B-cell immunity.
B-cell immunity involves several checkpoints, which are important to orchestrate and balance survival and apoptotic signals and control the quality and size of the B-cell compartment (1, 2). The earliest steps in B-cell development occur in the bone marrow, where assembly of the pre–B-cell receptor (pre-BCR) followed by the mature BCR occurs in distinct stages. Once the mature BCR is expressed, B lymphocytes then progress into the immature B-cell stage, where further selection checkpoints occur before the immature B cells transition to the periphery and mature into circulating B cells (2, 3). Mature B cells are maintained through tonic BCR, CD40, and B-cell–activating factor receptor (BAFFR) signaling (4). Activation by cross-linking the BCR leads to rapid proliferation, somatic hypermutation, and further differentiation of B cells (5, 6). The expansion of activated B-cell compartment is subsequently downmodulated through activation-induced cell death (AICD) (7, 8).
The death receptor Fas has been identified as a key regulator of AICD of B cells (9–11). Fas is highly expressed on activated B cells, particularly in the germinal center, where it mediates the deletion of autoreactive or unproductive somatic hypermutated B cells (12–14). Fas, as well as its ligand FasL, play critical roles in B-cell apoptosis. The selective removal of Fas from activated B cells results in lupus-like disease and autoantibody production (15–17). It has been a widely accepted view that the Fas/FasL mutations result in, at least in part, a failure to eliminate self-reactive GC B cells in autoimmune patients and animal models (12, 18). However, more recent studies show that FAS is in fact not required for the elimination of self-reactive GC B cells. Instead, Fas inactivation led to accumulation of a population of unconventional GC B cells that underwent somatic hypermutation and survived despite losing antigen reactivity (8). Nevertheless, it is clear that Fas-mediated B-cell death is critically involved in B-cell–mediated autoimmune diseases. However, the molecular mechanisms underlying how Fas-mediated B-cell death is regulated remain an enigma.
Hrd1 is one of the ER-associated E3 ubiquitin ligases involved in ERAD (endoplasmic reticulum-associated degradation), which is situated in the ER along with Sel1, Derlin1, Herp, and other proteins as part of an ERAD complex (19, 20). During ERAD, misfolded proteins within the ER bind to chaperones such as BiP and are retrotranslocated into the cytosol to be ubiquitinated by the cytosolic RING (really interesting new gene) domain of Hrd1 (19). Whereas Hrd1 is required for the clearance of misfolded proteins during ERAD, emerging evidence suggests that Hrd1 can also regulate cellular functions by controlling the availability of specific proteins, such as Nrf2, Blimp1, and PGC-1β (21–23). In this paper, we report that Hrd1 plays a crucial role at the B-cell survival checkpoint by degrading the death receptor Fas during peripheral B-cell activation to downmodulate AICD.
Results
Hrd1 Functions Are Required to Protect B Cells from Activation-Induced Apoptosis in Vitro.
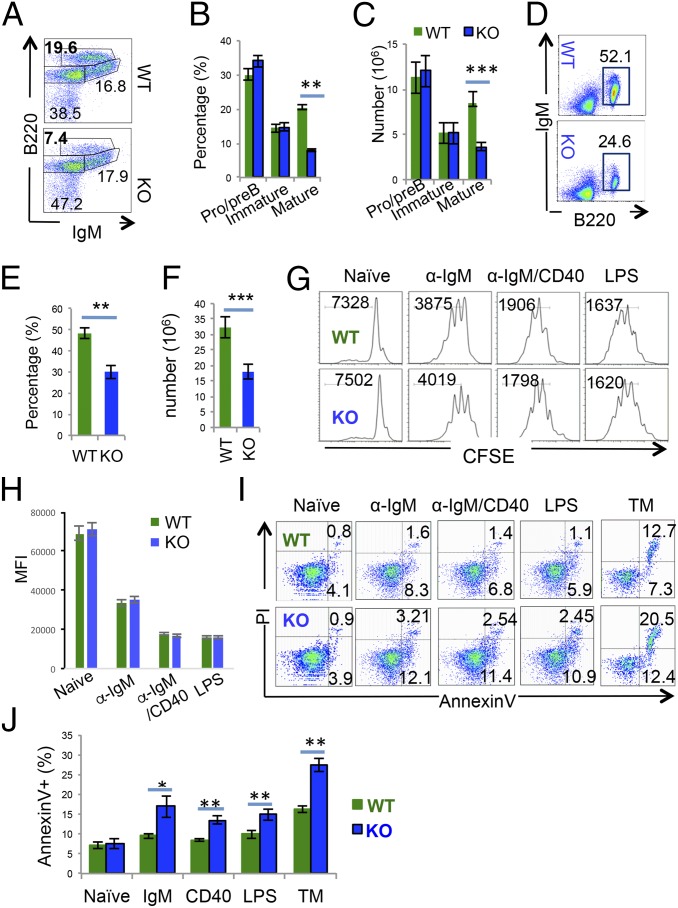
Real-time PCR analysis revealed approximately fivefold higher Hrd1 expression in splenic B cells than T cells, which was further elevated in B cells upon BCR stimulation (SI Appendix, Fig. S1A), implying a possible important role of Hrd1 in B-cell immunity. To investigate the functions of Hrd1 in B cells, mice bearing loxP-flanked Hrd1 allele (Hrd1f/f) were crossed with mice expressing CD19 promoter-driven Cre recombinase (CD19-Cre) to generate mice with a conditional B-cell–specific deletion of Hrd1. Real-time RT-PCR analysis detected a more than 90% reduction in Hrd1 expression in B cells from Hrd1 KO mice (SI Appendix, Fig. S1B), which was further confirmed at the protein level as determined by Western blotting (SI Appendix, Fig. S1C). B-cell development appears to be normal because both the percentages and the absolute cell numbers of the B220+IgM− pro/pre-B cells and B220lowIgM+ immature B cells are comparable between WT and Hrd1 KO mice (Fig. 1 A–C). However, deletion of Hrd1 resulted in significantly reduced number of B220HIIgM+ circulating mature B cells in the bone marrow (Fig. 1 A–C). Consequently, peripheral B220+ cells were reduced in the spleen (Fig. 1 D and F), indicating that Hrd1 functions are required for maintaining the B-cell compartment in mice. Further analysis of the splenic B cells revealed a significant reduction in the B220+IgMlowCD21INTCD23hi follicular, transitional B220+IgMHICD21−CD23− T1, B220+IgMHICD21HICD23+ T2, and B220+IgM+CD5+ B1 B cells, but a slight increase in IgMHICD21HICD23low marginal zone (MZ) B cells (SI Appendix, Fig. S1 D–G). Because Hrd1 deletion is specific to CD19-expressing cells, although proportionally higher due to the reduction of peripheral B cells, the number of CD3+CD4+ and CD3+CD8+ T cells, CD3−NK1.1+ NK, CD3lowNK1.1+ NKT, and CD11b+ myeloid cells in spleen were comparable in number between WT and Hrd1 KO mice (SI Appendix, Fig. S1 H–J). Therefore, loss of Hrd1 in CD19-expressing cells resulted in loss of mature B cells and particularly affected mature follicular B2 cells in the periphery.
Fig. 1.
Deletion of Hrd1 leads to reduced mature B cells and elevated B-cell death upon in vitro activation. (A–C) Bone marrow isolated from WT and Hrd1 KO mice was analyzed (A). Percentages (B) and absolute numbers (C) of B220LO IgM− pro-B and pre-B cells, B220LO IgM+ immature B cells, and B220HI IgM+ mature circulating B cells in A are indicated. (D–F) B220+ cells in spleen of WT and Hrd1 KO mice were analyzed (D). Percentages (E) and absolute numbers (F) of splenic B cells are indicated. (G–J) Splenic B cells were isolated from WT and Hrd1 KO mice. Purified B cells were stained with CFSE and cultivated with or without each indicated stimulation. The proliferation was analyzed by flow cytometry (G), and the MFI from five independent experiments are shown (H). Cell apoptosis was determined by AnnexinV and PI staining (I), and the average percentages of apoptotic cells from five independent experiments are shown (J). Error bars represent SD. n = 10. *P < 0.05, **P < 0.01, ***P < 0.001.
When stimulated with the antigenic stimuli α-IgM F(ab)2 or the addition of α-CD40 F(ab)2, WT and Hrd1 KO B cells proliferated at similar rates (Fig. 1 G and H), suggesting that Hrd1 is dispensable for B-cell proliferation upon in vitro stimulation. We then investigated if the reduction in peripheral B cells in Hrd1 KO mice was caused by increased apoptosis of B cells. Low frequencies of apoptotic cells as detected by AnnexinV and PI staining were found among freshly isolated splenic B cells from Hrd1 KO mice (Fig. 1 I and J). However, upon stimulation of BCR, CD40 costimulator, or Toll-like receptor (TLR4), we detected significantly increased apoptosis among activated B cells from Hrd1 KO mice (Fig. 1 I and J). As a positive control, when treated with the ER-stress inducer tunicamycin (TM), Hrd1 KO B cells underwent increased apoptosis compared with WT (Fig. 1 I and J). Therefore, Hrd1 functions are critical to protect B cells from both activation- and ER-stress–induced apoptosis.
Hrd1 Protects Peripheral B Cells from Activation-Induced Cell Death During Ag-Specific Immune Response.
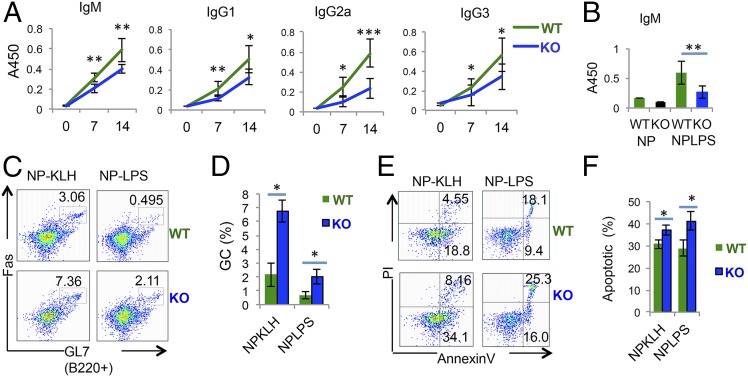
To study the function of Hrd1 in B cells in vivo, we immunized Hrd1 KO mice and their WT littermates with nitrophenylated-keyhole limpet hemacyanin (NP-KLH) or nitrophenylated-lipopolysaccharide (NP-LPS) to examine the thymus-dependent (TD) and thymus-independent (TI) humoral responses, respectively. Upon NP-KLH immunization, antigen-specific IgM, IgG1, IgG2a, and IgG were significantly reduced in the serum of Hrd1 KO mice compared with their WT littermates (Fig. 2A). Similarly, Hrd1 KO mice produced significantly fewer antigen-specific IgM antibodies compared with their WT littermates when immunized with NP-LPS (Fig. 2B). Interestingly, upon immunization with either TD or TI antigens, we detected an increase in the proportion of Fas (CD95)+ GL7+ germinal center (GC) B cells (Fig. 2 C and D). Despite this increase in Fas+ GL7+ GC B cells, we detected comparable frequencies of plasma cells (SI Appendix, Fig. S2 A–C) and NP-specific B cells (SI Appendix, Fig. S2 E–G) in immunized Hrd1 KO and WT mice. However, the absolute numbers of plasma cells (SI Appendix, Fig. S2D) and NP-specific B cells (SI Appendix, Fig. S2H) were dramatically reduced in immunized Hrd1 KO mice. Importantly, we detected a significantly increased amount of apoptotic cells in the B220+ compartment of immunized Hrd1 KO mice compared with WT littermates (Fig. 2 E and F), indicating that although Hrd1 is dispensable for B-cell clonal expansion and differentiation, it is required for survival of the B-cell compartment upon encountering antigen in vivo. Thus, both in vitro and in vivo studies revealed that Hrd1 is required for B-cell protection against AICD, possibly through Fas down-regulation.
Fig. 2.
Hrd1 KO B cells readily undergo apoptosis in vivo. (A) WT (black line) and Hrd1 KO mice were immunized with NP-KLH, and production of NP-specific Ig was analyzed by ELISA. (B) WT and KO mice were immunized with NP-LPS and NP-specific IgM detected by ELISA 7 d postimmunization. (C and D) Splenocytes were isolated from WT and KO mice immunized with NP-KLH (Left) or (NP-LPS) (Right) and analyzed for germinal center (GC) B cells by Fas and GL7 staining (C). Data from seven pairs of mice are shown (D). (E) Splenocytes isolated from WT and KO mice immunized with NP-KLH (Left) and NP-LPS (Right) mice were analyzed for apoptosis by AnnexinV and PI staining. (F) Quantification of AnnexinV+ apoptotic cells from seven pairs of mice as measured in E. Error bars represent SEM. For A and B, n = 9. For C–H, n = 5. *P < 0.05, **P < 0.01, ***P < 0.001.
Hrd1 Inhibits Fas Protein Cell Surface Expression During B-Cell Activation-Induced Apoptosis.
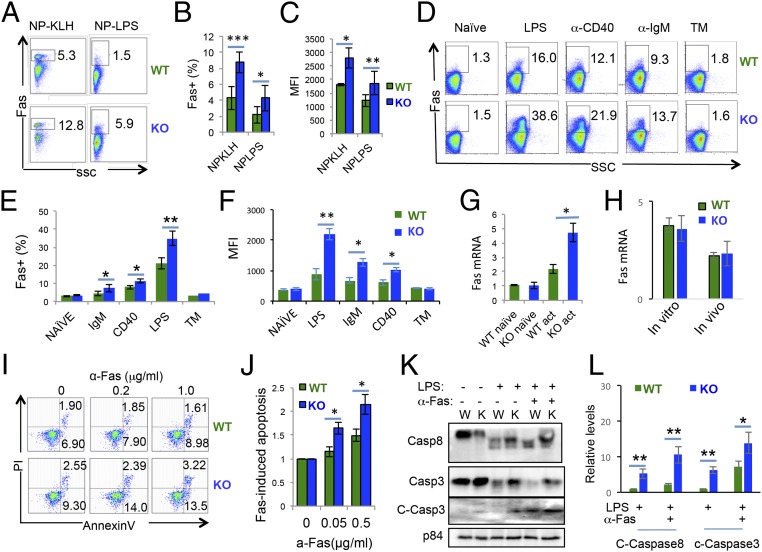
Fas is induced on activated B cells to downmodulate the immune response through AICD (12). When examining the splenocytes of immunized Hrd1 KO mice, we detected a significant increase in Fas expression on B cells in the spleen of mice immunized with either TI or TD antigens (Fig. 3 A and B). Importantly, the mean fluorescence identity (MFI) of Fas expression on the surface of Hrd1 KO B cells was significantly increased (Fig. 3D and SI Appendix, Fig. S3A), indicating that Hrd1 KO B cells undergo increased apoptosis upon activation due to increased Fas expression per cell base. Consistent with our in vivo experiments, we detected significantly increased Fas surface expression on Hrd1 KO B cells when activated in vitro by LPS, α-IgM, or α-CD40 (Fig. 3 D–F and SI Appendix, Fig. S3B), all of which have been shown to induce Fas expression on B cells (24). In contrast, tunicamycin treatment did not induce Fas expression on B cells (Fig. 3 D–F and SI Appendix, Fig. S3B), although Hrd1 KO B cells were more sensitized to ER-stress–induced cell death following tunicamycin treatment (Fig. 1 J and K). Upon LPS stimulation, Fas mRNA was also increased in activated Hrd1 KO B cells (Fig. 3G), possibly because of the increased percentages of Fas+ B cells in Hrd1 knockout mice. Indeed, Fas mRNA levels were comparable between WT and Hrd1 KO when we examined total mRNA from the sorted Fas+ B cells from either in vitro LPS stimulated B cells or from the spleen of NP-KLH–immunized mice (Fig. 3H). Therefore, Hrd1 appears to protect B cell from activation-induced cell death from downregulating Fas at the posttranscriptional level.
Fig. 3.
Fas is up-regulated in activated Hrd1 KO B cells. (A–C) Splenic B220+ cells were isolated from WT and Hrd1 KO mice immunized with NP-KLH (Left) or NP-LPS (Right) and analyzed for Fas expression (A). (B) Percentages of Fas+ cells (B) and MFI (C) of Fas as measured in A. (D–F) Splenic B cells were isolated from WT and KO mice and cultured with the indicated stimuli for 24 h. Fas expression was then measured by flow cytometry (D). Quantification of Fas+ cell percentages (E) and MFI (F) of Fas as measured in D. (G and H) quantitative PCR analysis of Fas mRNA in total naïve and LPS-stimulated WT and Hrd1 KO B cells (G) or sorted Fas+ B cells (H). (I) Splenic B cells were stimulated with LPS overnight, and agonistic anti-Fas antibody added for additional 4 h. Apoptosis was detected by AnnexinV and PI staining. (J) Apoptosis induced by Fas was calculated by normalizing to apoptosis induced by LPS alone. (K) Splenic B cells from WT and KO mice were either freshly isolated or cultured with LPS alone or LPS and anti-Fas. Cell lysates were then subjected to immunoblot, and Fas activation was detected based on the presence of cleaved caspase 8 (Top) and cleaved caspase 3 (Middle). P84 (Bottom) served as loading control (J). (L) The relative levels of cleaved caspase 8 and 3 from four independent experiments are shown. Error bars represent SEM. For A and B, n = 5. For C–H, n = 7. *P < 0.05, **P < 0.01, ***P < 0.001.
Consistent with increased Fas expression, treatment of LPS-stimulated B cells with agonistic Fas antibody resulted in increased apoptosis in Hrd1 KO B cells (Fig. 3 I and J), demonstrating that increased Fas expression in Hrd1 KO B cells sensitizes the cells to Fas-mediated apoptosis. To further support this conclusion, we observed that neutralization of the FasL significantly inhibited both WT and Hrd1 KO B-cell death upon LPS stimulation (SI Appendix, Fig. S3 C and D). Fas signaling leads to recruitment of FADD (Fas-associated protein with death domain) and cleavage of caspase-8, which then induces cleavage of proapoptotic caspase-3 (25). As expected, whereas addition of agonistic Fas antibody increased caspase-8 and caspase-3 cleavage in both WT and Hrd1 KO B cells, the levels of cleaved caspase-8 and caspase-3 were consistently higher in Hrd1 KO B cells, with LPS stimulation alone, or with LPS and anti-Fas antibody (Fig. 3 K and L). Thus, increased AICD in Hrd1 KO B cells is due to increased Fas expression and sensitization to Fas-mediated apoptosis.
Hrd1 Inhibits the Activation-Induced B-Cell Apoptosis Independent of ER Stress.
Hrd1 has been shown to protect against ER-stress–induced apoptosis through destructing the misfolded proteins (26, 27). To determine the contribution of UPR (unfolded protein response)-induced apoptosis in Hrd1 KO peripheral B cells, we examined the induction of ER-stress target genes. Whereas LPS stimulation induced expression of the ER-stress–related genes Xbp1s, Bip, Chop, Erdj3, and Wsj4, we did not detect a significant difference in their expression levels between WT and Hrd1 KO B cells (SI Appendix, Fig. S4 A–E), implying that Hrd1 gene deletion unlikely resulted in the accumulation of misfolded/unfolded protein under the physiological condition. To further validate this conclusion, we demonstrated that further deletion of the UPR-induced proapoptotic protein CHOP did not rescue the reduction in the B cells in spleen of Hrd1 KO mice (SI Appendix, Fig. S5 A and B). Upon activation in vitro by anti-IgM(Fab) stimulation, CHOP deletion did not enhance B-cell AICD, and further CHOP deletion failed to protect Hrd1-null B cells from the activation-induced apoptosis (SI Appendix, Fig. S5 C and D). Consistent to this notion, genetic deletion of CHOP did not affect Fas expression in activated B cells (SI Appendix, Fig. S5 E and F), further confirming that Hrd1 protects B cells from AICD independent of UPR response. We have recently discovered that Hrd1 suppresses ER-stress–induced cell death through targeting the downstream ER-stress sensor IRE1α for ubiquitination and degradation (27, 28). However, further deletion of IRE1α in Hrd1 KO mice could not rescue the reduction in the peripheral B cells in spleen (SI Appendix, Fig. S6 A and B). The up-regulation of Fas and activation-induced apoptosis was comparable between Hrd1 KO and DKO (Hrd1f/f Ire1af/f CD19-Cre) mice (SI Appendix, Fig. S6 C–F), indicating that Hrd1 KO B cells up-regulate Fas and are sensitized to Fas-mediated apoptosis through mechanisms other than the UPR pathway.
Hrd1 Is a Fas Ubiquitin Ligase in B Cells.
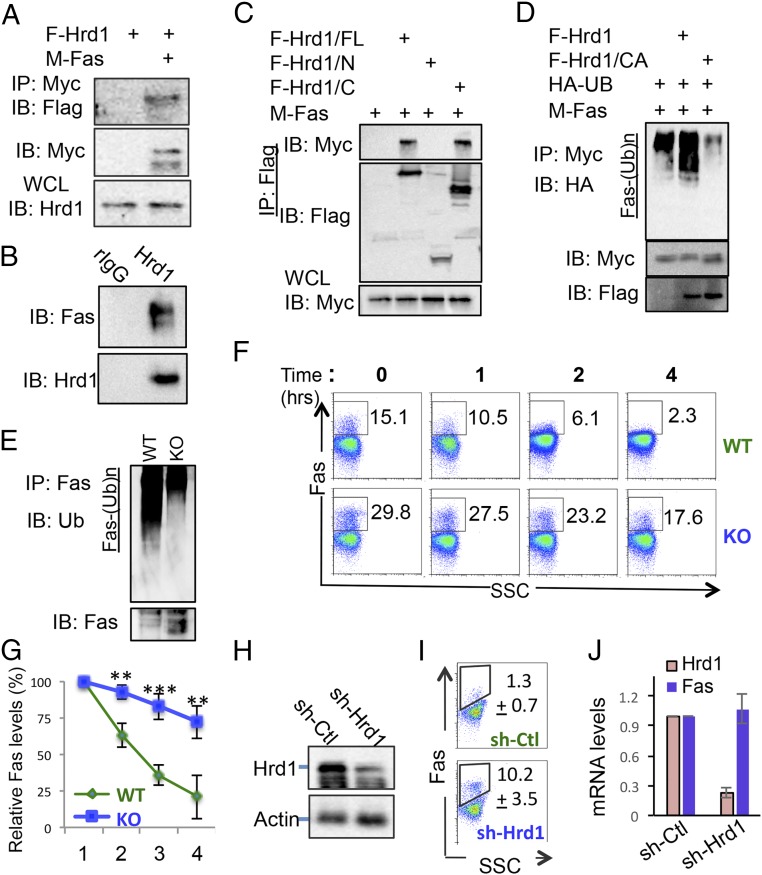
Because loss of Hrd1 expression resulted in the increased Fas protein expression, we speculated that Hrd1 functions as an E3 ubiquitin ligase to control Fas protein expression in B cells. In support of this hypothesis, we detected Hrd1 interaction with Fas in transiently transfected HEK293 cells (Fig. 4A). The interaction between endogenous Hrd1 and Fas was confirmed with coimmunoprecipitation of Hrd1 and Fas in A20 mature B cells (Fig. 4B). Furthermore, cotransfection of Flag-tagged Fas with full-length (FL) or truncated mutants of Hrd1 revealed that the cytoplasmic C terminus of Hrd1 is required and sufficient for its interaction with Fas (Fig. 4C), indicating that Hrd1 interacts with Fas through its cytoplasmic region. As E3 ligase often catalyzes the ubiquitin-conjugation to its interaction proteins, we then asked whether Hrd1 is an E3 ubiquitin ligase for Fas. As indicated in Fig. 4D, expression of Hrd1, but not its E3 ligase-inactive CA mutant, dramatically enhanced Fas ubiquitin conjugation. To further investigate whether Hrd1 ubiquitinates Fas under physiological conditions, we stimulated WT and Hrd1 KO B cells with LPS and immunoprecipitated the endogenous Fas receptor. Consistent with our in vitro results, we detected reduced levels of ubiquitination of Fas in LPS-stimulated Hrd1 KO B cells compared with WT cells, even though a higher amount of Fas was immunoprecipitated from Hrd1 KO B-cell lysates (Fig. 4E). Moreover, the reduction of Fas ubiquitination in Hrd1 KO B cells correlated with reduced Fas protein degradation, as LPS-stimulated Hrd1 KO B cells showed increased and more stable surface expression of Fas at 0, 1, 2, and 4 h after CHX treatment (Fig. 4 F and G). Taken together, our data indicate that Hrd1 recruits Fas protein through its cytoplasmic C terminus to catalyze Fas for ubiquitination and degradation. Therefore, Fas is a target of Hrd1 E3 ubiquitin ligase activity, and the loss of Hrd1 leads to stabilization and increased expression of Fas.
Fig. 4.
Hrd1 is an E3 ubiquitin ligase of Fas. (A) HEK293 cells were transfected with Flag-tagged Hrd1 (F-Hrd1) and Myc-tagged Fas (M-Fas). The interaction of Fas with Hrd1 was determined by co-IP and Western blotting (Top). The expression levels of Fas (Middle) and Hrd1 (Bottom) in transiently transfected A20 cells were analyzed. (B) A20 cell lysates were immunoprecipitated with either IgG control (ctrl) or anti-Fas antibody. Interaction with Hrd1 was detected by immunoblotting with anti-Hrd1 antibody. (C) Myc-tagged Hrd1 or its truncated mutants were cotransfected with Flag-tagged Fas into HEK293T cells, and their interaction was analyzed as in A. (D) Myc-tagged Fas was either cotransfected with Flag-tagged Hrd1 or its CA mutant and HA-tagged ubiquitin. Fas proteins in the lysates of transfected cells were then immunoprecipitated and their ubiquitination was detected by anti-HA antibody. (E) LPS-stimulated splenic B cells from WT and Hrd1 KO mice were lysed and immunoprecipitated with anti-Fas antibody. Ubiquitination of Fas was detected by immunoblotting with anti-Ub. (F) LPS-stimulated WT and KO splenic B cells were treated with CHX for 0, 1, 2, and 4 h, and Fas stability was measured by flow cytometry. (G) Degradation calculated as reduction in Fas+ cells compared with 0 h as measured in F. (H–J) A20 cells were transfected with Hrd1-specific shRNA, and GFP+ cells were sorted. Hrd1 expression was confirmed by Western blotting (H). The Fas expression was determined by flow cytometry, and data shown are the average percentages from four independent experiments (I, P < 0.001). The mRNA levels of Fas and Hrd1 in Hrd1 knockdown and control A20 cells were determined by real-time PCR (J). Error bars represent SEM. n = 11. *P < 0.05, **P < 0.01, ***P < 0.001. IB, immunoblotting; IP, immunoprecipitation; WCL, whole cell lysate.
The DNA recombinase expression driven under the CD19 promoter mediates deletion of floxed gene during the pro-B stage of B-cell development, raising a possibility that the elevated Fas expression in mature B cells from Hrd1 KO mice could be a consequence of B-cell developmental defect. To test this, we used a Hrd1-specific shRNA knockdown approach to inhibit Hrd1 expression in a human B-cell line, A20. Hrd1-specific shRNA expression resulted in an about 90% reduction in Hrd1 protein and its mRNA expression (Fig. 4 H and J). Of note, suppression of Hrd1 expression resulted in a significant increase in Fas+ A20 cells without affecting Fas mRNA expression (Fig. 4 I and J), clearly indicating that Hrd1 regulates Fas protein degradation in a cell-autonomous manner.
Deficient Fas Signaling in MRL/lpr Mice Abrogates Increased AICD in Hrd1 KO Mice.
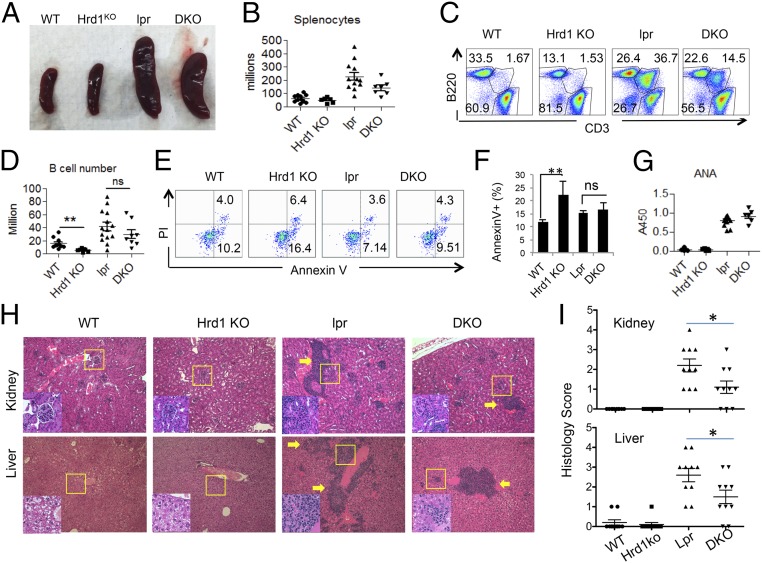
To confirm that Hrd1 protects B cells from AICD through degradation of Fas, we generated Fas-deficient Hrd1 KO (DKO) mice by crossing MRL/lpr Fas mutant mice (Fas KO) with B-cell–specific Hrd1 KO mice (Hrd1f/f CD19-Cre+Faslpr/lpr). Mice at the mixed genetic background were used, and littermates were used as controls. Defective Fas signaling in MRL/lpr mice has been reported to lead to splenomegaly and lymphadenopathy (29). Indeed, we observed that Fas KO mice at 8–16 wk of age exhibited splenomegaly, and, notably, further deletion of Hrd1 failed to alter this splenomegaly phenotype, as both the spleen sizes and total splenocyte numbers were comparable between Fas KO and DKO mice (Fig. 5 A and B). In contrast to the significant reduction in B-cell numbers in Hrd1 KO mice compared with WT mice, the CD3−B220+ B cells in Fas KO and DKO spleens were comparable, suggesting that the reduced B-cell phenotype is abrogated in DKO mice (Fig. 5 C and D). Surprisingly, Hrd1 deletion in CD19+ cells in Fas KO mice led to a dramatic reduction in CD3low B220+ T cells (29) (Fig. 5C), suggesting that, in contrast to a Fas-dependent role of Hrd1 in B cells, Hrd1 appears to regulate CD3low B220+ T cells in a Fas-independent manner.
Fig. 5.
Fas mutation in MRL/lpr mice rescue increased AICD phenotype in Hrd1 KO mice. (A) Spleens isolated from 16-wk-old WT, Hrd1 KO, lpr (Fas KO), and DKO mice. (B) Number of splenocytes in 8- to 16-wk-old WT, Hrd1 KO, lpr, and DKO mice. (C) Percentage of CD3− B220+ cells in WT, Hrd1 KO, Fas KO, and DKO spleens. (D) Number of B cells were calculated based on B-cell percentage and splenocyte number. (E) WT, Hrd1 KO, Fas KO, and DKO B cells were isolated from spleen and cultured in LPS overnight, followed by a 4-h incubation with anti-Fas antibody. Apoptotic B cells were then analyzed by AnnexinV and PI staining. (F) Percentage of AnnexinV+ apoptotic cells as analyzed in E. (G) Serum Ig from 16-wk-old WT, Hrd1 KO, Fas KO, and DKO mice was analyzed for ANA. (H) H&E staining of kidneys (Top) and liver (Bottom) isolated from mice from G. (I) Histological score for lymphocyte infiltration in the kidney (Top) and liver (Bottom). Error bars represent SEM. ns, not significant. n = 7. *P < 0.05, **P < 0.01, ***P < 0.001.
Consistent with our hypothesis that Hrd1 protects B cells from Fas-mediated AICD, upon stimulation, Hrd1 KO B cells underwent higher rates of apoptosis, but Fas KO and DKO B cells exhibited similar rates of apoptosis as WT B cells (Fig. 5 E and F). Moreover, loss of Fas-mediated apoptosis led to expansion of autoreactive B cells and increased autoantibody production in Fas KO mice (29). As expected, Fas KO mice had increased antinuclear antibodies (ANA), and DKO mice had similarly increased levels of ANA (Fig. 5 F and G), indicating that Hrd1-mediated Fas ubiquitination is important for elimination of self-reactive B cells. Of note, in contrast to the similar anti-ANA levels in Fas KO and DKO mice, lymphocyte infiltration in both the liver and kidney was partially attenuated by B-cell–specific Hrd1 deletion (Fig. 5 H and I). Thus, these DKO studies indicate that Hrd1 protects against apoptosis through degradation of Fas. Because Fas-mediated cell death is a crucial mechanism in various physiological processes, such as AICD and autoimmunity, our identification of Hrd1 as a regulator of Fas stability could provide insight for a wide variety of biological applications.
Discussion
Here we report that Hrd1 is required for protecting mature B-cell population from the activation-induced apoptosis through degradation of the death receptor Fas/CD95. This conclusion is validated by the following observations: (i) Conditional deletion of Hrd1 specifically in B-cell lineage results in reduced mature B cells in the peripheral lymphoid organs; (ii) Hrd1-deficiency sensitizes Fas-mediated activation-induced B-cell death, which can be largely blocked by FasL neutralization; (iii) Hrd1 inhibits per cell-based Fas protein but not mRNA expression in B cells; (iv) Hrd1 catalyzes Fas ubiquitination and promotes Fas protein degradation in B cells; and (v) Hrd1 regulates B-cell autoimmune response in a Fas-dependent manner in the lupus–prion MRL/lpr mice.
Hrd1 functions as a critical regulator in protecting B cells from AICD, based on our observation that apoptosis in Hrd1 KO peripheral B cells upon antigenic stimulation was significantly increased. Fas was up-regulated at both the mRNA and protein level, initially suggesting that Fas expression might be indirectly induced upon increase of Hrd1 substrates, such as p53 or IRE1α (27, 30). However, we did not detect a significant increase in the p53-target genes PUMA and p21 in activated Hrd1 KO peripheral B cells compared with WT. In addition, although we observed increased apoptosis with either antigenic stimuli or ER stress induced by tunicamycin, we found that Hrd1 KO peripheral B cells increased Fas expression upon antigenic stimulation, but not upon increased ER stress. Indeed, deletion of the UPR targets IRE1α and CHOP did not abrogate increased Fas expression or rescue the increased susceptibility to apoptosis of Hrd1 KO B cells, suggesting that up-regulation of Fas in activated Hrd1 KO B cells is unlikely to be caused by increased ER stress or p53 activation. The fact that per-cell Fas protein levels (MFI) but not mRNA expression levels were increased in Hrd1-null B cells during activation indicates that Hrd1 suppresses B-cell apoptosis through destruction of Fas protein. Indeed, we found that Hrd1 interacts directly with Fas protein, and loss of Hrd1 in B cells leads to decreased Fas ubiquitination, resulting in increased Fas protein stability upon antigenic stimulation.
Hrd1 regulates the B-cell immune response largely through the death receptor Fas-dependent manner. When cultured with LPS, both lpr (Fas KO) and Fas/Hrd1 double KO B cells had comparable apoptosis, indicating that Fas deficiency abrogated the proapoptotic phenotype induced by Hrd1 deletion. As a result, Fas KO and Fas/Hrd1 KO mice had similar B-cell numbers and comparable ANA levels. These discoveries provide a proof-of-principle for the Fas-dependent role of Hrd1 in AICD. However, while not largely abolished, lymphocyte infiltration was significantly reduced by further Hrd1 deletion in Fas KO mice. This reduction is unlikely due to the changes in autoantibody production, because the ANA levels were comparable between Fas KO and DKO mice. Interestingly, this reduction in lymphocyte infiltration was associated with a decrease in CD3lowB220+ cells, which are derived from thymus. Recent studies suggest that the CD3lowB220+ cells in lpr mice are innate lymphoid cells and play important roles in organ inflammation (31). It will be interesting to further study how Hrd1 regulates the development of CD3lowB220+ cells independent of Fas destruction.
Experimental Procedures
Animals.
Animal strains are detailed in SI Appendix. All mice used in this study were maintained and used at the Northwestern University Mouse Facility under pathogen-free conditions according to institutional guidelines. All of the animal studies including antigen immunization and collecting of the lymphoid organs have been approved by the Institutional Animal Care and Use Committee of Northwestern University. No human study is involved.
Primary B-Cell Isolation and Culture.
Primary B cells were negatively or positively isolated from 8- to 12-wk-old mice. Purified B cells were stimulated with goat F(ab)2 anti-mouse IgM (10 mg/mL; Jackson Immunoresearch), anti-CD40 (1 mg/mL; eBioscience), LPS (500 ng/mL), and tunicamycin as indicated. Cell proliferation and death were determined as detailed in SI Appendix.
Immunizations.
The antigen-specific B-cell immune response of WT and Hrd1 KO mice was analyzed as detailed in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Ira Tabas (Richard J. Stock Professor and Vice-Chairman of Research, Department of Medicine, Columbia University) for the CHOP-floxed mice. We thank members of the D.F. Laboratory for critical reading of the manuscript and constructive suggestions during our research. This work was supported by NIH R01 Grants AI079056, AI108634 and AR006634 (to D.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606742113/-/DCSupplemental.
References
- 1.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Levine MH, et al. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci USA. 2000;97(6):2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183(6):3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 4.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237(1):205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 5.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 7.Allen CD. Germinal center quality control: Death by Fas. Immunity. 2015;42(5):783–785. doi: 10.1016/j.immuni.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Butt D, et al. FAS inactivation releases unconventional germinal center B cells that escape antigen control and drive IgE and autoantibody production. Immunity. 2015;42(5):890–902. doi: 10.1016/j.immuni.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Scott DW, Grdina T, Shi Y. T cells commit suicide, but B cells are murdered! J Immunol. 1996;156(7):2352–2356. [PubMed] [Google Scholar]
- 10.Sharma K, et al. Death the Fas way: Regulation and pathophysiology of CD95 and its ligand. Pharmacol Ther. 2000;88(3):333–347. doi: 10.1016/s0163-7258(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 11.Ju S-T, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 12.Hao Z, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29(4):615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Germinal center B cells govern their own fate via antibody feedback. J Exp Med. 2013;210(3):457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe D, Suda T, Nagata S. Expression of Fas in B cells of the mouse germinal center and Fas-dependent killing of activated B cells. Int Immunol. 1995;7(12):1949–1956. doi: 10.1093/intimm/7.12.1949. [DOI] [PubMed] [Google Scholar]
- 15.Andrews BS, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher GH, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81(6):935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, et al. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98(5):1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aït-Azzouzene D, et al. Deletion of IgG-switched autoreactive B cells and defects in Fas(lpr) lupus mice. J Immunol. 2010;185(2):1015–1027. doi: 10.4049/jimmunol.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol Cell. 2007;28(4):544–554. doi: 10.1016/j.molcel.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christianson JC, et al. Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol. 2011;14(1):93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita H, et al. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1β. EMBO J. 2015;34(8):1042–1055. doi: 10.15252/embj.201489897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, et al. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med. 2014;211(12):2467–2479. doi: 10.1084/jem.20140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T, et al. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28(7):708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2(3):261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 25.Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85(6):817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 26.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao B, et al. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 2008;9(5):480–485. doi: 10.1038/embor.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun S, et al. IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat Cell Biol. 2015;17(12):1546–1555. doi: 10.1038/ncb3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S. Mutations in the Fas antigen gene in lpr mice. Semin Immunol. 1994;6(1):3–8. doi: 10.1006/smim.1994.1002. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki S, Yagishita N, Nishioka K, Nakajima T. The roles of synoviolin in crosstalk between endoplasmic reticulum stress-induced apoptosis and p53 pathway. Cell Cycle. 2007;6(11):1319–1323. doi: 10.4161/cc.6.11.4277. [DOI] [PubMed] [Google Scholar]
- 31.Rharbaoui F, et al. Characterization of a B220+ lymphoid cell subpopulation with immune modulatory functions in nasal-associated lymphoid tissues. J Immunol. 2005;174(3):1317–1324. doi: 10.4049/jimmunol.174.3.1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.