Pain is a major clinical, social, and economic problem, affecting more people than diabetes, heart disease, and cancer combined (1). Pain medicine represents a large family of compounds that are commonly prescribed or sold over-the-counter for treating various painful conditions. Among these medications, opioids stand out as the most efficacious for the control of moderately severe to severe pain and are widely used. Despite their effectiveness, opioid use has its “dark side”: opioids produce many clinically significant side effects, from pruritus, constipation, dependence, to respiration depression. In addition, opioids are among the most abused drugs and prescription opioid abuse has reached an epidemic level in recent years. Opioids are truly a double-edged sword. For decades, research pursued the “Holy Grail” of opioid analgesic research: the development of opioids that retain the analgesic efficacy with reduced side effects. In PNAS, Ding et al. (2) report a systematic evaluation of a novel opioid, BU08028, which may lead us one step closer to this ultimate goal.
Efforts to Create a Better Opioid Analgesic
Human use of opium poppy for religious rituals and for treating various ailments can be dated back thousands of years. A brief historical review on this topic was published in this journal (3), and no attempt will be made here to reiterate this history. Although morphine was discovered as the primary active component in opium poppy responsible for analgesia more than two centuries ago, scientific understanding of its pharmacology is a relatively recent event. There exists a fine-tuned endogenous opioid system in our body that is involved in various biological processes critical for our survival, which includes several endogenous peptides and four different receptors to which these peptides bind: µ, δ, κ, and nociceptin (NOP) receptors (4). Since the 1950s, dozens of synthetic and semisynthetic opioids have been developed and used clinically for pain management, treatment of opioid addiction, and rescue of opioid overdose. These opioids represent decades of collective efforts from medicinal chemists and pharmacologists, and are largely the result of tweaking the efficacy and affinity of the compounds on opioid receptors. They include compounds ranging from highly efficacious and potent opioids, such as fentanyl, and along the spectrum of efficacy all of the way down to opioid antagonists, such as naloxone. However, these compounds were mostly developed before the opioid receptors were identified and most primarily act on µ- or κ-opioid receptors. Because analgesia and many adverse effects of opioids are attributable to the activation of µ-opioid receptors, it is not surprising that the adverse effects, such as respiratory depression and abuse, are highly correlated with their analgesic efficacy. In fact, the drugs that are most widely used in the clinic such as hydrocodone, are among the most widely abused drugs.
The δ- (DOR) and µ-opioid receptors (MOR) were cloned in 1992 and 1993, respectively (5–7). Since then there has been an explosion in our understanding of how opioids work at the molecular level. In recent years, equipped with this new knowledge, great efforts have been made and many creative approaches have been tried to develop better drugs for treating pain. For example, MOR activation leads to two different signaling pathways: G protein signaling and β-arrestin recruitment, the former being associated with analgesia and the latter being associated with opioid adverse effects, such as respiratory depression and reduced gastrointestinal transit. The ability of drugs to activate, preferentially or not, two or more pathways is termed “functional selectivity” and an agonist that activates one pathway over the other is called a “biased agonist.” TRV130 is a G protein-preferring biased agonist at MOR that is currently under clinical development, and initial clinical trials demonstrated that TRV130 has analgesic activity that is similar to classic MOR agonists such as morphine, but produces less opioid-induced side effects (8). Another strategy that has been actively pursued is to retain the analgesic effects of opioids while reducing their abuse liability by using different abuse-deterrent techniques. For example, Suboxone is a sublingual tablet preparation that combines the MOR agonist buprenorphine and the MOR antagonist naloxone and is used to treat opioid dependence and pain. When taken orally, naloxone goes through extensive first-pass metabolism. However, if taken parenterally for nonmedical use by opioid-dependent patients, naloxone precipitates a withdrawal syndrome, thus deterring its abuse (9). It is yet to be seen whether these approaches will lead to breakthrough in the treatment of pain or addiction.
Bifunctional Opioid Agonists as Novel Analgesics
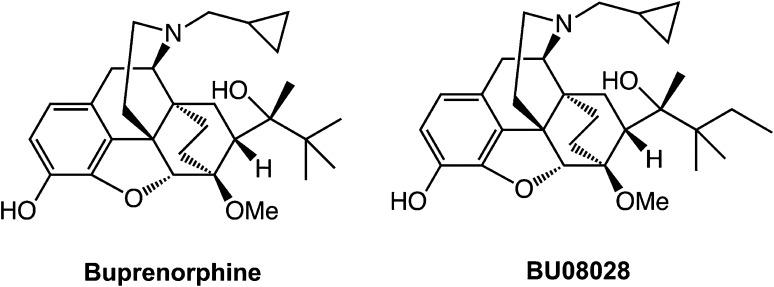
As reported by Ding et al. (2) in PNAS, BU08028 represents another innovative approach to develop efficacious yet less-addictive opioid analgesics. Most opioids bind to more than one opioid receptor. The earlier classification of opioid mixed agonist-antagonists include opioids that act as an agonist or partial agonist at one receptor and an antagonist at another (e.g., pentazocine and butorphanol) and opioids that act as a partial agonist at a single receptor (buprenorphine). Among these drugs, buprenorphine is particularly interesting (Fig. 1). Buprenorphine is a partial agonist at MOR and also has very low efficacy at the NOP receptor. Buprenorphine is widely used in both human and veterinary medicine to treat pain and is also used to treat opioid dependence (10). However, buprenorphine shares many adverse effects with other opioid receptor agonists, including abuse potential. Recent research suggests that pharmacological agonists of NOP receptors are effective in several rodent and nonhuman primate models of pain without effects that would predict abuse (11, 12). Therefore, NOP receptor agonists could represent a novel class of analgesics in their own right. An alternative reasoning is that if buprenorphine can be chemically modified to preserve its activity at MOR while improving its activity at NOP receptors, it may be possible to create a bifunctional µ/NOP opioid agonist that is superior to buprenorphine. The study by Ding et al. (2), supported by the National Institute of Drug Abuse/National Institutes of Health, which has a long-term history of supporting the development of nonaddictive analgesics, did just that.
Fig. 1.
The chemical structures of buprenorphine and BU08028.
Initial efforts to modify buprenorphine yielded BU72, a bridged pyrrolidinomorphina, which turned out to be a highly efficacious MOR agonist. BU72 is an effective analgesic with significant respiratory depression activity, which precluded it from further development (13). The same chemists' recent medicinal chemistry efforts yielded another buprenophine analog, BU08028 (Fig. 1), which in vitro has similar efficacy at MOR but much higher efficacy at NOP receptors compared with buprenorphine (14). In mice, BU08028 demonstrated long-lasting antinociceptive effects in a tail flick pain assay and the effect is primarily mediated through MOR (14). Importantly, BU08028 also produced conditioned place preference in mice, a paradigm that measures the rewarding effect of drugs, suggesting that BU08028 might have clinically relevant abuse liability (14). The report by Ding et al. (2) represents a significant advance from the prior study in using mice in that it used highly translational nonhuman primate models to systematically assess both therapeutic effects (analgesia) and several safety related effects that are associated with the clinical use of opioids.
Nonhuman primates may have better translational value than rodents because of their many similarities to humans in physiology, neuroanatomy, reproduction, and social complexity (15). Ding et al. (2) found that in two different monkey pain models, BU08028 dose-dependently produced antinociception (warm water tail withdrawal assay) and reduced thermal allodynia (capsaicin-induced thermal allodynia), being 10-fold more potent and lasting longer than buprenorphine. This finding contrasts what was observed in mice, where buprenorphine was at least 10-fold more potent than BU08028 (14). More importantly, the antinociceptive effect of BU08028 was significantly attenuated by the opioid receptor antagonist naltrexone and the NOP receptor antagonist J-113397. These findings also contrast results in mice, where there was no evidence that BU08028-induced antinociception was mediated through NOP receptors (14). These qualitative differences emphasize the importance of examining drug effects across multiple species before moving to human clinical trials, and further support the value of nonhuman primates in the development of analgesic drugs. The Ding et al. (2) study also examined several clinically significant adverse effects associated with opioid use: pruritus, abuse liability, respiratory depression, and physical dependence. The use of nonhuman primates to study these effects in the field of opioid behavioral pharmacology is time-consuming, technically challenging, but highly desirable, because over half a century of preclinical research has convincingly demonstrated the high predictive validity and translational value of these procedures (16). Unlike the MOR agonist fentanyl, which produced robust scratching at a dose that produced the maximal possible antinociceptive effect in both pain assays, BU08028 failed to produce scratching. This result might be due to the limited efficacy of BU08028 at MOR. In the intravenous self-administration procedure, an assay that is considered the “gold standard” for measuring the positive reinforcing effects of drugs (16), BU08028 failed to maintain self-administration behavior higher than saline, which is in contrast to high rates of self-administration maintained by cocaine and the MOR agonists remifentanil and buprenorphine. A progressive ratio procedure that measures reinforcing strength further confirms the limited positive-reinforcing effects of BU08028, suggesting that BU08028 has limited, if any, abuse liability. At a dose of BU08028 10 times larger than the dose that produced maximal antinociceptive effects, BU08028 failed to affect respiration rate, minute volume, or tidal volume, further demonstrating an excellent safety profile of BU08028 and confirming its limited efficacy at MOR. Finally, Ding et al. (2) examined the physical dependence potential of BU08028. Unlike morphine treatment, which produced significant physical dependence as evidenced by naltrexone-precipitated withdrawal signs that included increased respiratory and cardiovascular activity, neither naltrexone nor the NOP receptor antagonist J-113397 precipitated signs of withdrawal in monkeys treated with BU08028. This study describes a buprenorphine analog that is a bifunctional µ/NOP receptor partial agonist, with significant analgesic activity and a superior safety profile compared with buprenorphine. This finding validates µ/NOP receptor dual agonism as a viable strategy to develop next-generation safe and nonaddictive drugs for treating pain.
The use of nonhuman primates, the determination of full dose-effect curves, and the incorporation of both therapeutic and safety pharmacology assessments strengthen the potential value of these results and boost confidence of quickly translating these findings to clinical trials and eventually medical practice. This research strategy should be encouraged in the preclinical evaluation of other drugs for treating pain in particular, and any new chemical entities in general.
Acknowledgments
The author is supported by National Institute on Drug Abuse/National Institutes of Health Grants R01DA034806 and R21DA040777.
Footnotes
The author declares no conflict of interest.
See companion article on page E5511.
References
- 1.Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Ding H, et al. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci USA. 2016;113:E5511–E5518. doi: 10.1073/pnas.1605295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownstein MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toll L, Bruchas MR, Calo’ G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev. 2016;68(2):419–457. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258(5090):1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA. 1992;89(24):12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44(1):8–12. [PubMed] [Google Scholar]
- 8.Viscusi ER, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 9.Orman JS, Keating GM. Buprenorphine/naloxone: A review of its use in the treatment of opioid dependence. Drugs. 2009;69(5):577–607. doi: 10.2165/00003495-200969050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Raffa RB, et al. The clinical analgesic efficacy of buprenorphine. J Clin Pharm Ther. 2014;39(6):577–583. doi: 10.1111/jcpt.12196. [DOI] [PubMed] [Google Scholar]
- 11.Ko MC, et al. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34(9):2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiguchi N, Ding H, Ko MC. Central N/OFQ-NOP receptor system in pain modulation. Adv Pharmacol. 2016;75:217–243. doi: 10.1016/bs.apha.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neilan CL, et al. Characterization of the complex morphinan derivative BU72 as a high efficacy, long-lasting mu-opioid receptor agonist. Eur J Pharmacol. 2004;499(1-2):107–116. doi: 10.1016/j.ejphar.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 14.Khroyan TV, et al. The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): Characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther. 2011;336(3):952–961. doi: 10.1124/jpet.110.175620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips KA, et al. Why primate models matter. Am J Primatol. 2014;76(9):801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70(3) Suppl:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]