Significance
A potent opioid analgesic without addictive and respiratory adverse effects has been a predominant goal for opioid medicinal chemistry since the isolation of morphine from opium in the 19th century. Here we report a functional profile of a unique analog, BU08028, targeting a combination of a classical and nonclassical opioid receptors in monkeys. By examining behavioral, physiological, and pharmacologic factors, the present study demonstrates that BU08028 exhibits full antinociception and antihypersensitivity without reinforcing effects (i.e., abuse liability), respiratory depression, pruritus, adverse cardiovascular events, or acute physical dependence. Because monkey models provide the most phylogenetically appropriate evaluation of opioid receptor functions and drug effects, these findings provide a translational bridge for such ligands as effective analgesics without safety and abuse liability concerns.
Keywords: N/OFQ peptide receptor, respiratory depression, reinforcing effects, physical dependence, mu opioid peptide receptor
Abstract
Despite the critical need, no previous research has substantiated safe opioid analgesics without abuse liability in primates. Recent advances in medicinal chemistry have led to the development of ligands with mixed mu opioid peptide (MOP)/nociceptin-orphanin FQ peptide (NOP) receptor agonist activity to achieve this objective. BU08028 is a novel orvinol analog that displays a similar binding profile to buprenorphine with improved affinity and efficacy at NOP receptors. The aim of this preclinical study was to establish the functional profile of BU08028 in monkeys using clinically used MOP receptor agonists for side-by-side comparisons in various well-honed behavioral and physiological assays. Systemic BU08028 (0.001–0.01 mg/kg) produced potent long-lasting (i.e., >24 h) antinociceptive and antiallodynic effects, which were blocked by MOP or NOP receptor antagonists. More importantly, the reinforcing strength of BU08028 was significantly lower than that of cocaine, remifentanil, or buprenorphine in monkeys responding under a progressive-ratio schedule of drug self-administration. Unlike MOP receptor agonists, BU08028 at antinociceptive doses and ∼10- to 30-fold higher doses did not cause respiratory depression or cardiovascular adverse events as measured by telemetry devices. After repeated administration, the monkeys developed acute physical dependence on morphine, as manifested by precipitated withdrawal signs, such as increased respiratory rate, heart rate, and blood pressure. In contrast, monkeys did not show physical dependence on BU08028. These in vivo findings in primates not only document the efficacy and tolerability profile of bifunctional MOP/NOP receptor agonists, but also provide a means of translating such ligands into therapies as safe and potentially abuse-free opioid analgesics.
Pain, a symptom of numerous clinical disorders, afflicts millions of people worldwide. Despite the remarkable advances in the identification of potential targets as analgesics in the last decade, mu opioid peptide (MOP) receptor agonists remain the most widely used analgesics for pain management (1). Several side effects associated with MOP receptor agonists have severely limited the value of opioid analgesics, however (2). Owing to the abuse liability and the high mortality rate caused by respiratory arrest, opioid abuse not only has dire consequences, but also leads to mounting medical and economic burdens in our society (3–5). There is a clear, unmet need for safe analgesics without abuse liability in the global community.
Buprenorphine, a partial MOP receptor agonist, is considered a safe analgesic because of its ceiling effect on respiratory depression (6, 7). Buprenorphine is commonly used in both human and veterinary medicine to treat various pain conditions, including cancer pain and neuropathic pain (7, 8). However, buprenorphine is not devoid of reinforcing effects, the most devastating side effect of MOP receptor agonists. The abuse or misuse of buprenorphine has been documented, which limits its use worldwide (9–11). There has been a decades-long effort aimed at developing opioid analgesics with fewer side effects (12–15), but to date, safe opioid analgesics devoid of abuse potential remain undiscovered (16, 17).
Recent research on the nociceptin/orphanin FQ peptide (NOP) receptor could open an avenue for developing new analgesics. (17–20). Following spinal and systemic administration, NOP receptor agonists produce antinociception and antihypersensitivity comparable to those of MOP receptor agonists, but without reinforcing effects in nonhuman primates (21–24). More importantly, NOP agonists interact with buprenorphine in a synergistic manner to produce antinociceptive effects (25). Simultaneous activation of both NOP and MOP receptors to a small degree may produce desirable analgesic effects with fewer side effects—that is, a wider therapeutic window (17). Given that NOP receptor agonists have no abuse liability and/or can block or decrease the reinforcing effects of MOP receptor agonists (26–29), it is worth developing bifunctional MOP/NOP receptor agonists as potentially non-addictive analgesics.
Several medicinal chemistry groups have discovered such agonists with varying affinity and efficacy at MOP and NOP receptors (30–33). Subsequent to chemical modifications, some of these ligands exhibited antinociceptive and antihypersensitive efficacy with improved potency across different rodent pain models (31, 34–36). However, in vivo functional profiles of these ligands in primates are completely unknown. Among these recently developed ligands, BU08028 emerged from the orvinol series and displays a similar receptor binding profile to buprenorphine, but with better binding affinity (Ki, ∼8 nM) and efficacy (∼48% stimulation of [35S]GTPγS binding) on NOP receptors (37, 38). Therefore, BU08028 appears to be an ideal pharmacologic agent with mixed MOP/NOP agonist activity that merits further study.
Numerous studies have documented differences in the pharmacologic actions of MOP-related ligands (15, 39, 40) and NOP-related ligands (24, 41, 42) between rodents and nonhuman primates. In particular, the abuse liability and respiratory depression of MOP receptor agonists in humans can be most closely simulated in monkeys (43–45). Thus, it is extremely valuable to conduct functional studies in awake, behaving monkeys as a preclinical framework to validate the therapeutic profile of BU08028, and also to establish a translational bridge for the bifunctional MOP/NOP receptor agonists in humans. In the present study, we conducted large-scale experiments using various well-established behavioral and physiological assays to provide a comprehensive pharmacologic profile of BU08028 in nonhuman primates. Using prototypical MOP receptor agonists for comparison, we investigated the characteristics of BU08028 as an analgesic and its reinforcing effects and physiological functions, including pruritus, respiration, and cardiovascular activities. In addition, we applied a short-term repeated-dosing regimen to evaluate the development of acute physical dependence on BU08028.
Results
BU08028 Produces Potent and Long-Lasting Antinociceptive and Antiallodynic Effects.
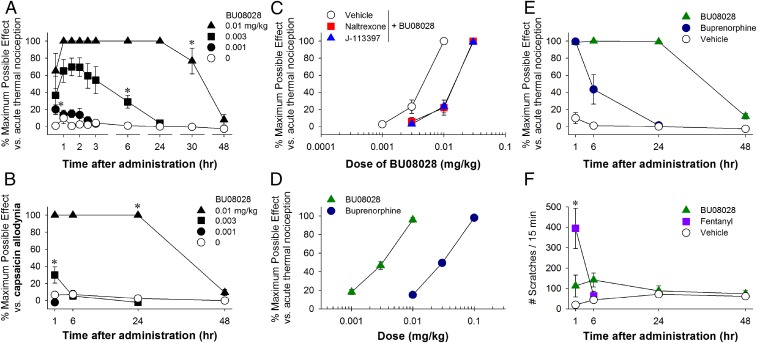
After s.c. administration, BU08028 produced antinociceptive effects against an acute noxious stimulus, 50 °C water, in a dose-dependent [F(3, 9) = 72.3; P < 0.05] and time-dependent [F(9, 27) = 16.1; P < 0.05] manner (Fig. 1A). The minimum effective dose of BU08028 to produce full antinociception was 0.01 mg/kg. The duration of action produced by this dose was 30 h, and it subsided by 48 h. To determine the antihypersensitive efficacy of BU08028, we used a clinically relevant model, capsaicin-induced allodynia, which has been widely applied to evaluate analgesics in humans (46, 47). Systemic BU08028 attenuated capsaicin-induced thermal allodynia in 46 °C water both dose-dependently [F(3, 9) = 360.8; P < 0.05] and time-dependently [F(3, 9) = 42.2; P < 0.05] (Fig. 1B).
Fig. 1.
Effects of systemic administration of BU08028 on modulating sensory processing in monkeys. (A) Antinociception against acute noxious stimulus (50 °C water). (B) Antihypersensitivity against capsaicin-induced allodynia (46 °C water). (C) Effects of MOP receptor antagonist naltrexone (0.03 mg/kg) and NOP receptor antagonist J-113397 (0.1 mg/kg) on BU08028-induced antinociception. (D) Comparison of the antinociceptive potency of BU08028 and buprenorphine. (E) Comparison of antinociceptive duration of BU08028 (0.01 mg/kg) and buprenorphine (0.1 mg/kg). (F) Comparison of the itch scratching responses elicited by BU08028 (0.01 mg/kg) and fentanyl (0.018 mg/kg) at antinociceptive doses. Each data point represents mean ± SEM (n = 4). All drugs were delivered by the s.c. route. *P < 0.05, a significant difference from the vehicle condition from the first time point to the corresponding time point.
We next conducted antagonist studies using a MOP receptor-selective dose of the opioid receptor antagonist naltrexone and the selective NOP receptor antagonist J-113397 (21, 23). Pretreatment with a single dose of naltrexone (0.03 mg/kg) or J-113397 (0.1 mg/kg) produced similar degrees (dose ratios approximately threefold) of the rightward shift of the dose–response curve for BU08028-induced antinociception (Fig. 1C). These findings indicate that MOP and NOP receptors contributed equally to the antinociceptive effects of BU08028. Using the same group of subjects, we further compared the potency and duration of action of BU08028 and buprenorphine. Based on the dose–response curves, systemic BU08028 was more potent than buprenorphine (ED50 = 0.003 mg/kg vs. 0.03 mg/kg) (Fig. 1D), and the antinociceptive duration of BU08028 0.01 mg/kg was much longer than that of buprenorphine 0.1 mg/kg (>24 h vs. 1–6 h) (Fig. 1E).
To examine whether BU08028 elicits itch sensation, we compared its effects with the MOP receptor agonist fentanyl, which was previously shown to elicit scratching responses in monkeys (48). Although BU08028 0.01 mg/kg produced potent and long-lasting antinociceptive and antihypersensitive effects, it did not significantly increase scratching responses [F(1, 3) = 4.5; P = 0.1]. In contrast, fentanyl 0.018 mg/kg elicited scratching responses in a time-dependent manner in the same subjects [F(1, 3) = 15.4; P < 0.05] (Fig. 1F). These findings strongly indicate that systemic BU08028 has a promising analgesic profile in primates.
BU08028 Does Not Have Reinforcing Effects.
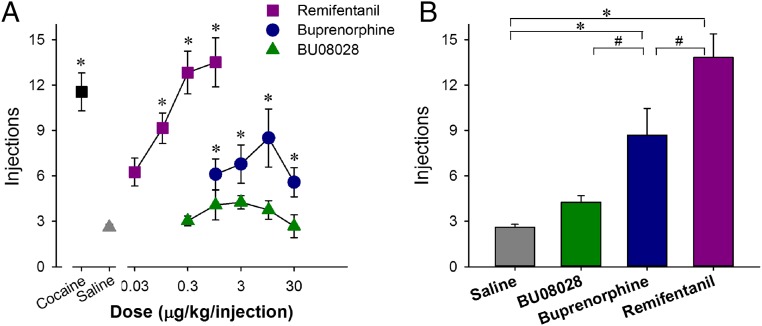
To examine and compare the reinforcing strengths of drugs, we used a progressive-ratio (PR) schedule of reinforcement that has been commonly used for evaluating abuse potential (49–51). For these studies, monkeys were trained to self-administer cocaine and various doses of the MOP receptor agonists remifentanil and buprenorphine, as well as BU08028. For all monkeys, substitution of saline for the maintenance dose of cocaine (0.03 mg/kg per injection) resulted in a low number of reinforcers (three or fewer injections) within approximately five sessions. There was a main effect of dose for remifentanil [F(4,12) = 21.8; P < 0.05] and buprenorphine [F(4,12) = 8.9; P < 0.05], but not for BU08028. Several doses of remifentanil and buprenorphine functioned as reinforcers (Fig. 2A). One-way ANOVA revealed an expected difference in peak reinforcers delivered across drugs [F(3,9) = 28.9; P < 0.05]. The peak number of reinforcers for remifentanil and buprenorphine differed significantly from each other, as well as from saline and BU08028. The peak numbers of self-administered injections of saline and BU08028 were not different (Fig. 2B). Overall, the reinforcing strength of BU08028 was significantly lower than that of cocaine, remifentanil, and buprenorphine and no different from that of saline.
Fig. 2.
Reinforcing effects of cocaine, remifentanil, buprenorphine, and BU08028 in monkeys. (A) Number of injections received as a function of dose in monkeys responding under a PR schedule of reinforcement. (B) Peak injections received for each drug. Each data point represents mean ± SEM (n = 4). *P < 0.05, a significant difference from saline in both A and B. #P < 0.05, a significant difference between drugs in B.
Higher Doses of BU08028 Do Not Compromise Physiological Functions.
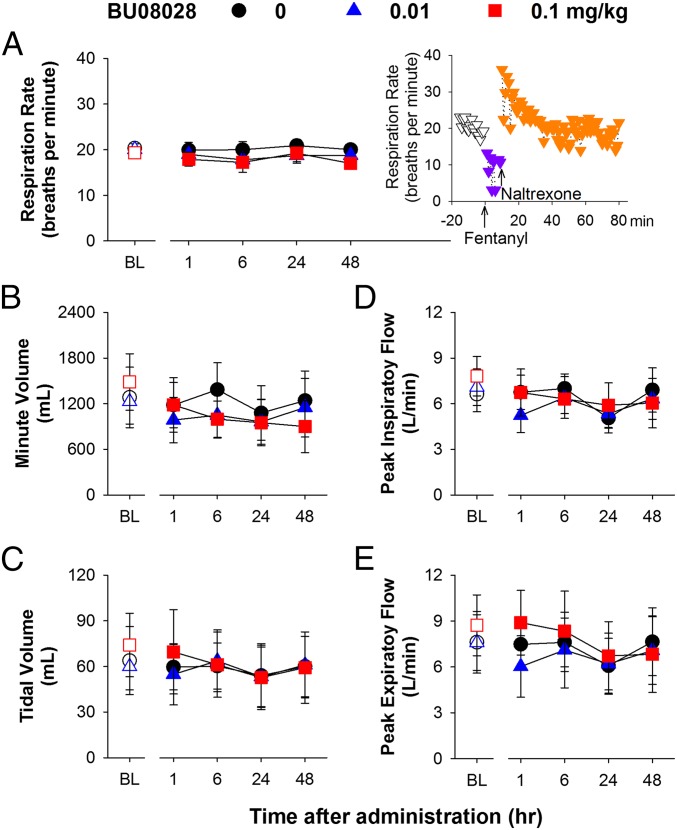
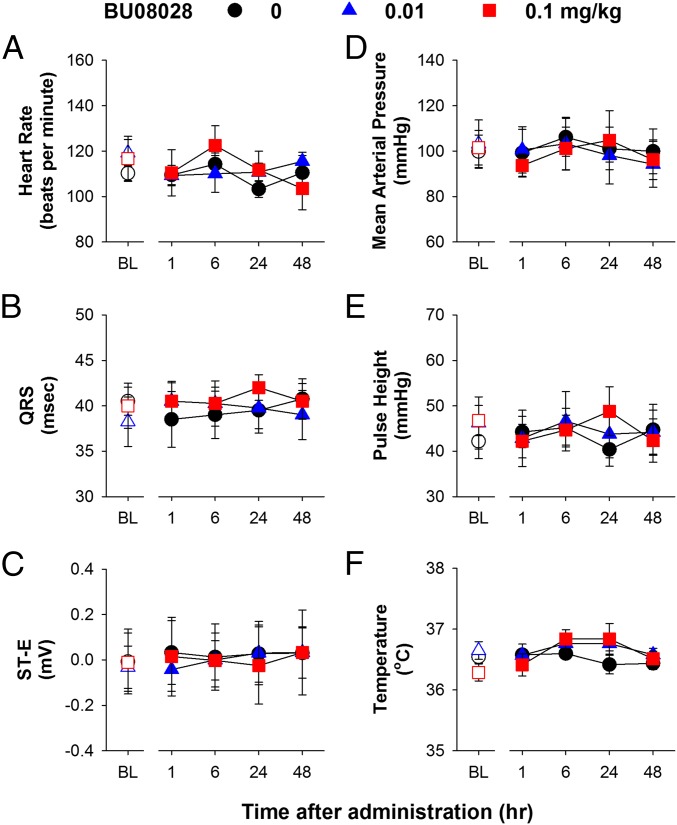
To characterize the safety profile of BU08028, we implanted radiotelemetric transmitters in monkeys for real-time measurements of different physiological functions (52). A systemic dose (0.01 mg/kg) of BU08028 that produced full antinociceptive effects did not significantly affect the respiratory function of freely moving monkeys, i.e., no respiratory depression (Fig. 3 A–E). More importantly, at a dose (0.1 mg/kg) that is ∼10–30 times higher than its antinociceptive doses (0.003–0.01 mg/kg), BU08028 did not cause any significant decreases across various respiratory parameters including respiratory rate, minute volume, tidal volume, peak inspiratory and expiratory flow (all F values 0.3–3; P > 0.1) during the 48-h observation period (Fig. 3 A–E). In stark contrast, an antinociceptive dose of fentanyl (0.056 mg/kg) (53) rapidly caused respiratory depression in a single monkey, which was reversed by a MOP receptor antagonist naltrexone (Fig. 3A, Inset). For safety reasons, this particular experiment was conducted in only one monkey. In addition, neither dose of BU08028 caused bradycardia or hypotension (Fig. 4 A and D). By examining various electrocardiography (ECG) parameters, we found that neither dose of BU08028 significantly changed any cardiovascular measures. Herein we show the ECG parameters such as QRS, ST-E, and pulse height, and the body temperature remained unchanged in monkeys receiving 0.1 mg/kg of BU08028 (Fig. 4 B, C, E, and F) (all F values 0.2–2; P > 0.3). These findings clearly illustrate that unlike standard MOP receptor agonists, BU08028 is a safe analgesic in primates.
Fig. 3.
Effects of systemic administration of BU08028 on respiratory parameters of freely moving monkeys implanted with telemetric probes. (A) Respiration rate. (Inset) Fentanyl (0.056 mg/kg)-induced decreases in respiration rate that were reversed by naltrexone (0.7 mg/kg) administration. (B) Minute volume. (C) Tidal volume. (D) Peak inspiratory flow. (E) Peak expiratory flow. Each data point represents mean ± SEM (n = 4) from each individual data value averaged from a 15-min time block. All drugs were delivered by the i.m. route. Open symbols represent baselines of different dosing conditions for the same monkeys before drug administration.
Fig. 4.
Effects of systemic administration of BU08028 on cardiovascular parameters of freely moving monkeys implanted with telemetric probes. (A) Heart rate. (B) QRS interval. (C) ST-E (ST elevation). (D) Mean arterial pressure. (E) Pulse height. (F) Body temperature. Each data point represents mean ± SEM (n = 4) from each individual data value averaged from a 15-min time block. All drugs were delivered by the i.m. route. Open symbols represent baselines of different dosing conditions for the same monkeys before drug administration.
Repeated Administration of BU08028 Does Not Produce Acute Physical Dependence.
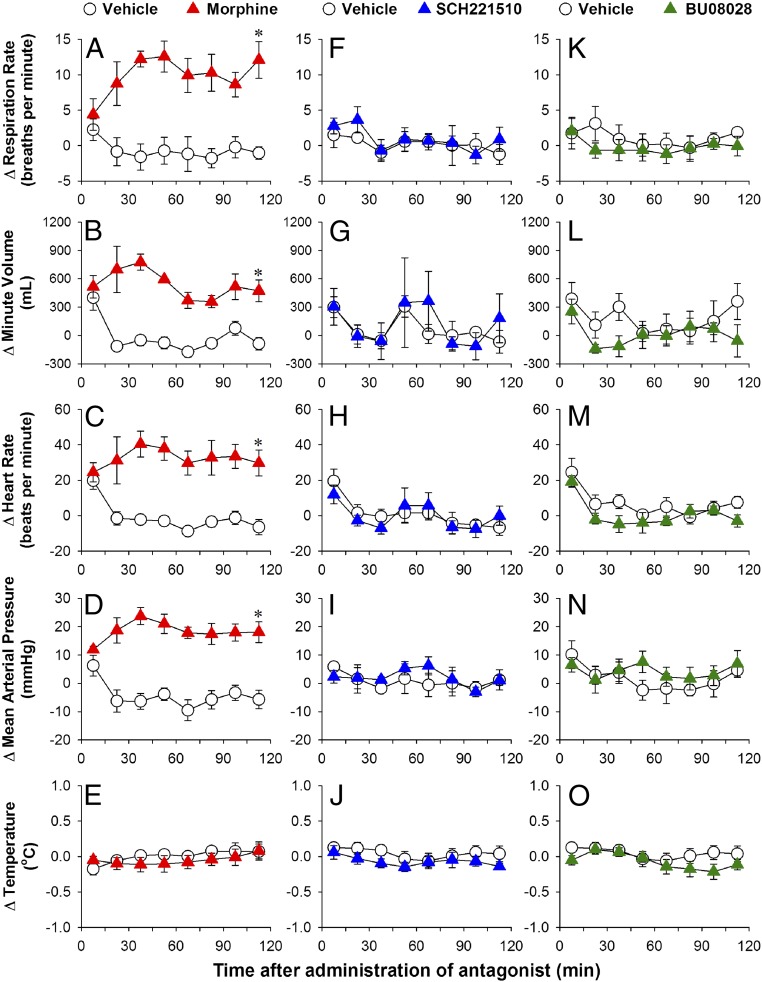
Following repeated exposure to antinociceptive doses of MOP receptor agonists, monkeys quickly develop acute physical dependence, as revealed by the emergence of withdrawal signs after administration of an opioid receptor antagonist (40, 54). Using similar repeated-dosing regimens, we compared the development of physical dependence on the MOP receptor agonist morphine, the NOP receptor agonist SCH221510, and the mixed MOP/NOP agonist BU08028 in the same subjects. Antagonist-precipitated withdrawal signs were measured in monkeys implanted with the telemetric device described above.
Compared with the vehicle-treated condition (0.1 mL/kg twice daily for 3 d), naltrexone (0.01 mg/kg) precipitated withdrawal signs on day 4 in morphine-treated (1.8 mg/kg twice daily for 3 d) monkeys. These withdrawal signs were manifested by increases in respiratory rate [F(1, 3) = 18.1; P < 0.05], minute volume [F(1, 3) = 124.3; P < 0.05], heart rate [F(1, 3) = 17.3; P < 0.05], and mean arterial pressure [F(1, 3) = 24.9; P < 0.05] without changes in body temperature [F(1, 3) = 0.4; P > 0.5] (Fig. 5 A–E). For a side-by-side comparison, following repeated administration of SCH221510 (0.01 mg/kg twice daily for 3 d), J-113397 (0.03 mg/kg) did not precipitate withdrawal signs, with no changes in all physiological parameters detected (all F values 0.02–0.8; P > 0.4) (Fig. 5 F–J). More importantly, after repeated administration of BU08028 (0.01 mg/kg once daily for 3 d), the combination of naltrexone and J-113397 at the same doses did not precipitate withdrawal signs (all F values 0.4–2.5; P > 0.1) (Fig. 5 K–O). Taken together, the foregoing findings indicate that, unlike morphine, selective NOP receptor agonist SCH221510 and bifunctional MOP/NOP receptor agonist BU08028 do not produce acute physical dependence following 3 d of repeated administration.
Fig. 5.
Comparison of precipitated withdrawal signs in monkeys from short-term repeated administration of morphine, SCH221510, or BU08028. Morphine (1.8 mg/kg) and SCH221510 (0.01 mg/kg) were administered twice daily for 3 d. Owing to its long duration, BU08028 (0.01 mg/kg) was administered once daily for 3 d. The antagonist naltrexone (0.01 mg/kg) or J-113397 (0.03 mg/kg) was used to precipitate withdrawal signs on day 4 for morphine-treated subjects (A–E) or SCH221510-treated subjects (F–J). Both antagonists, naltrexone (0.01 mg/kg) and J-113397 (0.03 mg/kg), were used to precipitate withdrawal signs on day 5 for BU08028-treated subjects (K–O). Antagonist-precipitated withdrawal signs were measured in monkeys implanted with telemetric probes before and after antagonist treatment. (A, F, and K) Respiration rate. (B, G, and L) Minute volume. (C, H, and M) Heart rate. (D, I, and N) Mean arterial pressure. (E, J, and O) Body temperature. Data are shown as changes from baseline values (i.e., before antagonist treatment). Each data point represents mean ± SEM (n = 4) from each individual data value averaged from a 15-min time block. All drugs were delivered by the i.m. route. *P < 0.05, significantly different from vehicle from 15–30 min to the corresponding time point.
Discussion
This first-in-primate study demonstrates that an orvinol analog (BU08028) with mixed MOP/NOP agonist activity displays a promising efficacy and tolerability profile as an analgesic following acute and repeated administration. The study provides four significant findings with direct translational impact on the development of safe opioid analgesics without abuse liability. First, BU08028 is highly potent, producing long-lasting antinociceptive and antihypersensitive actions mediated by both MOP and NOP receptors. Second, BU08028 does not have reinforcing effects under conditions in which other drugs with known abuse liability in the global community (including cocaine, remifentanil, and buprenorphine) function as reinforcers. Third, unlike the MOP receptor agonist fentanyl, BU08028 is safe and does not inhibit respiratory and cardiovascular activities at or above analgesic doses. Fourth, unlike the commonly used opioid analgesic morphine, repeated administration of BU08028 does not produce acute physical dependence.
BU08028 exhibits an extra-long duration of antinociceptive and antiallodynic actions, up to 30 h. To our knowledge, this is the sole analgesic with such a long duration of action in nonhuman primates. The high logP value of BU08028 could contribute to its unique pharmacokinetic profile (30, 38). More interestingly, antagonist studies have demonstrated that both MOP and NOP receptors contribute to BU08028-induced antinociception in primates. This is in contrast to rodent studies showing that pretreatment with a NOP receptor antagonist potentiated BU08028-induced antinociception (38). Nonetheless, the functional profile of BU08028 in monkeys provides proof of concept that a single molecule with mixed MOP and NOP agonist activity is more potent than selective ligands and devoid of MOP receptor-mediated side effects (17, 25).
On the other hand, capsaicin-induced allodynia has significant value in studying pain mechanisms and pharmacologic interventions in humans (46, 47). Given that capsaicin-sensitive nerve fibers are involved in a variety of pain conditions, the full effectiveness of BU08028 in inhibiting capsaicin-induced allodynia may indicate its clinically relevant analgesic efficacy. In addition, rodent studies have demonstrated the analgesic efficacy of NOP-related agonists across diverse pain modalities, including inflammatory pain, neuropathic pain, and sickle cell pain (18, 20, 22, 29). Cumulative evidence supports a broad application of NOP-related agonists as analgesics.
In dramatic contrast to cocaine, remifentanil, and buprenorphine, BU08028 does not possess detectable reinforcing strength when self-administration is studied under a PR schedule of reinforcement. Under a PR schedule, the response requirement for delivery of each reinforcer is successively increased. PR schedules measure how many responses subjects will emit to receive a drug injection before they cease to respond. The value of this schedule for assessing abuse liability is that it can provide an empirical differentiation among drugs that function as positive reinforcers (49–51). Remifentanil and buprenorphine exhibit strong and mild-to-moderate reinforcing strengths, respectively, in monkeys under the PR schedule, concordant with their abuse potential in humans (4, 9).
The present study also distinguishes between remifentanil and buprenorphine in terms of reinforcing strength. The lack of reinforcing effects of BU08028 under the same PR schedule as the other three drugs clearly suggests that this drug has lower abuse potential than buprenorphine in humans. Considering that the peak reinforcing strength of BU08028 does not differ from that of saline, the results go a step further to suggest that BU08028 has zero abuse potential. Given the decades-long effort aimed at developing abuse-free opioid analgesics (16, 17), BU08028 represents a major breakthrough for opioid medicinal chemistry.
Opioid analgesics are often associated with compromised physiological functions, particularly respiratory depression, which directly relates to mortality rates for pain management and overdose in addicted persons (4, 5, 55). The present study investigated the acute effects of BU08028 on physiological functions by measuring both respiratory and cardiovascular parameters simultaneously in the same group of freely moving monkeys implanted with a radiotelemetry device. Impedance-based measurement of respiratory function has been extensively described in humans, and the approach of combined cardiopulmonary assessments has been validated in monkeys (56). The basal values of these physiological parameters in this study are in line with those reported in rhesus monkeys (40, 52, 54). BU08028 at antinociceptive doses and ∼10- to 30-fold higher doses did not cause significantly respiratory depression or adverse cardiovascular events, whereas fentanyl produced a rapid, severe decrease in respiratory rate. Of note, a buprenorphine-like agonist, BU72, displayed a wide therapeutic window in mice, but its safety pharmacology profile could not be translated to primates owing to its respiratory depressant effects (15). BU08028 represents a safe opioid analgesic with a much wider therapeutic window than that of MOP receptor agonists.
Acute physical dependence occurs after fairly short-term (i.e., 1–3 d) administration of opioids in monkeys and humans (40, 54, 57). With precipitated withdrawal, distinctive withdrawal signs can be detected by administration of a cognate receptor antagonist. Using the same telemetry device, we found that administration of naltrexone significantly increased respiratory rate, minute volume, heart rate, and blood pressure in morphine-treated monkeys. These findings are consistent with previous studies demonstrating the development of acute physical dependence on morphine in nonhuman primates (40, 54), and validate respiratory and cardiovascular parameters as reliable and quantitative indicators of antagonist-precipitated withdrawal. More importantly, under the same repeated-dosing regimen with doses producing full antinociception, neither the NOP receptor agonist SCH221510 nor BU08028 produced acute physical dependence. These results further support the notion that unlike MOP receptor agonists, NOP-related agonists have less liability to develop physical dependence following a short-term exposure.
In the context of chronic pain management, development of opioid analgesic tolerance occurs and is attributed to several factors, including aggravation of pain, neuroadaptation of MOP receptors, and opioid-induced hyperalgesia (3, 4, 58). Owing to ethical concerns, there is no chronic pain model in nonhuman primates. Nonetheless, repeated intrathecal or i.p. administration of drugs with dual MOP/NOP receptor agonist activity (e.g., SR16435, cebranopadol) was found to result in slower development of tolerance to their antiallodynic effects compared with MOP agonists in rodents under neuropathic pain (35, 36). Morphine and buprenorphine have different analgesic potencies and tolerance development in humans, with a faster onset of tolerance to morphine (6, 59). Based on well-justified doses and duration of action, future studies will further determine whether chronic administration of BU08028 has a slower development of tolerance to its antinociceptive effects compared with morphine and buprenorphine in nonhuman primates.
To our knowledge, the present study provides the first functional evidence in nonhuman primates that BU08028 with mixed MOP/NOP agonist activities is an effective and safe analgesic without apparent abuse liability or other opioid-associated side effects. These in vivo findings strongly support the hypothesis that coactivation of MOP and NOP receptors can widen the therapeutic window through their synergistic interactions (17). In recent years, medicinal chemists have developed bifunctional MOP/NOP receptor agonists with a wide range of efficacies (i.e., from low to moderate to full efficacy) (19, 29, 30, 33). Because ligands with differential intrinsic efficacies for activating MOP and NOP receptors have distinct integrated functional outcomes, it is essential that their efficacy and tolerability profiles (e.g., abuse liability, respiratory depression, physical dependence) be studied in awake, behaving monkeys. Such pharmacologic studies not only will validate the therapeutic profiles of bifunctional MOP/NOP receptor agonists, but also will translate candidate drugs into therapies as safe, abuse-free analgesics that are long overdue in the global community.
Materials and Methods
Animals.
All animal care and experimental procedures were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by Wake Forest University’s Institutional Animal Care and Use Committee. This study is reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting experiments involving animals (60). Twelve adult male and female rhesus monkeys (Macaca mulatta), age 10–17 y and weight 6.5–13 kg, were kept at an indoor facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animals were housed individually in species-specific rooms with environmental controls set to maintain 21–25 °C, 40–60% relative humidity, and a 12-h light (06:30–18:30)/12-h dark cycle. The daily diet consisted of ∼20–28 biscuits (Purina Monkey Chow; Ralston Purina) and fresh fruit and water ad libitum. Small amounts of nonhuman primate treats and various cage-enrichment devices were supplied as forms of environmental enrichment. Animals were not exposed to any opioid compound for 1 mo before the present study.
Nociceptive Responses.
Acute thermal nociception.
The warm water tail-withdrawal assay was used to evaluate thermal antinociceptive effects of BU08028 and MOP receptor agonists (21). Monkeys were seated in primate restraint chairs in a designated procedure room, and the lower parts of their shaved tails (∼15 cm) were immersed in a thermal flask containing water maintained at 42, 46, or 50 °C. Water at 42 and 46 °C was used as nonnoxious stimuli, and water at 50 °C served as an acute noxious stimulus. All tail-withdrawal latencies were measured at each temperature using a computerized timer by investigators who were unaware of the experimental conditions. If a monkey did not remove its tail within 20 s (cutoff), the flask was removed, and a maximum time of 20 s was recorded.
Test sessions began with baseline measurements at each temperature. Subsequent tail-withdrawal latencies were measured at multiple time points after s.c. administration of a single dose of test compound. For dose–response curves, the test compound was administered by a cumulative dosing procedure with a 30-min interinjection interval. Tail-withdrawal latencies were measured at 20 min after each injection. A single dose of MOP receptor-selective antagonist naltrexone (0.03 mg/kg) or NOP receptor-selective antagonist J-113397 (0.1 mg/kg) was administered s.c. at 15 min before determination of dose–response curves to compare their antagonist effects against BU08028-induced antinociception. The doses and pretreatment time for both naltrexone and J-113397 were chosen based on previous studies (23, 25).
Capsaicin-induced thermal allodynia.
At multiple time points after s.c. administration of BU08028, 0.3 mL of capsaicin at 1.2 mg/mL was administered topically via a bandage attached on the terminal 3–5 cm of the tail for 15 min (61). The allodynic effects of capsaicin peak at 15 min after removal of the capsaicin bandage, and this is the time at which to measure the tail-withdrawal latency in 46 °C water to evaluate the antiallodynic effects of the test compound. This allodynic response was manifested as reduced tail-withdrawal latency from a maximum value of 20 s to ∼2–3 s in 46 °C water (21, 61).
Itch Scratching Responses.
The monkeys’ behaviors were recorded in their home cages for scratching activity, which has been associated previously with itch sensation (48). Each 15-min recording session was conducted at multiple time points after s.c. administration of BU08028 or fentanyl. A scratch was defined as one brief (<1 s) episode of scraping contact of the forepaw or hindpaw on the skin surface. Total scratches were counted and summed for each 15-min time block by experimenters who were unaware of the experimental conditions.
Drug Self-Administration.
Four monkeys with indwelling i.v. catheters and s.c. vascular access ports were used. Monkeys had been trained previously to self-administer (−)cocaine HCl (National Institute on Drug Abuse) under a PR schedule of reinforcement in sessions that started at ∼3:00 PM each day. Before each session, the pump was operated for ∼30 s to fill the catheter with the drug solution available for that session. Under the PR schedule, white lights were illuminated above the right lever and 50 responses resulted in a 10-s injection, extinguishing of white lights and illumination of red lights for 10 s. During a 10-min timeout period, no lights were illuminated and responding had no scheduled consequences. The response requirement for subsequent injections was determined by the exponential equation used by Richardson and Roberts (1996), ratio = [5 × e(R × 0.2)] – 5, where e is the mathematical constant and R is equal to the reinforcer number.
For the present study, the first response requirement (50 responses) corresponds to the 12th value given by this equation and was followed by 62, 77, 95,117, 144, 177, 218, 267, 328, 402, 492, 602, 737, 901, 1,102, 1,347, etc. Sessions ended when 2 h elapsed without an injection. Initially, the monkeys’ responding was maintained by injections of 0.03 mg/kg cocaine until responding was stable (mean ± three injections for three consecutive sessions with no trend). Next, dose-effect curves were determined in each monkey by substituting saline and a range of doses of remifentanil (0.03‒1 μg/kg per injection), buprenorphine (1‒30 μg/kg per injection) or BU08028 (0.3‒30 μg/kg per injection) for the maintenance dose in a quasi-random order. Doses were available for at least five consecutive sessions and until responding was deemed stable. The dependent variable of primary interest was the number of drug injections earned under the PR schedule. The order of testing was remifentanil, buprenorphine, and BU08028.
Surgical Implantation of Telemetry Device.
Before surgery, animals were given atropine (0.04 mg/kg s.c.), buprenorphine (0.01–0.03 mg/kg i.m.), and cefotaxime (500 mg i.v.) for pain management and to prevent inflammation and infection. The animals were then anesthetized with ketamine (10 mg/kg i.m.), and a catheter was placed in a saphenous vein for administration of lactated Ringer’s solution during the surgery. The animals were intubated and maintained under anesthesia with inhaled isoflurane or sevoflurane (1–2% in 1 L/min O2). The animals were placed in dorsal recumbency for surgery. The surgical sites were prepared for strict aseptic surgery by cleansing with povidone-iodine, followed by 70% isopropyl alcohol. Vital signs, including heart rate, respiration rate, indirect blood pressure, and body temperature, were monitored during the surgery and in the immediate postoperative recovery period. The animals received buprenorphine (0.003–0.02 mg/kg i.m.) and meloxicam (0.15 mg/kg s.c.) as postoperative analgesics and ceftiofur (2.2 mg/kg i.m.) as the postoperative antibiotic. Postoperative care and incision site observations were performed daily for 14 d or until healing was complete.
A cardiopulmonary telemetry transmitter [model TL11M3-D70-PCTR; Data Sciences International (DSI)] was surgically implanted into each of four monkeys. This transmitter device was placed between the internal and external abdominal oblique muscles on the flank, and the green ground lead was placed intramuscularly near the device body. The blood pressure catheter was inserted into the femoral artery with the pressure-sensing tip in the aorta. The positive ECG lead was placed on the abdominal side of the diaphragm near the apex of the left ventricle. The solid-tip negative ECG lead was placed into the cranial vena cava via the right internal jugular vein. The final depth of positioning was determined based on optimization of a live telemetry signal. Two respiratory impedance leads were placed intramuscularly on each side of the chest along the transverse plane of the seventh rib. Both negative leads (white stripes) were placed on the right side of the chest, and both positive leads (solid colors) were placed on the left side. The violet leads were placed cranial to the seventh rib, and the turquoise leads were placed caudal to the seventh rib. All of the leads were cut to the appropriate length. The insulation was removed, and the exposed wire was looped and secured with suture before placement. After initial placement of leads and before the leads were secured to the tissue, base impedance was optimized by repositioning the leads, and the impedance value was verified by monitoring a live respiratory signal using DSI hardware and software. The depth of breathing was verified to ensure signal quality.
Physiological Responses.
Four freely moving monkeys implanted with the D70-PCTR telemetry transmitter were used to evaluate the effects of BU08028 and MOP receptor agonists on physiological parameters. Respiration, blood pressure, ECG, and temperature were measured and analyzed with Ponemah version 5.2 (Data Sciences International). For acute drug effects, data from the 30-min interval before drug administration were collected as baseline and then at each time point (i.e., 1, 6, 24, and 48 h) after drug administration. For detecting precipitated withdrawal signs, data from the 30-min duration before administration of antagonist were collected and then continuously for 2 h after antagonist administration. The mean value of each 15-min time block was generated from each subject to represent the measure outcome for each single data point.
Data Analysis.
Mean ± SEM values were calculated from individual-subject data for all behavioral endpoints. Comparisons were made for the same monkeys across all test sessions in the same experiment. All data except self-administration data were analyzed by repeated-measures two-way ANOVA, followed by Bonferroni’s multiple-comparisons test. For each drug used in the self-administration paradigm, repeated-measures one-way ANOVA was conducted with the post hoc Dunnett’s test to determine which doses functioned as reinforcers. In addition, maximum reinforcing strength, defined as the peak number of reinforcers that a monkey earned regardless of dose, was compared across drugs using repeated-measures one-way ANOVA and a Holm–Sidak multiple-comparisons test. The criterion for significance for all tests was set at P < 0.05. To analyze nociceptive responses, individual tail-withdrawal latencies were converted to the percentage of maximum possible effect. The formula for calculating the percentage of maximum possible effect was as follows: [(test latency ‒ control latency)/(cutoff latency, 20 s ‒ control latency)] × 100.
Drugs.
BU08028 HCl (37) (University of Bath) was dissolved in a solution of dimethyl sulfoxide/10% (mass/vol) (2-hydroxypropyl)-β-cyclodextrin in a ratio of 3:97. Morphine sulfate, buprenorphine HCl, fentanyl HCl, remifentanil HCl, and naltrexone HCl (National Institute on Drug Abuse) were dissolved in sterile water. The (‒)cocaine HCl (National Institute on Drug Abuse) was dissolved in sterile 0.9% saline. J-113397 and SCH221510 (Tocris Bioscience) were dissolved in a solution of dimethyl sulfoxide/Tween 80/sterile water in a ratio of 1:1:8. Capsaicin (Sigma-Aldrich) was dissolved in 70% (vol/vol) ethanol. For systemic administration, drugs were administered at a volume of 0.1 mL/kg. There was a minimum 1-wk interval between drug administrations.
Acknowledgments
We thank Drs. Megan Fine, Vince Mendenhall, and Heather DeLoid for their excellent surgical procedures and Colette Cremeans, Erin Gruley, Jade Lackey, Jillian Odom, and Emily Whitaker for their technical assistance with animal training and data collection. The research reported in this paper was supported by the National Institutes of Health, National Institute on Drug Abuse (Grants DA023281, DA032568, and DA035359) and the US Department of Defense (Contract W81XWH-13-2-0045).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10225.
References
- 1.Nuckols TK, et al. Opioid prescribing: A systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: An update. Am J Psychiatry. 2016;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, McLellan AT. Opioid abuse in chronic pain: Misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 4.Cheatle MD. Prescription opioid misuse, abuse, morbidity, and mortality: Balancing effective pain management and safety. Pain Med. 2015;16(Suppl 1):S3–S8. doi: 10.1111/pme.12904. [DOI] [PubMed] [Google Scholar]
- 5.Dart RC, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 6.Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209–219. doi: 10.1016/j.suponc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Hans GH. Buprenorphine in the treatment of neuropathic pain. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Ligands. Vol 1131. American Chemical Society; Washington, DC: 2013. pp. 103–123. [Google Scholar]
- 8.Raffa RB, et al. The clinical analgesic efficacy of buprenorphine. J Clin Pharm Ther. 2014;39(6):577–583. doi: 10.1111/jcpt.12196. [DOI] [PubMed] [Google Scholar]
- 9.Lavonas EJ, et al. Abuse and diversion of buprenorphine sublingual tablets and film. J Subst Abuse Treat. 2014;47(1):27–34. doi: 10.1016/j.jsat.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug Alcohol Depend. 2003;70(2) Suppl:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14(3-4):363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 12.Mohacsi E, O’Brien J, Blount J, Sepinwall J. Acylmorphinans: A novel class of potent analgesic agents. J Med Chem. 1985;28(9):1177–1180. doi: 10.1021/jm00147a009. [DOI] [PubMed] [Google Scholar]
- 13.Archer S, Albertson NF, Harris LS, Pierson AK, Bird JG. Pentazocine: Strong analgesics and analgesic antagonists in the benzomorphan series. J Med Chem. 1964;7:123–127. doi: 10.1021/jm00332a001. [DOI] [PubMed] [Google Scholar]
- 14.Husbands SM, et al. 3-Alkyl ethers of clocinnamox: Delayed long-term mu-antagonists with variable mu efficacy. J Med Chem. 1998;41(18):3493–3498. doi: 10.1021/jm9810248. [DOI] [PubMed] [Google Scholar]
- 15.Neilan CL, et al. Characterization of the complex morphinan derivative BU72 as a high-efficacy, long-lasting mu-opioid receptor agonist. Eur J Pharmacol. 2004;499(1-2):107–116. doi: 10.1016/j.ejphar.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 16.Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: The exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006;147(Suppl 1):S153–S162. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4(2):214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schröder W, Lambert DG, Ko MC, Koch T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol. 2014;171(16):3777–3800. doi: 10.1111/bph.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calò G, Guerrini R. Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the nociceptin/orphanin FQ receptor. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Ligands. Vol 1131. American Chemical Society; Washington, DC: 2013. pp. 275–325. [Google Scholar]
- 20.Toll L, Bruchas MR, Calò G, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev. 2016;68(2):419–457. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu E, Calò G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148(1):107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiguchi N, Ding H, Ko MC. Central N/OFQ-NOP receptor system in pain modulation. Adv Pharmacol. 2016;75:217–243. doi: 10.1016/bs.apha.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko MC, et al. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34(9):2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhtankar DD, Lee H, Rice KC, Ko MC. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 2014;231(7):1377–1387. doi: 10.1007/s00213-013-3341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cremeans CM, Gruley E, Kyle DJ, Ko MC. Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther. 2012;343(1):72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flau K, Redmer A, Liedtke S, Kathmann M, Schlicker E. Inhibition of striatal and retinal dopamine release via nociceptin/orphanin FQ receptors. Br J Pharmacol. 2002;137(8):1355–1361. doi: 10.1038/sj.bjp.0704998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33(4):877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- 28.Sukhtankar DD, Lagorio CH, Ko MC. Effects of the NOP agonist SCH221510 on producing and attenuating reinforcing effects as measured by drug self-administration in rats. Eur J Pharmacol. 2014;745:182–189. doi: 10.1016/j.ejphar.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaveri NT. Nociceptin opioid receptor (NOP) as a therapeutic target: Progress in translation from preclinical research to clinical utility. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.1025b01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husbands SM. Buprenorphine and related orvinols. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Ligands. Vol 1131. American Chemical Society; Washington, DC: 2013. pp. 127–144. [Google Scholar]
- 31.Molinari S, et al. [Dmt1]N/OFQ(1-13)-NH2: A potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol. 2013;168(1):151–162. doi: 10.1111/j.1476-5381.2012.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaveri NT, Jiang F, Olsen C, Polgar WE, Toll L. Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP receptor-selective scaffolds: Part I. Bioorg Med Chem Lett. 2013;23(11):3308–3313. doi: 10.1016/j.bmcl.2013.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schunk S, et al. Discovery of a potent analgesic NOP and opioid receptor agonist: Cebranopadol. ACS Med Chem Lett. 2014;5(8):857–862. doi: 10.1021/ml500117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khroyan TV, et al. Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: Studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther. 2011;339(2):687–693. doi: 10.1124/jpet.111.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linz K, et al. Cebranopadol: A novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther. 2014;349(3):535–548. doi: 10.1124/jpet.114.213694. [DOI] [PubMed] [Google Scholar]
- 36.Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/μ-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther. 2013;346(1):11–22. doi: 10.1124/jpet.113.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cami-Kobeci G, Polgar WE, Khroyan TV, Toll L, Husbands SM. Structural determinants of opioid and NOP receptor activity in derivatives of buprenorphine. J Med Chem. 2011;54(19):6531–6537. doi: 10.1021/jm2003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khroyan TV, et al. The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): Characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther. 2011;336(3):952–961. doi: 10.1124/jpet.110.175620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowen CA, Fischer BD, Mello NK, Negus SS. Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther. 2002;302(1):264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- 40.Ko MC, Divin MF, Lee H, Woods JH, Traynor JR. Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther. 2006;316(2):772–779. doi: 10.1124/jpet.105.094409. [DOI] [PubMed] [Google Scholar]
- 41.Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: Behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318(3):1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- 42.Ding H, et al. Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol. 2015;172(13):3302–3312. doi: 10.1111/bph.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 44.Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301(2):698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- 45.Butelman ER, France CP, Woods JH. Apparent pA2 analysis on the respiratory depressant effects of alfentanil, etonitazene, ethylketocyclazocine (EKC) and Mr2033 in rhesus monkeys. J Pharmacol Exp Ther. 1993;264(1):145–151. [PubMed] [Google Scholar]
- 46.Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86(6):1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63(2):163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 48.Ko MC, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310(1):169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 49.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 50.Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: Antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 2000;153(1):1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- 51.Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36(2):539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins GT, et al. Repeated administration of a mutant cocaine esterase: Effects on plasma cocaine levels, cocaine-induced cardiovascular activity, and immune responses in rhesus monkeys. J Pharmacol Exp Ther. 2012;342(1):205–213. doi: 10.1124/jpet.112.194639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko MC, Butelman ER, Woods JH. The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998;286(1):150–156. [PMC free article] [PubMed] [Google Scholar]
- 54.Kishioka S, Paronis CA, Woods JH. Acute dependence on, but not tolerance to, heroin and morphine as measured by respiratory effects in rhesus monkeys. Eur J Pharmacol. 2000;398(1):121–130. doi: 10.1016/s0014-2999(00)00279-x. [DOI] [PubMed] [Google Scholar]
- 55.Lee LA, et al. Postoperative opioid-induced respiratory depression: A closed claims analysis. Anesthesiology. 2015;122(3):659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 56.Authier S, Haefner P, Fournier S, Troncy E, Moon LB. Combined cardiopulmonary assessments with implantable telemetry device in conscious freely moving cynomolgus monkeys. J Pharmacol Toxicol Methods. 2010;62(1):6–11. doi: 10.1016/j.vascn.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA. Acute opioid physical dependence in humans: Effect of naloxone at 6 and 24 hours postmorphine. Pharmacol Biochem Behav. 1990;36(2):393–399. doi: 10.1016/0091-3057(90)90421-d. [DOI] [PubMed] [Google Scholar]
- 58.Koyyalagunta D, et al. A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician. 2012;15(3) Suppl:ES39–ES58. [PubMed] [Google Scholar]
- 59.Khanna IK, Pillarisetti S. Buprenorphine: An attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859–870. doi: 10.2147/JPR.S85951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group Animal research: Reporting in vivo experiments. The ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311(1):155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]