For southern African grasslands, many hypotheses have been posed and contested to explain bare circular areas (“fairy circles”) (1). Getzin et al. (2) “discovered” similar bare areas in arid grasslands of Australia and investigated their causes. Their data and modeling supported the hypothesis that soil crusting, water flow, and plant biomass feedbacks drove self-organizing vegetation patterns. Alternative causal factors, including termites, were investigated but rejected (2). Although we accept that water redistribution occurs between bare and vegetated areas in Australian desert grasslands, we have evidence that bare patches are subterranean termitaria, both active and inactive (abandoned).
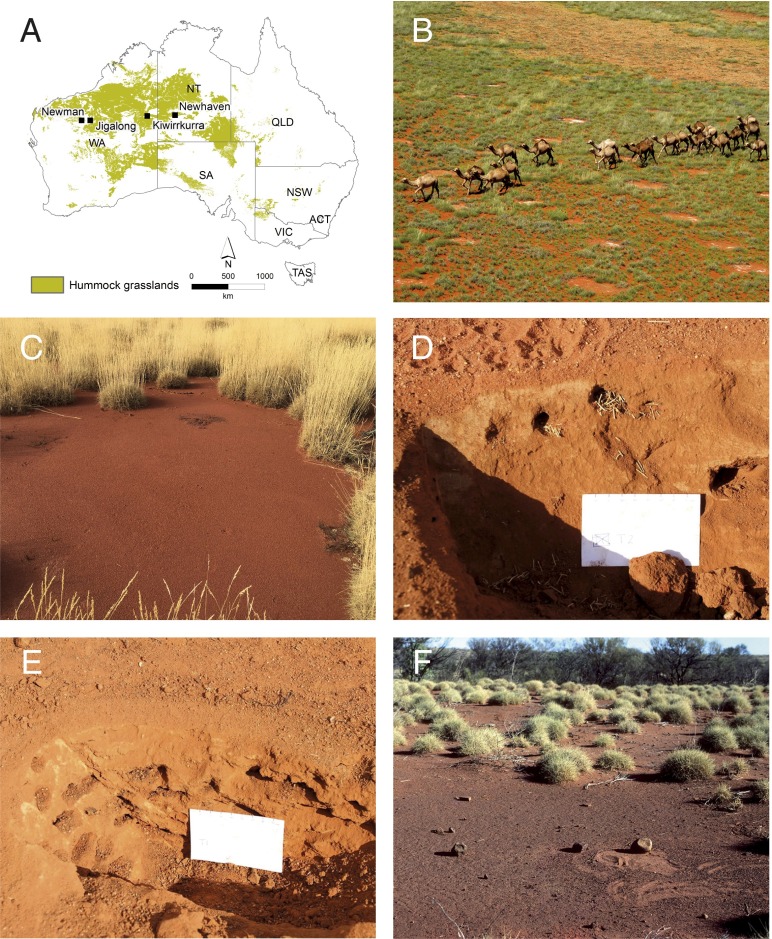
We have frequently observed bare circular areas that appear as “gaps” as defined by Getzin et al. (2) but are actually pavement termitaria (3). These gaps are common across more than 1,200 km from Newman (Western Australia) to Newhaven (Northern Territory) (Fig. 1 A and B) in Triodia spp. (“spinifex”) hummock grasslands with scattered Acacia aneura (“mulga”) shrublands. Our preliminary excavations beneath circles at four locations (Table 1) revealed all gaps had termitaria typical of Drepanotermes harvester termites (3, 4). Termite chambers occurred within 5 cm of the surface within a metastable matrix (Fig. 1 D and E).
Fig. 1.
Circular bare areas at oblique, ground level, and subterranean views in Australian desert spinifex grasslands. (A) Australian distribution of Triodia hummock grasslands. Our excavations of circular bare areas near Newman, Jigalong, Kiwirrkurra, and Newhaven revealed that all were termitaria. Map by N. Raisbeck-Brown (Commonwealth Scientific and Industrial Research Organisation, Geelong, Australia). (B) Triodia hummock grassland with circular bare areas and a recent burn (background right) at Newhaven (NT, Australia). Feral camels give scale. Image by J.S. (C) Bare area in Triodia basedowii grassland at Newman (23.43730°S, 119.81839°E). An aeolian surface sand layer obscures most of the termitarium pavement, but a low termite mound is present on the far side (pocket knife indicates scale). Image by P.K. (D) At Newhaven, one bare area (22.75037°S, 131.26035°E) was excavated to a depth of ∼20 cm. Shadowed hollows are exposed termite chambers less than about 5 cm in diameter. Upper chambers included grass chaff typical of Drepanotermes spp. harvester termites. Image by J.S. (E) At Newhaven, another bare area (22.75006°S, 131.26014°E) excavated to a depth of ∼20 cm shows the aeolian sands over a cemented matrix with termite chambers. Image by J.S. (F) Pavement termitarium with artifacts used by Martu people for seed processing (McKay Range, WA, Australia). Bare area surrounded by T. basedowii hummocks with A. aneura fringing a shallow watercourse. People swept these pavements clean of aeolian sands to provide wide, flat, hard surfaces suited to threshing, food processing, and artifact production. Image by F.J.W.
Table 1.
Characteristics of excavations of pavement termitaria at four locations
| Locations | Approximate area surveyed, ha | No. of bare circles examined at surface | No. with mound present | No. of bare circles excavated* | No. of bare circles with termite galleries |
| Newman Airport 23.437°S, 119.818°E | 1 | 10 | 10 | 2 | 2 |
| Jigalong Road 22.979°S, 119.997°E | 1 | 10 | 9 | 2 | 2 |
| Kiwirrkurra Indigenous Protected Area 22.776°S, 127.540°E | 1 | 20 | 6 | 10 | 10 |
| Newhaven Wildlife Sanctuary 22.750°S, 131.260°E | 1 | 20 | — | 3 | 3 |
—, data not available.
Bare circles were excavated by hand with crowbars. Pavement hardness and the physical effort required in excavating them contributed to limits on our sample sizes.
Gap-termite associations in Australian deserts are cryptic to the unfamiliar. Drepanotermes are leaf harvesters, surface-active only during cooler or humid conditions (4), and thus ecologically different from Namibian root eater and sand termite guilds (1). Termitaria pavements are flat and cemented, appearing simply as hard clear ground. Pavements are often partially or fully obscured under shallow wind-blown sands (Fig. 1C). Mounds are variable. They may be absent from pavements or hidden in fringing spinifex; when present, mounds can be as little as 2 cm high.
Pavements are very hard, withstanding fire, flood, and road grader blades; on worn-down vehicle tracks, the termitaria stand as discrete walled structures. Termitaria appear long-lived even when periodically or permanently abandoned; such “ghosts of termitaria” may persist for decades or longer. Pavement termitaria inhibit plant growth due to their hardness and resistance to surface water infiltration (5). Although rainfall redistribution may contribute to growth of grasses and subshrubs encircling gaps (2), we argue gaps originate as termitaria.
Termites are fundamental to Australian desert ecosystem function, having major pedogenic, nutrient cycling, and food web roles (6). Drepanotermes termitaria can occur at densities up to 1,000 ha−1 (7). Australian Aboriginal people have long recognized pavement termitaria and use them as sitting areas, walking paths, and food and artifact processing sites (Fig. 1F). Thus, these pavements have names in Aboriginal languages (e.g., linyji in Manjilyjarra).
What complex of ecological processes shapes patterns of termitaria and desert vegetation? We look forward to collaborations aiming to understand causes of diverse and dynamic patterns of pavement termitaria and vegetation at multiple scales (8). Excavations, ethnography, and aerial imagery interpretation would describe ecological and spatial characteristics of termitaria and vegetated areas. Investigations into Drepanotermes intercolony foraging competition, termitaria occupancy dynamics, the influences of wild and anthropogenic fire in spinifex-mulga mosaics (9), and interactions between these processes could describe the drivers of patterning in termitaria and spinifex of Australian deserts.
Acknowledgments
Alan Andersen, Doug Bird, Garry Cook, and two anonymous reviewers commented on this letter. It was informed from doctoral research by Melinda Hillery and Anna Petts plus observations by Stephen van Leeuwen, Danae Moore, Gareth Catt, other field ecologists, and Aboriginal experts. Excavations on the Newhaven Sanctuary and Kiwirrkura Indigenous Protected Area were conducted with owner permissions. The Commonwealth Scientific and Industrial Research Organization (CSIRO), Western Australian Department of Parks and Wildlife, and the Australian Wildlife Conservancy fund our respective positions. No project or fieldwork funds have yet been allocated to this research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tschinkel WR. Experiments testing the causes of Namibian fairy circles. PLoS One. 2015;10(10):e0140099. doi: 10.1371/journal.pone.0140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getzin S, et al. Discovery of fairy circles in Australia supports self-organization theory. Proc Natl Acad Sci USA. 2016;113(13):3551–3556. doi: 10.1073/pnas.1522130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson JAL, Perry DH. The Australian harvester termites of the genus Drepanotermes (Isoptera: Termitinae) Aust J Zool Suppl Series. 1981;29(78):1–153. [Google Scholar]
- 4.Watson JAL. Distribution, biology and speciation in the Australian harvester termites, Drepanotermes (Isoptera: Termitinae) In: Barker WR, Greenslade PJM, editors. Evolution of the Flora and Fauna of Arid Australia. Peacock Publishers; Adelaide, Australia: 1982. pp. 263–265. [Google Scholar]
- 5.Watson JAL, Gay FJ. The role of grass-eating termites in the degradation of a mulga ecosystem. Search. 1970;1(1):45. [Google Scholar]
- 6.Morton SR, et al. A fresh framework for the ecology of arid Australia. J Arid Environ. 2011;75(4):313–329. [Google Scholar]
- 7.Watson JAL, Lendon C, Low BS. Termites in mulga lands. Tropical Grasslands. 1973;7(1):121–126. [Google Scholar]
- 8.Bonachela JA, et al. Ecological feedbacks. Termite mounds can increase the robustness of dryland ecosystems to climatic change. Science. 2015;347(6222):651–655. doi: 10.1126/science.1261487. [DOI] [PubMed] [Google Scholar]
- 9.Bliege Bird R, Bird DW, Codding BF, Parker CH, Jones JH. The “fire stick farming” hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc Natl Acad Sci USA. 2008;105(39):14796–14801. doi: 10.1073/pnas.0804757105. [DOI] [PMC free article] [PubMed] [Google Scholar]