Significance
Transition metals are micronutrients that all organisms use in essential metabolic processes. The ubiquitous Natural resistance-associated macrophage protein (Nramp) family facilitates the acquisition of these metal ions by transporting them across cellular membranes, including dietary iron absorption in mammals. We show that a conserved methionine, an unusual metal-binding residue found in the Nramp metal-binding site, is not essential for the transport of physiological environmentally scarce transition metals like iron and manganese. Instead, it confers selectivity against the abundant alkaline earth metals calcium and magnesium, with the tradeoff of making the toxic metal cadmium a preferred substrate. Using protein structure information, biochemical results, molecular dynamics simulations, and inorganic chemistry theory, we propose a model for how metal discrimination is enforced.
Keywords: transition metals, MntH, divalent metal transporter DMT1, hard-soft acid-base theory, ion selectivity filters
Abstract
Natural resistance-associated macrophage protein (Nramp) family transporters catalyze uptake of essential divalent transition metals like iron and manganese. To discriminate against abundant competitors, the Nramp metal-binding site should favor softer transition metals, which interact either covalently or ionically with coordinating molecules, over hard calcium and magnesium, which interact mainly ionically. The metal-binding site contains an unusual, but conserved, methionine, and its sulfur coordinates transition metal substrates, suggesting a vital role in their transport. Using a bacterial Nramp model system, we show that, surprisingly, this conserved methionine is dispensable for transport of the physiological manganese substrate and similar divalents iron and cobalt, with several small amino acid replacements still enabling robust uptake. Moreover, the methionine sulfur’s presence makes the toxic metal cadmium a preferred substrate. However, a methionine-to-alanine substitution enables transport of calcium and magnesium. Thus, the putative evolutionary pressure to maintain the Nramp metal-binding methionine likely exists because it—more effectively than any other amino acid—increases selectivity for low-abundance transition metal transport in the presence of high-abundance divalents like calcium and magnesium.
All organisms require transition metal ions as cofactors in proteins that perform a variety of essential cellular tasks. Through evolution, organisms have developed mechanisms to acquire, transport, and safely store essential metals such as manganese, iron, cobalt, and zinc. The natural resistance-associated macrophage protein (Nramp) family of metal transporters represents a common transition metal acquisition strategy conserved across all kingdoms of life (1). The first discovered mammalian Nramp (Nramp1) is expressed in phagosomal membranes and likely extracts essential metals to help kill engulfed pathogens (2, 3). Mammals use Nramp2, an essential gene also called DMT1, to absorb dietary iron into the enterocytes that line the small intestine (4) and to extract iron from transferrin-containing endosomes in all tissues. Bacteria express their own Nramp homologs, which they typically use to scavenge manganese and other first row divalent transition metals (5, 6). Last, most plants have several Nramp homologs that take up iron and manganese, the essential cofactor in photosystem II, from the soil or vacuolar stores (7, 8).
Nramps are generally thought to function as metal-proton symporters (1) and are able to bind and/or transport a wide range of divalent transition metal substrates, including the biologically useful metals Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+, as well as the toxic heavy metals Cd2+, Pb2+, and Hg2+ (4, 9–13). Nramps do discriminate against the divalent alkaline earth metal ions Mg2+ and Ca2+ (9, 14), which are typically several orders of magnitude more abundant than the transition metals (15). Using a bacterial Nramp homolog, Deinococcus radiodurans MntH, we demonstrate the role of the conserved metal-binding site methionine in conferring specificity against Ca2+ and Mg2+, thus explaining at the molecular level the metal selectivity of Nramps.
Results
Conserved Metal-Binding Site Methionine Is Not Required for Transition Metal Transport.
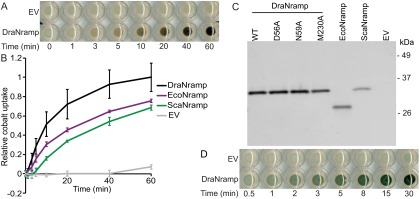
To validate Deinoccocus radiodurans MntH (DraNramp) as a model system, we developed a cell-based cobalt uptake assay adapted from a similar assay in yeast (16). In this colorimetric assay, we quantify the relative Co2+ accumulation in Nramp-expressing E. coli at various time points by precipitating the transported Co2+ to the black solid cobalt (II) sulfide (Fig. S1A). We observed time-dependent Co2+ uptake for DraNramp, Escherichia coli MntH (EcoNramp), and Staphylococcus capitis MntH (ScaNramp) (Fig. S1B), which roughly correlated with expression levels of these His-tagged proteins (Fig. S1C).
Fig. S1.
Validation of DraNramp using an in vivo cobalt transport assay. (A) E. coli-expressing DraNramp accumulated Co2+ over time, whereas cells with an empty vector control (EV) did not. We detected relative Co2+ transport by adding (NH4)2S to form the black solid CoS, which darkens the cell pellets in a quantifiable manner. (B) Overexpressing DraNramp, EcoNramp, or ScaNramp enabled cobalt uptake into E. coli. Error bars are SD from the mean from four independent experiments. (C) DraNramp expressed better than EcoNramp or ScaNramp when detected via Western blot using an anti-His fluorescent antibody, which correlates with the observed in vivo cobalt transport differences. Notably, WT, D56A, N59A, and M230A DraNramp variants are all expressed similarly. (D) We can detect Fe2+ transport in an analogous assay via the formation of the dark-green FeS precipitate.
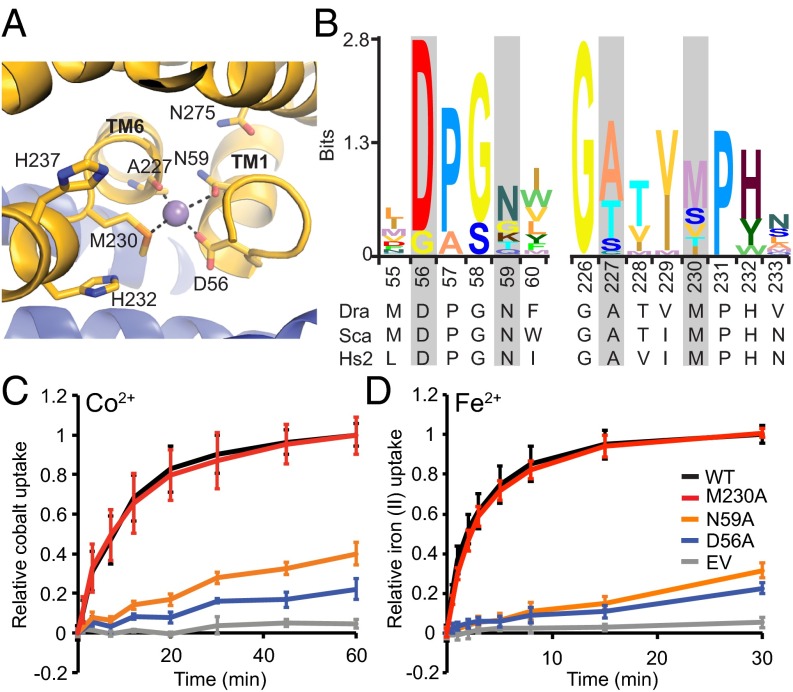
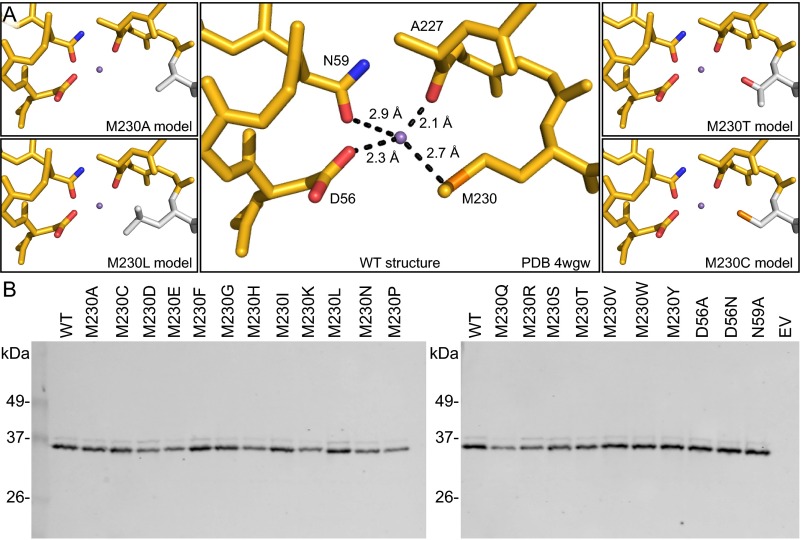
In the inward-facing conformational state revealed by the ScaNramp crystal structures, three conserved side chains and a backbone carbonyl group formed the coordination sphere of bound transition metals (9). In DraNramp, these correspond to D56, N59, M230, and the backbone carbonyl of A227 (Fig. 1 A and B). The conserved methionine in the metal-binding site was postulated to stabilize transition metal substrates, facilitating their transport (9). To better understand the roles of the three metal-coordinating side chains, we mutated each residue to alanine and measured Co2+ transport by the resulting DraNramp variants (Fig. 1C). Loss of a metal-coordinating residue that uses oxygen to bind the metal, D56 or N59, was detrimental to Co2+ uptake, consistent with the previously demonstrated importance of these residues to the transport of Co2+, Fe2+, Mn2+, and Cd2+ in bacterial and eukaryotic Nramp homologs (9, 17–20).
Fig. 1.
The conserved metal-binding site methionine is not required for transport Co2+ and Fe2+. (A) The ScaNramp structure identified four residues that directly bind metal substrate as D56, N59, A227, and M230 (DraNramp numbering). (B) Sequence logo of metal-binding site from 2,691 related sequences. Alignment was generated using HMMER with the DraNramp sequence as the search sequence within the UniprotRef database, with an E-value cutoff of 1 × 10−9. Metal-coordinating residues are identified by gray rectangles. (C) In vivo Co2+ transport was greatly reduced for D56A and N59A compared with WT DraNramp, but M230A retained full activity. (D) Phenotypes for in vivo Fe2+ uptake were similar to Co2+. Error bars are SDs (n = 4).
In stark contrast to D56A and N59A, M230A, which lacks the metal-binding thioether sulfur, transported Co2+ at rates and levels similar to WT DraNramp. We also observed this unexpected mutant phenotype with another essential metal, as M230A and WT transported Fe2+ similarly, whereas D56A and N59A were both severely impaired (Fig. 1D and Fig. S1D).
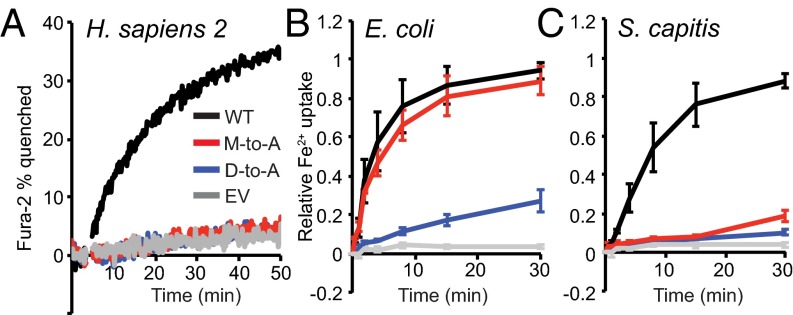
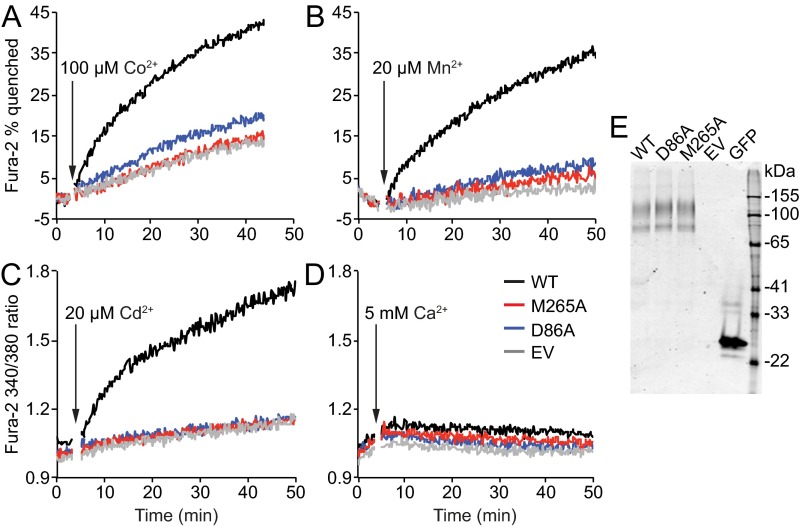
To test the generality of this robust transport activity of the M-to-A mutant, we used Fura-2 fluorescence to measure metal transport by HEK cells expressing WT human Nramp2 or binding-site mutants D86A and M265A. WT Nramp2 enabled Fe2+ and Co2+ transport (Fig. 2A and Fig. S2A), in agreement with our DraNramp results, as well as Mn2+ and Cd2+ (Fig. S2B and Fig. S2C), but not Ca2+ (Fig. S2D), consistent with previous studies with mammalian Nramp2 homologs (9, 11, 14, 21). However, in contrast to DraNramp M230A, M265A did not transport any of the tested metals, similarly to the D86A loss-of-function phenotype, even though both variants expressed as well as WT (Fig. S2E).
Fig. 2.
The M-to-A mutation has species-specific effects on Nramp-dependent Fe2+ transport. (A) The M-to-A mutant in human Nramp2 does not transport Fe2+. We monitored Fe2+ uptake in HEK 293 cells transfected with WT, D86A, or M265A HsNramp2, or an empty vector (EV), using Fura-2 quenching on binding Fe2+. Traces are representative of observed metal uptake activity (n = 3). (B and C) The M-to-A mutation in the bacterial homologs EcoNramp (B) and ScaNramp (C) display starkly different phenotypes for Fe2+ transport when expressed in E. coli. Error bars are SD (n = 3). Mutants all expressed similarly to their respective WT (Figs. S2E and S3B).
Fig. S2.
The M-to-A mutant in human Nramp2 does not transport any tested metal substrates. We monitored metal uptake in HEK 293 cells transfected with WT, D86A, or M265A HsNramp2, or an empty vector (EV), using Fura-2 quenching on binding Co2+ (A) or Mn2+ (B) or 340-nm/380-nm excitation ratio increase on binding Cd2+ (C) or Ca2+ (D). WT HsNramp2 efficiently transported Fe2+, Co2+, Mn2+, and Cd2+, whereas no significant above-EV transport was observed for binding-site mutants D86A or M265A. No significant Ca2+ transport was observed for WT or either mutant. Traces are representative of metal uptake activity observed in at least three independently transfected samples. (E) The three HsNramp2 variants expressed similarly in HEK 293 cells (GFP tag used for detection).
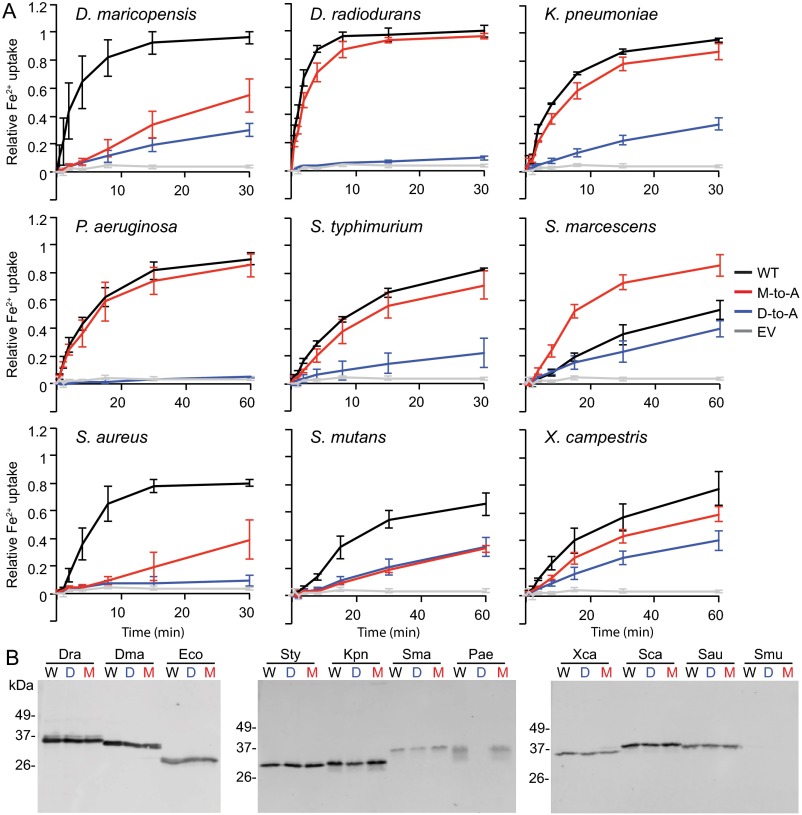
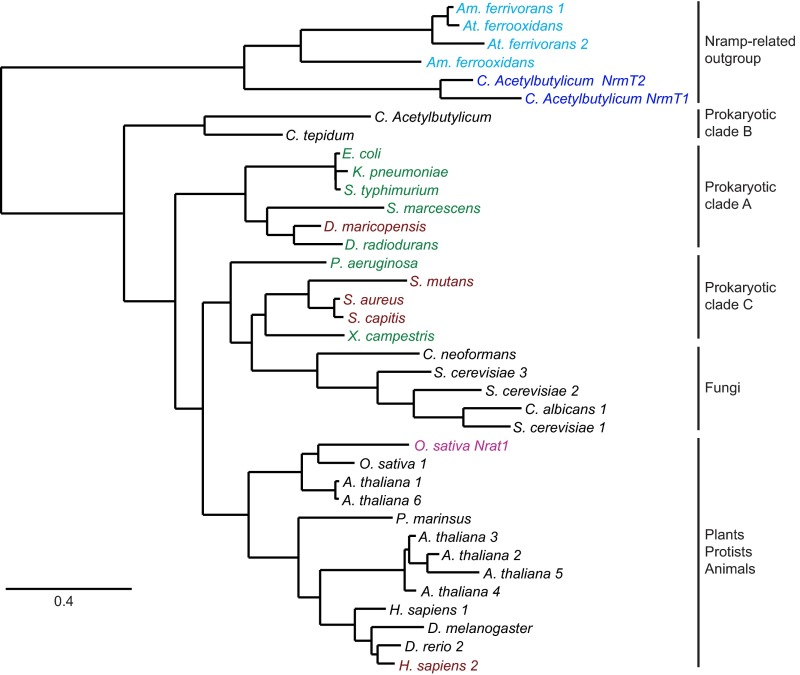
To determine whether DraNramp represented an evolutionary outlier, we tested Fe2+ transport by a variety of bacterial Nramps and their corresponding M-to-A and D-to-A mutants, expressed in E. coli (Fig. 2 B and C and Fig. S3). Like DraNramp, the E. coli, Salmonella typhimurium, Klebsiella pneumonia, Serratia marcescens, Pseudomonas aeruginosa, and Xanthomonas campestris M-to-A mutants exhibited similar or enhanced Fe2+ transport relative to their WT counterpart. In contrast, the S. capitis, Staphylococcus aureus, Streptococcus mutans, and Deinococcus maricopensis M-to-A mutants showed significantly impaired Fe2+ transport. The M-to-A mutation phenotype does not cluster in the phylogenetic tree; members of two distinct evolutionary clades of Nramps tolerate an M-to-A substitution for Fe2+ transport (Fig. S4) (22, 23). Thus, the observed differences are unlikely due to a broad mechanistic divergence within the Nramp family, and instead likely depend on sequence and structure context. We therefore decided to use DraNramp and its transport-competent methionine-less mutant to test the hypothesis that a primary role of this methionine is to specifically discriminate against binding or transport of certain metals.
Fig. S3.
Bacterial Nramp homologs display a range of M-to-A mutant phenotypes. (A) Fe2+ uptake by E. coli cells expressing various bacterial Nramp homologs and their respective binding-site mutants. Error bars represent SDs from the mean from three independent experiments. (B) Western blots show that D-to-A (D) and M-to-A (M) mutants express similarly to WT (W) counterpart for each species, except for the P. aeruginosa D-to-A mutant which may not express. S. mutans WT and mutants are poorly detected on Western blots which makes expression level comparison difficult.
Fig. S4.
The distinct methionine mutant phenotypes are phylogenetically dispersed. Prokaryotic Nramp homologs can be divided into three clades (22) in a maximum-likelihood phylogenetic tree generated using phylogeny.fr (23), with eukaryotic Nramps most closely related to clade C. Nramp homologs for which the M-to-A mutants displayed similar or enhanced Fe2+ transport compared with their WT counterpart are distributed in both prokaryotic clade A and clade C (green). Homologs for which the M-to-A mutation led to significantly reduced Fe2+ transport include species in both clades A and C and human Nramp2 (maroon). Also highlighted are the Oryza sativa Nramp-related aluminum transporter Nrat1 (magenta), two Clostrididium acetylbutylicum Nramp-related magnesium transporters (NrmTs; blue), and four Nramp-related genes from the heavy metal and acid tolerant extremophiles Acidimicrobium ferrooxidans, Acidithiobacillus ferrooxidans, and Acidithiobacillus ferrivorans (cyan) (see discussion). Given that homologs with transport-active M-to-A mutants do not segregate in any one evolutionary clade, it is unlikely that a broad mechanistic divergence in metal binding or selectivity exists between specific clades of the Nramp family. We speculate that species-specific features of the environment surrounding the metal binding-site affect the relative importance of the methionine residue for metal binding and transport. For example, the binding-site environment may better facilitate water-mediated metal coordination in the M-to-A mutant in some species than in others, resulting in transport-impaired M-to-A mutants providing a suboptimal metal coordination sphere. Alternatively, the methionine may have a more important role in conformational change and/or the outward-open state in some species than in others, such that impaired transport in some M-to-A mutants may result from altered protein dynamics rather than impaired metal binding. Further experiments will be required to test these hypotheses.
The Methionine Reduces Alkaline Earth Metal Competition.
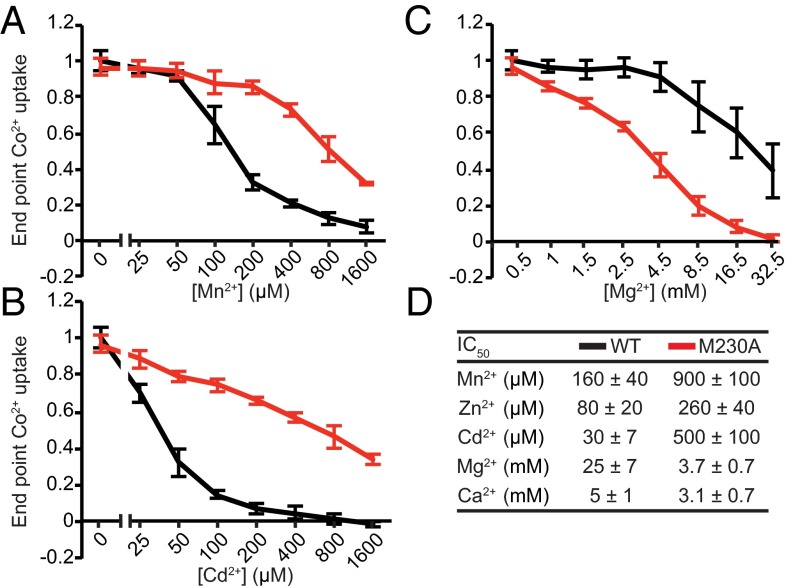
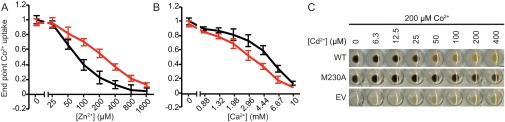
To test our metal-discrimination hypothesis, we compared Co2+ transport by WT DraNramp and M230A in the presence of various concentrations of competing Mg2+, Ca2+, Mn2+, Zn2+, and Cd2+, none of which form dark sulfide salts, and determined IC50 values for their inhibition of Nramp-dependent Co2+ transport (Fig. 3). Consistent with our hypothesis, both alkaline earth metals Mg2+ and Ca2+ inhibited Co2+ transport by M230A more effectively relative to WT (Fig. 3 C and D and Fig. S5B). In contrast, the first row transition metals Mn2+ (the physiological substrate) and Zn2+, as well as the second row transition metal Cd2+, competed Co2+ transport more effectively in WT relative to M230A (Fig. 3 A, B, and D and Fig. S5A). For most tested metals, this assay cannot discriminate between simple inhibition of cobalt transport or uptake of the competing metal, as their sulfide salts are white (MgS, CaS, ZnS) or light brown (MnS). However, WT DraNramp-expressing cells exposed to equal concentrations of Cd2+ and Co2+ turned yellow on (NH4)2S addition, due to formation of the yellow solid cadmium (II) sulfide, whereas M230A cells remained black (Fig. S5C). Thus, WT DraNramp preferentially transports Cd2+ over Co2+, whereas M230A preferentially transports Co2+ over Cd2+. In summary, the metal-binding site methionine reduces the competition from the environmentally abundant Mg2+ and Ca2+ ions while also altering the relative substrate preferences among the rarer transition metals.
Fig. 3.
The metal-binding site methionine dictates DraNramp substrate competition against Co2+. Uptake of 200 μM Co2+ by E. coli expressing either WT (black) or M230A (red) DraNramp was measured in the presence of varying concentrations of competing divalent metals. The transition metals Mn2+ (A), Cd2+ (B), and Zn2+ (Fig. S5A) compete more effectively for WT than for M230A DraNramp Co2+ transport. Conversely, the alkaline earth metals Mg2+ (C) and Ca2+ (Fig. S5B) compete more effectively for M230A than for WT DraNramp Co2+ transport. Error bars are SD (n = 4). (D) IC50 values with SE for added metals calculated for each metal, assuming a Hill coefficient of 1.
Fig. S5.
Competition of additional divalent metals against Co2+ for WT and M230A DraNramp. Uptake of 200 μM Co2+ by E. coli expressing either WT (black) or M230A (red) DraNramp was measured in the presence of varying concentrations of competing Zn2+ (A), which competes WT Co2+ transport more efficiently than M230A, or Ca2+ (B), which competes M230A Co2+ transport more efficiently than WT. (C) WT DraNramp transports Cd2+ in the presence of Co2+. WT DraNramp-expressing cell pellets turned yellow under higher competing Cd2+ concentrations due to the formation of the yellow CdS precipitate and the lack of significant Co2+ uptake. In contrast, M230A-expressing cells still took up significant amounts of Co2+ even in the presence of excess competing Cd2+, whereas cells containing an empty vector (EV) took up neither metal to a significant level.
The Methionine Is Crucial for Both Promoting Cadmium and Reducing Calcium Transport.
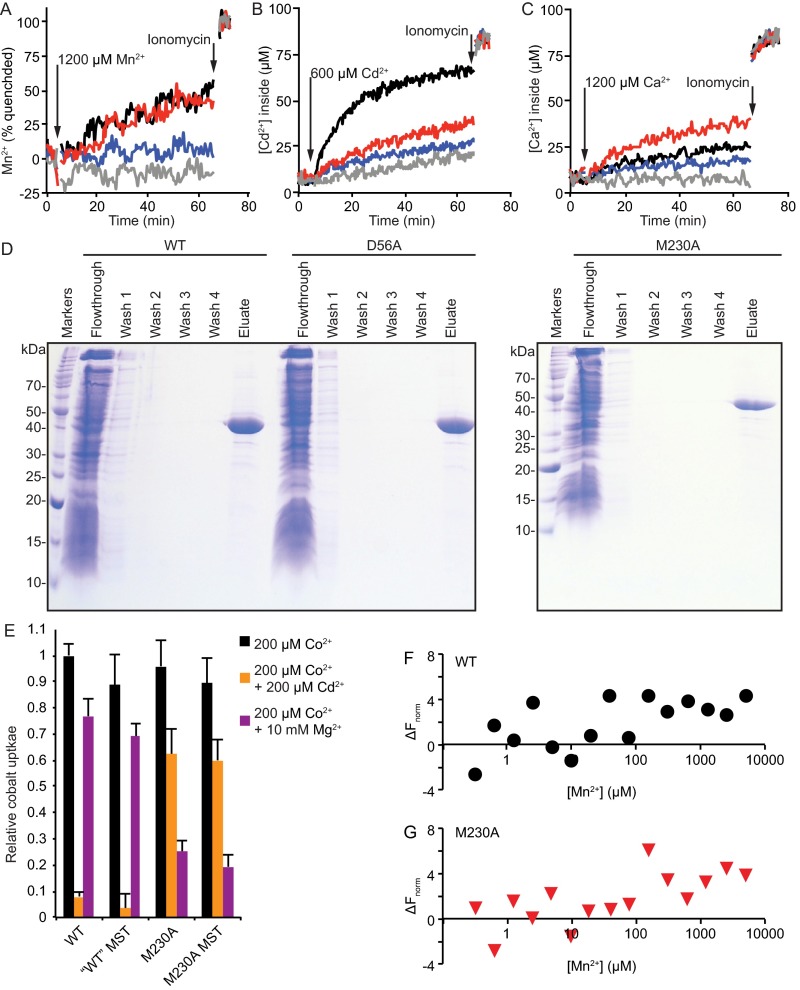
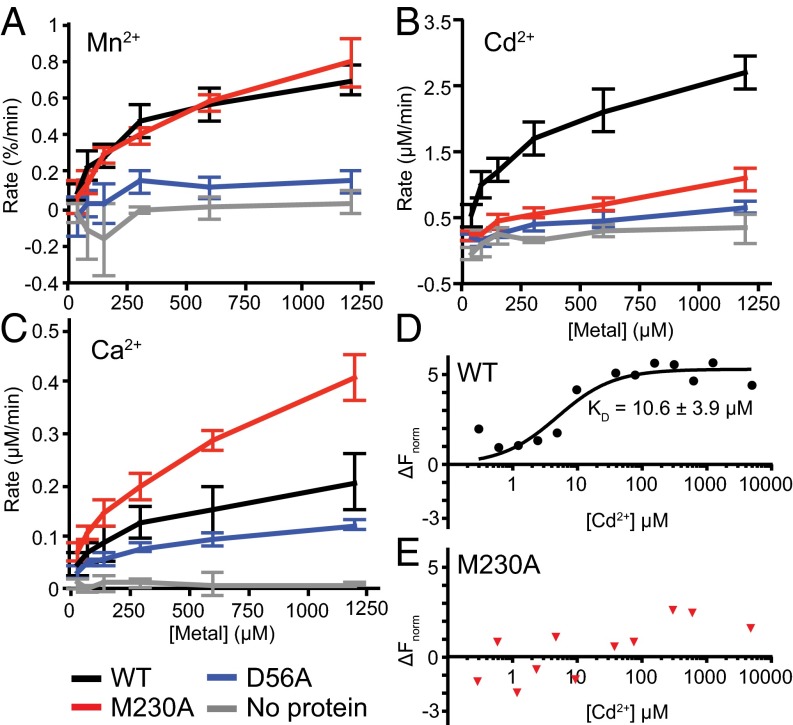
To directly test transport of additional metals, we reconstituted purified DraNramp into Fura-2-loaded proteoliposomes and monitored DraNramp-dependent transport over a range of Cd2+, Mn2+, or Ca2+ concentrations. Mn2+ quenches Fura-2 fluorescence at 340 nm, allowing relative influx measurements, and we determined intraliposome Cd2+ or Ca2+ concentrations using ratiometric Fura-2 fluorescence (Fig. S6 A–D). Mn2+ was transported similarly by WT and M230A, whereas D56A showed little Mn2+ transport activity (Fig. 4A). In contrast, WT transported Cd2+ much more efficiently than either M230A or D56A (Fig. 4B), which demonstrates the importance of both residues to making Cd2+ a good substrate. Importantly, M230A transported Ca2+ more efficiently than either WT or D56A (Fig. 4C), showing how this methionine serves to deter Ca2+ transport. Our in vitro data suggest that the binding-site methionine is dispensable for transport of the biological substrate Mn2+, consistent with our in vivo observations for the similar metals Co2+ and Fe2+. However, the methionine is essential to both promote transport of the toxic metal Cd2+ and prevent transport of the alkaline earth metal Ca2+. Of note, the D56A mutation impaired transport of all tested substrates, demonstrating the importance of the conserved aspartate to the general metal transport mechanism. In contrast, the M230A mutation retains transport function, although with altered metal transport preferences, suggesting the methionine functions as a selectivity filter.
Fig. S6.
Sample time traces of metal influx into proteoliposomes, purification of DraNramp variants for reconstitution in liposomes, and validation of DraNramp constructs used for microscale thermophoresis experiments. (A–C) Indicated concentrations of Mn2+ (A), Cd2+ (B), or Ca2+ (C) is added at 5 min to proteoliposomes loaded with Fura-2 and ionomycin at 65 min to measure maximal Fura-2 signal. (D) Coomassie-stained SDS/PAGE gels showing the purification of WT, D56A, and M230A DraNramp proteins by Ni-affinity chromatography. Washes were performed with buffer containing 75 mM imidazole, and proteins were eluted using 450 mM imidazole buffer. (E) MST constructs display the same cobalt uptake levels and cobalt competition assay metal preferences in in vivo transport assay as the analogous WT and M230A constructs used in other experiments. Cobalt uptake and cobalt competition data with MST constructs were collected in the same experiments as the data presented in Fig. 5. Data are averages of four independent experiments with error bars showing SD from the mean. (F) WT and (G) M230A MST constructs show similar binding trends with Mn2+. Because the noise is higher than observed with Cd2+, we did not calculate KD values. Data are representative of three independent experiments.
Fig. 4.
In vitro transport and binding assays reveal M230’s role in metal selectivity. (A–C) Initial transport rates for Mn2+ (A), Cd2+ (B), and Ca2+ (C) are shown for WT DraNramp (black), M230A (red), D56A (blue), and empty (no protein) liposomes (gray). Error bars are SD from n = 3 or 4. (D and E) Binding isotherms showing WT DraNramp (D) and M230A (E) affinity for Cd2+. Representative data are shown (n = 4) and the error on KD is SD.
To further investigate the underlying cause of the pronounced difference in Cd2+ transport by WT and M230A DraNramp, we measured the in vitro Cd2+-binding affinity of these two proteins using microscale thermophoresis (Fig. 4 D and E). We used purified Strep-tagged protein that was Cy5-maleimide–labeled at a lone cysteine replacing R211 in extracellular loop 5–6; this R211C/C382S construct shows WT-like Co2+ transport activity and similar Cd2+ and Mg2+ competition phenotypes (Fig. S6E). WT protein bound Cd2+ with a KD of 10.6 ± 3.6 μM, similar to the 29 ± 10 μM value determined for WT ScaNramp using ITC (9). In contrast, M230A showed no clear Cd2+-binding signal. This result demonstrates the importance of M230 for Cd2+ binding in WT DraNramp and suggests that reduced binding affinity directly causes Cd2+ transport impairment in M230A. In contrast, we observed a similar trend in binding behavior of Mn2+ (which WT and M230A both transport) for WT and M230A (Fig. S6 F and G), although we did not calculate KD values due to the higher noise in the data.
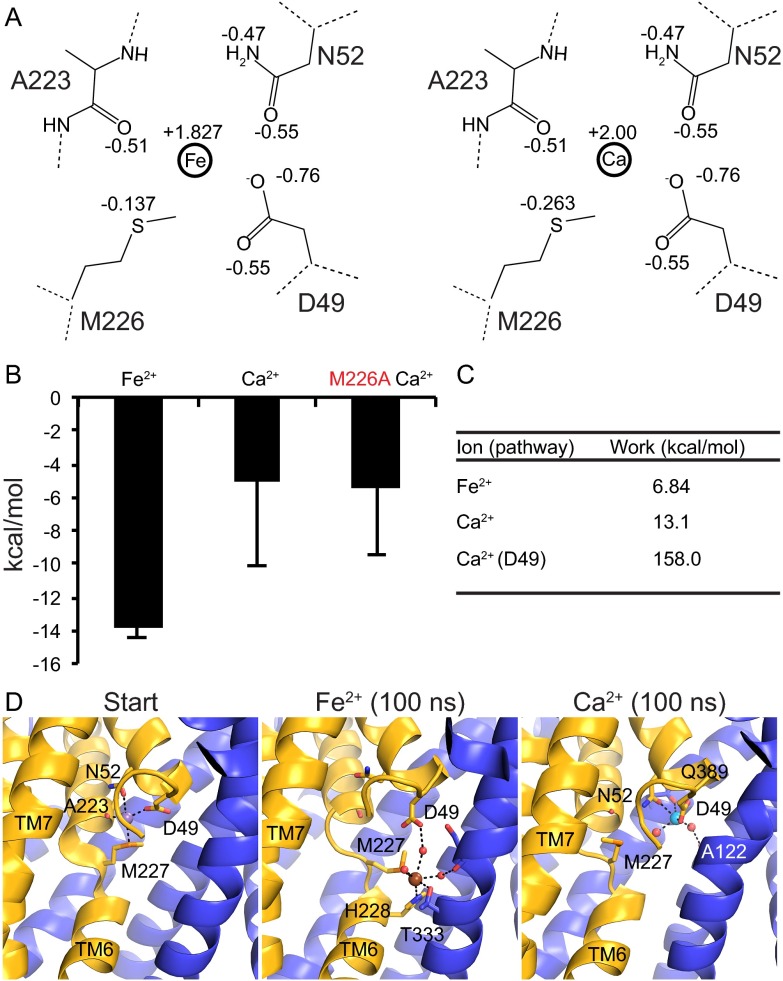
To extend these observations regarding the differential importance of the methionine to the transport of different metals, we performed a series of molecular dynamics simulations, which we discuss in depth in SI Discussion, using the available ScaNramp structure to compare how different ions equilibrate in and exit the conserved binding site (Fig. S7). These simulations showed that if an ion can interact favorably with the methionine, it experiences greater stabilization in the binding site and a lower-energy exit pathway in the intracellular conformation than an ion that cannot interact with the methionine.
Fig. S7.
MD simulations reveal lower overall energies for ions that favorably interact with methionine sulfur. (A) Charge distribution for the MD simulations for Fe2+ (Left) and Ca2+ (Right). Residue numbers correspond to ScaNramp, and dotted lines indicate continuing backbone. (B) Equilibrium MD Gibbs free energy values for Ca2+ and Fe2+ ions in the WT (black) or M226A (red) ScaNramp metal-binding site. Fe2+ was more stable in a WT site, whereas Ca2+ was less stable. (C) Work values from SMD simulations for WT ScaNramp. Ca2+ (D49) refers to the work value for a Ca2+ to follow the Fe2+ pathway. (D) Configuration of ions at the start and end (75 ns) of SMD simulations. Start is the initial binding configuration from PDB ID code 4WGW. Waters are shown as red spheres, Fe2+ as brown sphere, and Ca2+ as cyan sphere.
Only the Native M230 Allows Transition Metal Transport in High Mg2+ Concentrations.
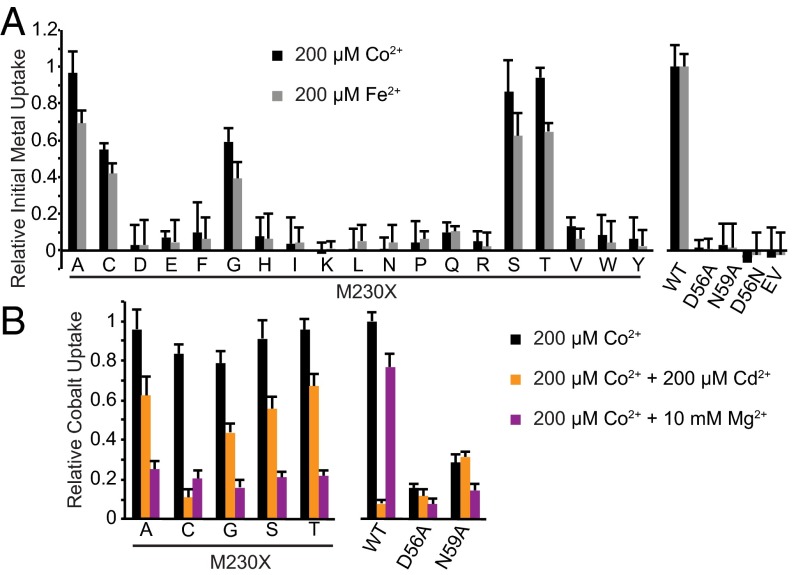
To determine how the unique chemical properties of the binding-site methionine affect metal transport and selectivity, we used our in vivo assay to measure Co2+ and Fe2+ uptake by a panel of 20 DraNramp variants, with each possible amino acid substituted at position 230 (Fig. 5A and Fig. S8). The clear trend is that only small residues—glycine, alanine, serine, threonine, and cysteine—or the native methionine enabled efficient metal transport. This result suggests that other electron-donating functional groups can substitute for the thioether sulfur while preserving transition metal transport. For example, alanine or glycine could provide enough room for one or more water molecules to coordinate the metal (Fig. S8A). Similarly, the threonine or serine hydroxyl group may either directly bind the metal or help align a metal-binding water molecule, but the striking functional difference between the isosteric threonine and valine residues demonstrates the importance of this side chain hydroxyl. Additionally, little metal transport was observed when we replaced M230 with the sterically similar but purely aliphatic isoleucine or leucine side chain, emphasizing the importance of having an electron-pair donor at this position to stabilize the metal substrate. Interestingly, we observed little metal uptake with larger residues that do contain common metal-binding functional groups (asparagine, glutamine, histidine, aspartate, and glutamate). This result may reflect the inability for large polar side chains to optimally orient within the metal-binding site.
Fig. 5.
Substitutions to small amino acids at position 230 retain metal transport ability. (A) Initial Co2+ (black bars, 5-min time point) and Fe2+ (gray bars, 1.5-min time point) transport levels measured for WT DraNramp or variants containing substitutions of M230 to each of the 19 other amino acids expressed in E. coli (Fig. S8B). We observed significant transport (relative to WT) of both metals with the small residues alanine, cysteine, glycine, serine, and threonine replacing M230. (B) Although several mutants transported Co2+ efficiently over the course of 60 min (black bars), only WT retains significant Co2+ transport with a moderate concentration of competing Mg2+ (magenta bars). However, only WT and M230C showed drastic reduction in Co2+ transport with an equal concentration of Cd2+ (gold bars). We used competing Mg2+ and Cd2+ concentrations showing significant differences between WT and M230A (Fig. 3). Error bars are SD (n = 4).
Fig. S8.
Mutation of DraNramp M230 largely did not impact protein expression. (A) The crystal structure of Mn2+-bound ScaNramp (PDB ID code 4WGW) facilitates interpretation of our functional data with various substitutions at position 230. In WT ScaNramp (Center; DraNramp numbering), the four conserved binding-site residues closely approach the bound Mn2+ ion (purple sphere) to form stabilizing ligand-metal bonds. We hypothesize that replacement of M230 with alanine in DraNramp may create enough space for one or more water molecules to coordinate the metal (Upper Left). The M230T threonine hydroxyl may directly interact with the metal or help align a water (Upper Right). The M230L large aliphatic side chain may exclude water from the binding site (Lower Left). For M230C (Lower Right) our data suggest the cysteine can directly interact with the metal to favor Cd2+. (B) Western blots show all M230 mutants expressed well, although replacement with the larger hydrophilic side chains Asp, Glu, His, Lys, Asn, Gln, and Arg, as well as Pro, led to slightly decreased expression compared with WT.
For WT DraNramp and the most active mutants, we measured end point Co2+ uptake in the absence or presence of the competing metals Cd2+ or Mg2+ (Fig. 5B). Strikingly, 10 mM Mg2+ inhibits all mutants, with only WT facilitating significant Co2+ transport. In contrast, 200 μM Cd2+ (1:1 ratio with Co2+) severely reduced WT DraNramp Co2+ uptake, but did not impair the M230A, G, T, or S mutants to the same degree. Interestingly, Co2+ transport by M230C was inhibited by Cd2+ similarly to WT, which suggests the cysteine thiol group can directly coordinate the metal. Thus, the presence of a binding-site sulfur, whether from cysteine or methionine, is essential to making the toxic metal Cd2+ a preferred DraNramp substrate. In conclusion, although five small amino acid side chains can effectively replace the methionine to enable transition metal transport, only the native methionine offers robust selectivity in favor of transition metals in the presence of competing Mg2+.
The Methionine Confers Tolerance to Ca2+ and Mg2+ While Facilitating Cd2+ Toxicity.
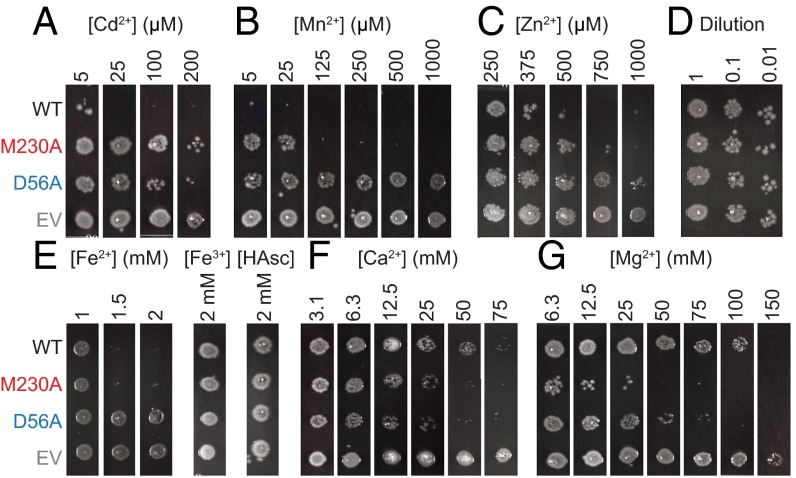
To explore the biological relevance of metal selectivity, we determined the impact of different metal-binding site configurations on growth of DraNramp-expressing E. coli strains in the presence of various metals using a plate-based toxicity assay. We attribute impaired growth of Nramp-expressing E. coli to toxic intracellular accumulation of metal substrates. M230A- and D56A-expressing strains similarly tolerated Cd2+ at levels which prevented growth of WT-expressing cells (Fig. 6A), confirming the importance of both residues observed for Cd2+ uptake in liposomes. Likewise, Mn2+ inhibited growth of WT- and M230A-expressing strains at high micromolar concentrations that the D56A-expressing strains tolerated (Fig. 6B), which also echoes our liposome assay results. Interestingly, at low micromolar Mn2+ concentrations, M230A-expressing cells grew better than WT-expressing cells, which suggests that M230 is important for Mn2+ uptake at low concentrations and/or among competing alkaline earth metals (Ca2+ and Mg2+ are both present at hundreds of micromolar in LB agar) (24). Additionally, WT- and M230A-expressing cells were more susceptible to Zn2+, and Fe2+ but not equivalent concentrations of Fe3+, than their D56A counterparts (Fig. 6 C and E).
Fig. 6.
Metal toxicity assays demonstrate the importance of M230 in filtering out abundant divalent metals. E. coli overexpressing either WT, M230A, or D56A DraNramp, or an empty vector control (EV) in liquid culture were spotted on LB agar containing indicated divalent metal concentrations, and grown overnight. (A) M230A- and D56A-expressing cells tolerated Cd2+ concentrations toxic to WT-expressing cells. (B) Low Mn2+ concentrations inhibited growth of WT-expressing cells, whereas M230A-expressing cells were inhibited at higher concentrations of Mn2+ that D56A-expressing cells tolerated. (C) Zn2+ inhibited cells expressing WT > M230A > D56A. (D) Control dilutions plated in the absence of added divalent metals. (E) Fe2+, but not Fe3+, inhibited growth of WT- and M230A-expressing cells equally, but not D56A-expressing cells. (F) WT-expressing cells grew on higher concentrations of Ca2+ than either M230A- or D56A-expressing cells. (G) WT-expressing cells tolerated much higher Mg2+ concentrations than their M230A, and to a lesser extent, D56A counterparts. Images representative of n = 3 results.
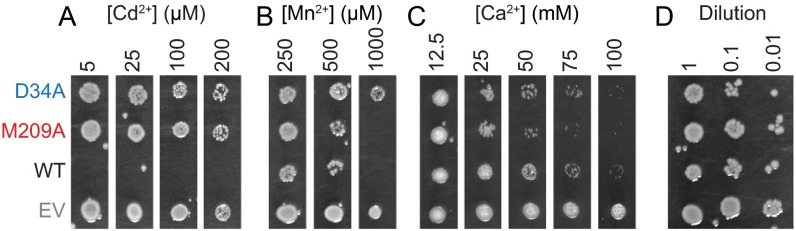
Notably, M230A-expressing cells fared worse at concentrations of Ca2+ or Mg2+ that WT-expressing cells tolerated (Fig. 6 F and G), supporting our Co2+ competition assay data. Interestingly, D56A-expressing cells grew noticeably less than WT-expressing cells at high Mg2+ or Ca2+ concentration, with a similar Ca2+-mediated impairment as M230A. This result suggests that removing either of these two residues may allow nondiscriminatory metal inflow at high concentration gradients, with the intact binding site perhaps functioning as the principal metal transport gate. These metal toxicity observations were not unique to DraNramp, as EcoNramp-expressing cells likewise showed increased Cd2+, decreased Ca2+, and similar Mn2+ toxicity compared with the EcoNramp M-to-A mutant-expressing cells (Fig. S9). Overall, these metal toxicity assays provide indirect evidence of metal transport in a biological setting that largely corroborates our in vitro metal transport findings and emphasizes the importance of the methionine residue in selecting against the environmentally abundant Mg2+ and Ca2+, but also increasing the ability to transport the toxic heavy metal Cd2+.
Fig. S9.
Metal toxicity assays with EcoNramp demonstrate the same trends as seen with DraNramp. E. coli overexpressing either WT, M209A, or D34A EcoNramp, or an empty vector control (EV) in liquid culture were spotted onto LB agar containing indicated concentrations of divalent metals and grown overnight. (A) M209A- and D34A-expressing cells tolerated Cd2+ concentrations toxic to WT-expressing cells. (B) Mn2+ similarly inhibited growth of WT- and M209A-expressing cells, whereas D34A-expressing cells were less sensitive. (C) Ca2+ inhibited the growth of M209A-expressing cells to a greater extent than WT-expressing cells, with D34A-expressing cells somewhere in the middle. (D) Control dilutions plated in the absence of added divalent metals. Images are representatives of results from three independent experiments.
SI Discussion
Molecular Dynamics Simulations Illustrate Effects of the Methionine in Metal Selectivity.
To further explore the molecular basis for our experimental observations, we performed a series of molecular dynamics (MD) simulations using the available ScaNramp structure (9). Note that although the transport properties of DraNramp and ScaNramp differ, both share the same conserved metal-binding residues. Parameters for Ca2+ were obtained from CHARMM (38, 39). Fe2+ parameters were deduced from those of heme-Fe2+ (40) using charge-transfer corrections (41), in particular the methionine sulfur charge was redistributed onto the iron to reduce its effective positive partial charge (Fig. S7A). Although this method is ad hoc, offering a static view of charge-transfer phenomena, it has provided useful models for the energetic and bonding interaction distributions for different metal ions in different configurations and binding site geometries.
Equilibrium MD simulations confirmed that Fe2+ experienced a more favorable interaction than Ca2+ in the same configuration of the WT metal-binding site (Fig. S7). The Ca2+-interaction energies remained essentially unchanged in simulations of an in silico M-to-A mutation, suggesting that Ca2+-methionine interactions contribute only marginally to the Ca2+-interaction energies in WT Nramp.
We also performed steered MD (SMD) simulations to explore the initial unbinding of the metal ion by lowering the coordination number of the binding site over 100 ns (42, 43). Although we did not observe complete unbinding within the timescale of the simulation, the metal-ion movements did lead to structural changes in the coordinating groups. Consistent with equilibrium simulations, a sulfur-mediated unbinding pathway seen with Fe2+, in which the methionine flips to allow ion exit, was less energetically expensive than a non–sulfur-mediated pathway seen with Ca2+, where a glutamine interacts with the exiting Ca2+ (Fig. S7 C and D). Thus, these simulations, in which interactions with the methionine sulfur—modeled as stabilizing transition metal ions—yielded overall lower-energy unbinding pathways, are consistent with and provide an atomic-level explanation for our experimental observations that transition metal ions are preferentially transported by WT DraNramp, whereas the M230A mutant is less selective.
Equilibrium MD Simulations Suggest a Stronger Metal-Sulfur Interaction Yields Higher Metal Ion Binding Affinity.
In our equilibrium MD simulations, we observed that the metal ion interaction energy, averaged over 100 ns (simulations in triplicate), was strongest for Fe2+, compared with Ca2+ (−13.8 ± 0.7 and –5.2 ± 5.0 kcal/mol, respectively; Fig. S7B). Decomposing the force-field interaction energy into various components, the increased stability of Fe2+ over Ca2+ stems from its stronger van der Waals interaction with the M226 sulfur (−0.40 ± 0.02 vs. 5.3 ± 0.6 kcal/mol); the sidechain-metal ion electrostatic interactions within the binding pocket are similar for Fe2+ and Ca2+ (−14.2 ± 0.7 to –10.5 ± 4.4 kcal/mol). Furthermore, the Ca2+-interaction energies remained unchanged in simulations of an in silico M-to-A mutation (−5.4 ± 4.0 kcal/mol), suggesting that Ca2+-methionine interactions contribute little to the Ca2+-interaction energies in WT Nramp.
SMD Simulations Suggest the Softer Ions Access Sulfur-Mediated Exit Pathway.
The SMD simulation of Fe2+ in the ScaNramp binding site, in which we lowered the coordination number of the binding site over 100 ns, revealed a M226-mediated exit pathway for the metal ion: The methionine sidechain flipped out of the binding site, essentially guiding the ion out of the binding pocket toward the intracellular vestibule (Fig. S7D). The work required to induce this exit path was 6.8 kcal/mol. In contrast, Ca2+ accessed a D49-mediated pathway, which involved more work, 13.1 kcal/mol, than the Fe2+ exit (Fig. S7 C and D). In addition, after exiting the pocket through the higher-work pathway toward the extracellular side, Ca2+ found a secondary binding site, interacting with Q389.
We then performed another simulation in which we steered the Ca2+ ion to exit in the same direction as the Fe2+ ion. The work required for Ca2+ to exit through this methionine-mediated pathway was an order of magnitude more expensive than its D49-mediated pathway (158.0 vs. 13.1 kcal/mol). This increase in work is likely explained by conformational changes: the M226 shifted its backbone (ϕ,ψ) angles to allow Ca2+ to exit. Taken together, a direct exit of an ion to the water pool from the binding site via a methionine-mediated flip seems more energetically feasible than a methionine-independent pathway. Because methionine interacts more strongly with softer atoms (Fig. S7), the low-work exit pathway is selectively accessible to softer ions such as Fe2+. These simulations therefore suggest that methionine interactions and conformations at equilibrium underlie the ion selectivity of Nramp transport.
Discussion
Our study demonstrates the role of the conserved metal-binding site methionine as the selectivity filter in Nramps. In contrast to the other binding site residues, it is not essential for transport of many biologically useful transition metals in DraNramp and several other homologs, although a mutation to alanine does slightly impair Mn2+ transport in some conditions. In addition, while this mutation drastically decreases transport of the toxic metal Cd2+, it increases transport of Mg2+ and Ca2+, which would likely be deleterious to most organisms.
Mutations of the binding site methionine have previously been investigated in a few species. In EcoNramp, an M-to-I or M-to-K mutation eliminated Mn2+ transport (25), which agrees with our lack of observed Fe2+ and Co2+ transport for those mutants in DraNramp. Consistent with our findings regarding the methionine’s importance for Cd2+ binding and transport in DraNramp, an M-to-A mutation in ScaNramp significantly decreased Cd2+ binding affinity, and the analogous mutation in human Nramp2 reduced Cd2+ transport (9), a phenotype we replicated and extended by showing loss of Co2+, Mn2+, and Fe2+ transport. We discovered a range of relative Fe2+ transport abilities for M-to-A mutants in different bacterial homologs (Fig. 2 and Fig. S3) that stretched from no apparent activity for M226A ScaNramp to approximately WT-level transport in M230A DraNramp and M209A EcoNramp to enhanced transport in M236A S. marcescens Nramp.
Our DraNramp results suggest that the main purpose of Nramp’s binding site methionine is to reduce Mg2+ and Ca2+ transport, thus avoiding their overaccumulation and preventing competition with the rarer transition metals that are Nramp’s intended substrates. Inorganic chemistry theory helps explain how many proteins, including Nramp, evolved to discriminate among similar metal ions by tuning their binding-site chemical and structural properties. Interactions between metals and electron-donating ligands are typically strongest when either the ionic or covalent nature of the bond is maximized (26). Thus, highly electronegative ligands such as oxygen form strong bonds with “hard” metal ions like Mg2+, which has a high charge in a small radius. Conversely, less electronegative, more polarizable ligands such as sulfur form the most stable bonds with “soft” metal ions like Cd2+, which better share electron density in a covalent manner. In the empirical classification of metal ions as hard (favoring ionic interactions), intermediate (capable of both ionic and covalent interactions), or soft (favoring covalent interactions), Nramp’s biological substrates—the first row transition metals—are considered intermediate, whereas Ca2+ and Mg2+ are hard, and Cd2+, Pb2+, and Hg2+ are soft. In Nramp’s binding site, the soft methionine sulfur is the sole exception; all other metal-binding ligands are hard oxygens better suited to ionic bonds. The methionine therefore selectively stabilizes transition metals capable of covalent and semicovalent interactions.
Our data show that this methionine provides significant stabilization necessary for the binding and transport of Cd2+, which like other soft toxic heavy metals can forge strong covalent-like interactions with sulfur. Additionally, our results suggest that the magnitude of the net methionine stabilization may be minimal for Nramp’s biological substrates (the intermediate first row transition metals), whereas the ionic-interacting hard alkaline earth metals Ca2+ and Mg2+ (which become better substrates upon the methionine’s removal) are effectively destabilized by its presence.
One potential explanation for the M-to-A mutant phenotype is that alanine enables substrate metal ions to retain one or more water ligands that they would otherwise shed upon coordination by the WT binding site. The M-to-A mutation would therefore both improve the binding environment for metals that prefer hard oxygen ligands (Mg2+ and Ca2+) and impair the ability of a metal like Cd2+ that prefers soft ligands to bind while having only a minor effect on net affinity for intermediate metals that lack a strong preference between a hard and soft ligand. Our M-to-X substitution panel results support this model (Fig. 5): Small residues, which could leave free space for water, all enabled transport of intermediate metals Co2+ and Fe2+ while remaining susceptible to Mg2+ competition and decreasing Cd2+ preference (with the exception of the sulfur-containing cysteine), whereas larger residues such as leucine, which may not provide space for water, severely impaired transport.
Methionine is rarely found in binding sites for most metals, with the notable exception of copper (27, 28). However, the putative metal-binding site in the iron exporter ferroportin contains a methionine (29), suggesting a strategy similar to Nramps' for selective transition metal transport. Underscoring the methionine’s key metal selectivity role, a functionally diverged Nramp-related transporter of the hard metal Al3+ in rice has a threonine (providing a hard oxygen ligand) in place of the methionine while retaining the other binding-site residues (30). Similarly the methionine is also replaced with threonine in two bacterial Nramp-related Mg2+ transporters (31). Intriguingly, Nramp homologs from several extremophilic prokyarotes that flourish in highly acidic and heavy metal rich environments (32) have an alanine in place of the methionine, suggesting this substitution is favored under the right selective pressure.
In this evolutionary context, Nramp’s binding site methionine likely represents a tradeoff. We propose that this residue was selected and conserved primarily for its ability to deter competition from and transport of Ca2+ and Mg2+, rather than for it being an optimal ligand for its intended substrates such as Mn2+ and Fe2+. The alkaline earth metals are highly abundant in the environment (the ocean contains 50 mM Mg2+ and 10 mM Ca2+) (33), and intracellular Ca2+ concentration is tightly regulated, with unchecked import disrupting cell viability, thus providing a strong selective pressure to guard against accidental transport. In addition, as the first row transition metals are approximately six orders of magnitude less abundant than the alkaline earth metals (33), organisms require a robust discrimatory mechanism to find those essential metals within a sea of Ca2+ and Mg2+. However, the binding site methionine also serves as an optimal ligand for the toxic metal Cd2+, enabling it to become an ideal substrate for Nramps: a detrimental property as Nramp activity has been linked to cadmium poisoning in mammals (34, 35). Nevertheless, the normally low environmental abundance of Cd2+ (two orders of magnitude below Mn2+) likely did not provide significant selective pressure against the methionine on evolutionary time scales. In conclusion, our results explain the ability of Nramps to transport a variety of transition metals while highlighting the role of the conserved methionine in specifically discriminating against the abundant hard alkaline earth metals.
Methods
Additional methods are described in SI Methods.
DraNramp Microscale Thermophoresis Binding Assay.
Purified strep-tagged fluorescently labeled DraNramps were diluted to 100 nM in 150 mM NaCl, 10 mM Hepes, pH 7.5, and 0.03% β-dodecylmaltoside (β-DDM), mixed 1:1 with 14 serially diluted CdCl2 solutions, and loaded into Premium Coated capillaries (NanoTemper Technologies). Thermophoresis data were obtained with a NanoTemper Monolith NT.115 at 70% light-emitting diode, 80% microscale thermophoresis (MST) power and 30-s MST time, and analyzed using GraphPad prism.
DraNramp Reconstitution and Proteoliposome Activity Assays.
Lipids [50:35:15 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE):1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC):1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-rac-glycerol (POPG); Avanti Polar Lipids] and freshly purified DraNramp at a 1:500 mass ratio and 5 mM β-decylmaltoside (β-DM) were dialyzed against 10 mM Mops, pH 7.0, and 100 mM KCl at 4 °C for 2–3 d and at room temperature for 1 d. After three freeze-thaw cycles to incorporate Fura-2 (100 μM), liposomes were extruded through a 400-nm filter, separated from bulk dye on a PD-10 column (GE Life Sciences), and diluted threefold into 10 mM Mops, pH 7.0, and 100 mM NaCl in a 96-well black clear-bottom assay plate (Greiner). After measuring baseline fluorescence for 5 min, metal substrate was added, along with 50 nM valinomycin to establish a negative internal potential. Fluorescence (λex = 340 and 380 nm; λem = 510 nm) was monitored at room temperature for 70 min, adding ionomycin (0.25 μM) at 60 min to measure maximum signal. The intraliposome concentration of Cd2+ or Ca2+ was determined using [M2+]inside = ([M2+]free[Fura-2]total)/(KD + [M2+]free) + [M2+]free, where KD is for M2+ and Fura-2 (Cd2+ = 1 pM; Ca2+ = 135 nM) (36, 37). Transport rates were determined by linear regression of the data for the first 10 min after adding Cd2+ or 60 min after adding Mn2+ or Ca2+.
In Vivo Metal Uptake Assays in E. coli.
E. coli expressing bacterial Nramps at OD = 5.26 in 190 μL assay buffer [50 mM Hepes, pH 7.3, or 2-(N-morpholino)ethanesulfonic acid (Mes), pH 6.4, for Fig. 2 and Fig. S3 data, 60 mM NaCl, 10 mM KCl, 0.5 mM MgCl2, and 0.217% glucose] were distributed into 96-well plates at 37 °C. To initiate uptake, 10 μL 20× metal solution [4 mM Co(NO3)2, 4 mM freshly made FeSO4 in 4 mM ascorbic acid, or a mixture of 4 mM Co(NO3)2 with competing metal as chloride salt] was added. To quench uptake, 10 μL 100 mM EDTA (20 μL 200 mM EDTA when Ca2+ or Mg2+ was included) was added. Cells were pelleted and washed three times before precipitating the metal with 1% (NH4)2S. Scanned images of the resulting pellets were converted to black and white, and pellet darkness was determined using ImageJ64 (NIH).
In Vivo Metal Uptake Assays in HEK293 Cells.
Fifty microliters of 150 mM NaCl, 4.5 mM KCl, 0.2 mM MgCl2, 10 mM glucose, and 20 mM Mes, pH 5.5, was added to transfected Fura-2–loaded HEK293 cells. Fluorescence (λex = 340 and 380 nm; λem = 510 nm) was monitored for a 2- to 4-min baseline and 45 min after adding 25 μL 3× metal solution [Co(NO3)2, MnCl2, CdCl2, CaCl2, or FeSO4 + ascorbic acid].
Metal Toxicity Assay.
Isopropyl β-d-1-thiogalactopyranoside (IPTG)-induced E. coli expressing bacterial Nramps (1 μL of dilutions to OD600 = 0.01, 0.001, or 0.0001 in LB) were plated onto LB ampicillin agar containing various concentrations of added metal, and the plates were imaged using an AlphaImager system after overnight incubation at 37 °C. Gamma values were adjusted the same way for all images to increase contrast.
SI Methods
Cloning of Nramp Constructs.
pET21-N8H was derived from pET21a (Novagen) vector by adding an 8xHis-tag followed by a new NdeI site between the NdeI and BamHI sites and mutating the original NdeI site: replacing multiple cloning site region 5′-CATATGGCTAGCATGACTGGTGGACAGCAAATGGGTCGCGGATCC-3′ with 5′-CTTATGCATCATCACCATCACCATCACCATATGTTGGAAAAGGGATCC-3′. The DraNramp coding sequence was amplified from genomic DNA (ATCC) and inserted in the NdeI and NotI sites of pET21-N8H or an analogously prepared pET21-NStrep vector containing a StrepII-tag. ScaNramp was obtained from Raimund Dutzler, ETH Zürich, Zurich (9), and the coding sequence was transferred to pET21-N8H. EcoNramp (from Mathieu Cellier, INRS-Institut Armand Frappier Research Center, Laval, Canada) was transferred to a similarly derived pET21-N10H vector and lacked the first eight residues of the WT sequence. Nramp genes from Salmonella typhimurium, Klebsiella pneumonia, Pseudomonas aeruginosa, and Deinococcus maricopensis (amplified from genomic DNA), and from Serratia marcescens, Xanthomonas campestris, and Streptococcus mutans (from Mathieu Cellier) were inserted into pET21-N11H. Staphylococcus aureus Nramp (from Nancy Andrews, Duke School of Medicine, Durham, NC) was inserted into a pET21-C10H vector. Human Nramp2 (from DNASU) was inserted into pCDNA3 vectors with or without a C-terminal eGFP. Mutagenesis restored the highly conserved Q119, replacing the lysine present in the DNASU plasmid. Quikchange mutagenesis (Stratagene) was used to make mutations. Coding sequences were confirmed by DNA sequencing.
Expression and Purification of Nramp Constructs.
Bacterial proteins were expressed for purification and all functional assays in E. coli C41(DE3) cells. Although this strain is WT for the E. coli Nramp gene (MntH), available expression data indicate that the endogenous MntH is expressed primarily when iron is scarce or H2O2 is present (ref. 44 and references therein). Consistent with this expression pattern, our Co2+ and Fe2+ uptake data show very little background uptake, further supporting our assumption that the overexpressed Nramp variant is responsible for the observed signals. For in vivo assays, E. coli were grown in lysogeny broth (LB) + 100 mg/L ampicillin at 37 °C, inducing at OD600 = 0.6 with 100 µM IPTG. To allow for Nramp expression, cells were shaken for 75 min (most in vivo metal uptake and competition experiments), 90 min (for plate-based toxicity assays), or 4 h (Fe2+ uptake experiments in Fig. 2 and Fig. S3). For in vivo metal uptake assays cells were washed twice in Co2+ assay buffer before plating and initiating metal uptake.
For protein purification, terrific broth with 10% (wt/vol) glycerol and 100 mg/L ampicillin was inoculated by diluting overnight culture 1:50. Cells were grown at 37 °C to OD600 = 1.0, induced with 100 µM IPTG for 4 h, harvested, flash frozen in liquid nitrogen, and stored at −20 °C. Protein purification was done at 4 °C. Cells from a 12-L culture were lysed by sonication in three volumes of lysis buffer [20 mM NaPO4, pH 7.0, 75 mM imidazole-HCl, pH 7.0, 500 mM NaCl, 10% (vol/vol) glycerol] plus 1 mM PMSF, 1 mM benzamidine, 0.3 mg/mL DNaseI, and 0.3 mg/mL lysozyme. After removing debris by 20-min centrifugation at 27,200 × g in a JA-20 rotor (Beckman), membranes were pelleted in 70 min at 235,000 × g in a Ti-45 rotor (Beckman Coulter) and resuspended in 35 mL lysis buffer + 1% (wt/vol) DDM. After 1 h, insoluble material was removed by 35-min centrifugation at 142,000 × g in a Ti-45 rotor. The supernatant was filtered through a 0.45-μm filter and then loaded onto 5 mL Ni-Sepharose (GE Healthcare) pre-equilibrated with lysis + 0.03% β-DDM, and washed thrice with 50 mL lysis + 0.03% β-DDM. Protein was eluted in 25 mL by increasing imidazole to 450 mM and concentrated to ∼1 mL in a 50-kDa cutoff centrifugal concentrator. Ni-affinity purification results are shown in Fig. S6D. Following buffer exchange on Superdex S200 (GE Healthcare) to 20 mM Hepes, pH 7.5, 150 mM NaCl, and 0.1% (wt/vol) β-decylmaltoside (DM), protein-containing fractions were analyzed using SDS/PAGE, pooled, and concentrated to ∼1 mg/mL.
Purification and Fluorescent Labeling of Strep-Tagged Nramp Constructs.
Solubilized membranes of cells expressing Strep-tagged DraNramp with two additional mutations (R211C and C382S) that serve to insert a single cysteine in an extracellular loop were prepared as above, replacing lysis buffer with buffer W (100 mM Tris, pH 8.0, 150 mM NaCl), and then loaded onto a 3-mL Strep-Tactin Superflow (IBA) column pre-equilibrated with buffer W + 0.03% β-DDM, and washed five times with 3 mL buffer W + 0.03% β-DDM. Protein was eluted in six 1.5-mL fractions with buffer W + 0.03% β-DDM + 2.5 mM desthiobiotin. Protein-containing fractions were buffer-exchanged into 150 mM NaCl, 10 mM Hepes, pH 7.5, and 0.03% β-DDM. Protein was concentrated to ∼5 mg/mL in a 50-kDa cutoff centrifugal concentrator. The protein was labeled with the red fluorescent maleimide dye NT-647 (NanoTemper Technologies) by incubating 30 μM dye with 10 μM protein for 30 min. Labeled protein was separated from free dye using a desalting column. Protein fluorescence was measured and both WT and M230A eluted proteins were diluted to a concentration of 100 nM into buffer containing 150 mM NaCl, 10 mM Hepes, pH 7.5, and 0.03% β-DDM.
Data Analysis for in Vivo Metal Uptake Assays in E. coli.
ImageJ64 was used to determine the pellet darkness for each sample at each time point. For the time course experiments, the average of WT DraNramp endpoint darkness (60 min for Co2+ or 30 min for Fe2+) was set as 100% transport, with other mutants and other species compared relatively. For the WT vs. M230A metal competition experiments (Fig. 3), the average of five replicate WT 60-min Co2+-only darkness values was set to 100% transport. The empty vector darkness values for each metal combination was subtracted from the corresponding WT or M230A value to determine DraNramp-dependent Co2+ uptake. IC50 values were calculated in Prism (Graphpad Software). For these fits, the average relative cobalt transport in the absence of competing metal (except 0.5 mM Mg2+) was set as the upper threshhold (1.000 for WT, 0.967 for M230A), no cobalt transport (0.00) was set as the lower threshhold, and the Hill slope was set to 1 as only one metal binding site is expected. Data were similarly treated (minus IC50 value calculations) for the M230X Co2+ competition experiments in Fig. 5B.
Transfection and Dye Loading of HEK293 Cells for in Vivo Metal Uptake Assays.
HEK293 cells were grown until confluent in DMEM supplemented with GlutaMAX, penicillin, streptomycin, and 10% (vol/vol) FBS. To transfect cells, 2 μg plasmid was mixed with 200 μL 250 mM CaCl2 and added to 220 μL 2× Hepes-buffered phosphate (250 mM NaCl, 10 mM KCl, 40 mM Hepes, 12 mM dextrose, 1.4 mM Na2HPO4, pH 7.05) and then added dropwise to 3.3 × 105 cells in 2.2 mL media and mixed. Cells (200 μL/well) were plated in a poly-d-lysine–coated 96-well assay plate (Corning) and incubated overnight in a 37 °C before changing the media and transferring to 30 °C for 48 h. Media were then replaced by 50 μL 5 μM Fura-2AM in dye-loading buffer (150 mM sodium gluoconate, 10 mM NaCl, 10 mM glucose, 2 mM CaCl2, 10 mM Hepes, pH 7.2, and 0.02% pluronic F-127). Cells were incubated for 45–60 min then washed with 180 μL of dye-loading buffer for at least 60 min to remove excess dye. For the metal uptake assay, loading buffer was then replaced with 50 μL assay buffer (150 mM NaCl, 4.5 mM KCl, 0.2 mM MgCl2, 10 mM glucose, 20 mM Mes, pH 5.5).
Western Blots.
Cells at OD600 = 5.26 were spun down and resuspended in the same volume of 6 M urea, 0.5% SDS, and 0.1 M Tris⋅HCl, pH 7. SDS sample buffer (4×) was added, and a 8- to 12-μL sample run on 12% SDS/PAGE and then transferred to PVDF membranes (GE Life Sciences). At room temperature, membranes were blocked in TBST (20 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) + 3% (wt/vol) BSA for 1 h, stained with Anti-Penta-His Alexa Fluor 647 Conjugate (QIAGEN) at a 1:1,500 ratio in TBST + 1% BSA for 1 h, washed thrice in TBST, and imaged on a GE Amersham Typhoon Imager at an excitation of 633 nm and emission of 670 nm.
In Gel Fluorescence Measurements.
HEK293 cells were transfected as above (using C-terminal eGFP tagged constructs) and 2 mL/well was plated in a 12-well dish. After 48 h at 30 °C, cells were detached by replacing the media with 1 mL ice-cold PBS. Cells were pelleted and resuspended in 200 μL lysis buffer (150 mM NaCl, 50 mM Tris, pH 8.0, 1% Triton X-100, 0.1% SDS, 5 mM EDTA, with 1× Protease Inhibitor Mixture; biotool.com), and the total protein concentration of the lysate was determined by Bradford Assay. Equal amounts of protein were mixed with 4× SDS loading buffer, and 27 μg/well was run on a 12% SDS/PAGE gel. In gel fluorescence was measured using a GE Amersham Typhoon Imager at an excitation of 488 nm and emission of 520 nm.
MD Simulation System Preparation.
All MD simulations were performed with NAMD 2.10 using our XSEDE allocations on the Stampede supercomputer. The ScaNramp structure [Protein Data Bank (PDB) ID code 4WGW] was inserted into a lipid membrane, solvated, and ionized using the Membrane Builder tools on CHARMM-GUI (45). Although the ScaNramp structure was determined as a dimer in the asymmetric unit, the simulations were performed on the functionally relevant monomeric state corresponding to chain A. To determine the transmembrane orientation of a monomeric ScaNramp, a search was performed on the orientation of known LeuT protein monomers (PDB ID codes 4WGW , 2X79, 3GIA, 3TT3, and 4C7R), including both inward-facing and outward-facing states, using CHARMM-GUI. The orientation of all these proteins were comparable within an azimuthal angle deviation of 15° about the membrane normal z axis. Finally 4WGW-A was aligned to the five structures to derive its membrane orientation. The lipid composition was 3 POPE: 2 POPC: 1 POPG, as has been successfully used for modeling bacterial membranes (46). An initial membrane of surface area 110 × 110 Å was constructed in the XY plane about the protein. The protein-lipid construct was solvated with 25-Å-thick layers of water along the Cartesian Z direction and ionized to charge neutralization using Monte Carlo sampling of Na+ and Cl− ions at 0.15 M concentration. The overall system size was 103,189 atoms. Before simulation, the system was subjected to 10,000 steps of conjugate gradient energy minimization, followed by 100 ps of thermalization and 25 ns of equilibration. During the first 10 ns of the equilibration stage, the protein was kept fixed, allowing the lipids, ions, and water molecules to equilibrate, whereas the subsequent 15 ns of equilibration also included the protein.
Force-Field Parameters.
All simulations were performed using the CHARMM36 force field for the proteins and lipids (47). The TIP3P model was used to simulate water (48). The parameters for the Nramp-bound metal ions are described in some detail here. Our biochemical results and previous work (9) implicated M226 (ScaNramp numbering) in increasing transport or binding propensity of Mn2+, Fe2+, Cd2+, and Zn2+ over Ca2+ ions. We performed MD simulations primarily to validate the role of the methionine in selectivity. To fully capture the effects of electron delocalization on metal ion binding would require more rigorous quantum-chemical calculations to develop appropriate polarizability and charge-transfer force field parameters, which is beyond the scope of the current study. We thus used established methods to model metal ions, remaining in the pairwise-additive approximation. Classical force field parameters are unavailable for Mn2+, but some parameters are available for Ca2+ and Fe2+. The commonly used reduced heme parameters (40), which are also distinct from the Ca2+ ones, were corrected for partial charges using Klein’s suggested corrections (41) to model Fe2+ in the binding site. In particular, the methionine sulfur charge was delocalized on the iron to reduce its effective positive partial charge (Fig. S7A). However, we refrained from including charge delocalization effects between the cation and binding-site acidic groups as relevant information was only available for Fe2+ and not for Ca2+. Thus, at the force field level, the ions only differ by their interactions with the methionine sulfur. Specifically, Fe2+ is predefined to be more strongly interacting than Ca2+. We thus simulated metal-ion binding using classical force fields that approximated different extents of the metal ion-M226 interaction: Ca2+ interacts minimally, and Fe2+ interacts substantially via explicit accounting of charge redistribution through modifying conventional Coulomb and van der Waals parameters. Both the Ca2+ and Fe2+ parameters have been used in several past metalloenzyme simulations over a range of coordination sites (49–52).
Simulation Parameters.
For equilibrium MD, systems were kept at constant temperature (T = 310 K) using Langevin dynamics for all nonhydrogen atoms with a Langevin damping coefficient of 5 ps−1. A constant pressure of 1 atm was maintained using the Nosé–Hoover Langevin piston with a period of 100 fs and damping timescale of 50 fs. Simulations were performed with an integration time step of 1 fs where bonded interactions were computed every time step, short-range nonbonded interactions every two steps, and long range electrostatic interactions every four steps. A 12-Å cutoff was used for van der Waals and short-range electrostatic interactions, with a switching function starting at 10 Å for van der Waals interactions to ensure a smooth cutoff. The simulations were performed under periodic boundary conditions, with full-system, long-range electrostatics calculated by using the PME method with a grid point density of 1/Å. The unit cell was large enough that adjacent copies of the protein did not interact via short-range interactions. We then performed 500 ns of MD simulation at 310 K. The final 100 ns was repeated thrice to examine the statistical significance of the result.
SMD simulations were performed with the equilibrium MD parameters, reported above, together with the coordination number collective variable within NAMD’s colvar module (42, 43). The coordination number of the metal ion (Fe2+ and Ca2+) with binding-site residues (D49, N52, and A223) was set to zero over a timescale of 200 ns, using a force constant of 5 kcal/mol per Ångstrom, and the resulting exit pathway of the metal ion was monitored to investigate its interactions with the M226 sidechain.
Nonequilibrium work values were calculated from the SMD trajectories to yield a semiquantitative measure of the relative energy cost for metal ion unbinding. The work performed by the harmonic force was computed by numerical integration of the force applied by the potential over time multiplied by the total displacement (53). Pathways requiring lower work to unbind a metal ion are expected to be statistically more amenable.
Acknowledgments
We thank Ming-Feng Tsai, Chris Miller, and Ute Hellmich for assistance with developing the liposome assay; Ali Raja, Gunes Bozkurt, and Hao Wu for assistance with the microscale thermophoresis system; Jack Nicoludis and members of the R.G. laboratory for discussions; Mathieu Cellier for providing several bacterial Nramp clones; and Raimund Dutzler for sharing the ScaNramp coordinates before release and providing the ScaNramp gene. The work was funded in part by a Basil O’Connor Starter Scholar research award from the March of Dimes Foundation (to R.G.), NIH Grant 9P41GM104601 (to K.S.), and a Beckman Postdoctoral Fellowship (to A.S.). We gladly acknowledge supercomputer time from the Texas Advanced Computing Center via Extreme Science and Engineering Discovery Environment Grant National Science Foundation (NSF)-MCA93S028.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607734113/-/DCSupplemental.
References
- 1.Courville P, Chaloupka R, Cellier MFM. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem Cell Biol. 2006;84(6):960–978. doi: 10.1139/o06-193. [DOI] [PubMed] [Google Scholar]
- 2.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9(14-15):1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Vidal S, Belouchi AM, Cellier M, Beatty B, Gros P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mamm Genome. 1995;6(4):224–230. doi: 10.1007/BF00352405. [DOI] [PubMed] [Google Scholar]
- 4.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 5.Agranoff D, Monahan IM, Mangan JA, Butcher PD, Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J Exp Med. 1999;190(5):717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36(5):1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 7.Cailliatte R, Schikora A, Briat JF, Mari S, Curie C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell. 2010;22(3):904–917. doi: 10.1105/tpc.109.073023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colangelo EP, Guerinot ML. Put the metal to the petal: Metal uptake and transport throughout plants. Curr Opin Plant Biol. 2006;9(3):322–330. doi: 10.1016/j.pbi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol. 2014;21(11):990–996. doi: 10.1038/nsmb.2904. [DOI] [PubMed] [Google Scholar]
- 10.Milner MJ, et al. Root and shoot transcriptome analysis of two ecotypes of Noccaea caerulescens uncovers the role of NcNramp1 in Cd hyperaccumulation. Plant J. 2014;78(3):398–410. doi: 10.1111/tpj.12480. [DOI] [PubMed] [Google Scholar]
- 11.Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem. 2000;275(46):35738–35745. doi: 10.1074/jbc.M005387200. [DOI] [PubMed] [Google Scholar]
- 12.Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012;287(36):30485–30496. doi: 10.1074/jbc.M112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez M, Vélez D, Devesa V, Puig S. Participation of divalent cation transporter DMT1 in the uptake of inorganic mercury. Toxicology. 2015;331:119–124. doi: 10.1016/j.tox.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Jin J, DeFelice LJ, Andrews NC, Clapham DE. A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biol. 2004;2(3):E50. doi: 10.1371/journal.pbio.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan JA. The Biological Chemistry of Magnesium. VCH; New York: 1995. p. xvi. [Google Scholar]
- 16.Myers BR, Bohlen CJ, Julius D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron. 2008;58(3):362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaloupka R, et al. Identification of functional amino acids in the Nramp family by a combination of evolutionary analysis and biophysical studies of metal and proton cotransport in vivo. Biochemistry. 2005;44(2):726–733. doi: 10.1021/bi048014v. [DOI] [PubMed] [Google Scholar]
- 18.Courville P, et al. Solute carrier 11 cation symport requires distinct residues in transmembrane helices 1 and 6. J Biol Chem. 2008;283(15):9651–9658. doi: 10.1074/jbc.M709906200. [DOI] [PubMed] [Google Scholar]
- 19.Haemig HAH, Brooker RJ. Importance of conserved acidic residues in mntH, the Nramp homolog of Escherichia coli. J Membr Biol. 2004;201(2):97–107. doi: 10.1007/s00232-004-0711-x. [DOI] [PubMed] [Google Scholar]
- 20.Lam-Yuk-Tseung S, Govoni G, Forbes J, Gros P. Iron transport by Nramp2/DMT1: pH regulation of transport by 2 histidines in transmembrane domain 6. Blood. 2003;101(9):3699–3707. doi: 10.1182/blood-2002-07-2108. [DOI] [PubMed] [Google Scholar]
- 21.Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102(5):1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- 22.Cellier MF, Bergevin I, Boyer E, Richer E. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 2001;17(7):365–370. doi: 10.1016/s0168-9525(01)02364-2. [DOI] [PubMed] [Google Scholar]
- 23.Dereeper A, et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucl Acids Res. 2008;36(Web Server Issue):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abelovska L, et al. Comparison of element levels in minimal and complex yeast media. Can J Microbiol. 2007;53(4):533–535. doi: 10.1139/W07-012. [DOI] [PubMed] [Google Scholar]
- 25.Haemig HAH, Moen PJ, Brooker RJ. Evidence that highly conserved residues of transmembrane segment 6 of Escherichia coli MntH are important for transport activity. Biochemistry. 2010;49(22):4662–4671. doi: 10.1021/bi100320y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson RG. Hard and soft acids and bases. J Am Chem Soc. 1963;85(22):3533–3539. [Google Scholar]
- 27.Zheng H, Chruszcz M, Lasota P, Lebioda L, Minor W. Data mining of metal ion environments present in protein structures. J Inorg Biochem. 2008;102(9):1765–1776. doi: 10.1016/j.jinorgbio.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding MM. The architecture of metal coordination groups in proteins. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 5):849–859. doi: 10.1107/S0907444904004081. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi R, et al. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat Commun. 2015;6:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA. 2010;107(43):18381–18385. doi: 10.1073/pnas.1004949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JH, et al. Transport of magnesium by a bacterial Nramp-related gene. PLoS Genet. 2014;10(6):e1004429. doi: 10.1371/journal.pgen.1004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangold S, Potrykus J, Bjorn E, Lovgren L, Dopson M. Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains. Extremophiles. 2013;17(1):75–85. doi: 10.1007/s00792-012-0495-3. [DOI] [PubMed] [Google Scholar]
- 33.Fisher M. The Sea, Volume 2: The Composition of Sea-Water Comparative and Descriptive Oceanography. Harvard Univ Press; Cambridge, MA: 1963. [Google Scholar]
- 34.Park JD, Cherrington NJ, Klaassen CD. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol Sci. 2002;68(2):288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Shu Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci. 2015;16(1):1484–1494. doi: 10.3390/ijms16011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinkle PM, Shanshala ED, 2nd, Nelson EJ. Measurement of intracellular cadmium with fluorescent dyes. Further evidence for the role of calcium channels in cadmium uptake. J Biol Chem. 1992;267(35):25553–25559. [PubMed] [Google Scholar]
- 37.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 38.Mayaan E, Moser A, MacKerell AD, Jr, York DM. CHARMM force field parameters for simulation of reactive intermediates in native and thio-substituted ribozymes. J Comput Chem. 2007;28(2):495–507. doi: 10.1002/jcc.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Lopes PEM, Mackerell AD., Jr Recent developments and applications of the CHARMM force fields. Wiley Interdiscip Rev Comput Mol Sci. 2012;2(1):167–185. doi: 10.1002/wcms.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Autenrieth F, Tajkhorshid E, Baudry J, Luthey-Schulten Z. Classical force field parameters for the heme prosthetic group of cytochrome c. J Comput Chem. 2004;25(13):1613–1622. doi: 10.1002/jcc.20079. [DOI] [PubMed] [Google Scholar]
- 41.Dal Peraro M, et al. Modeling the charge distribution at metal sites in proteins for molecular dynamics simulations. J Struct Biol. 2007;157(3):444–453. doi: 10.1016/j.jsb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Granata D, Camilloni C, Vendruscolo M, Laio A. Characterization of the free-energy landscapes of proteins by NMR-guided metadynamics. Proc Natl Acad Sci USA. 2013;110(17):6817–6822. doi: 10.1073/pnas.1218350110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiorin G, Klein ML, Henin J. Using collective variables to drive molecular dynamics simulations. Mol Phys. 2013;111(22-23):3345–3362. [Google Scholar]
- 44.Martin JE, Waters LS, Storz G, Imlay JA. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet. 2015;11(3):e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J Comput Chem. 2008;29(11):1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 46.Ash WL, Zlomislic MR, Oloo EO, Tieleman DP. Computer simulations of membrane proteins. Biochim Biophys Acta. 2004;1666(1-2):158–189. doi: 10.1016/j.bbamem.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Klauda JB, et al. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J Phys Chem B. 2010;114(23):7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79(2):926–935. [Google Scholar]
- 49.Duarte F, et al. Force field independent metal parameters using a nonbonded dummy model. J Phys Chem B. 2014;118(16):4351–4362. doi: 10.1021/jp501737x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LeBard DN, Martin DR, Lin S, Woodbury NW, Matyushov DV. Protein dynamics to optimize and control bacterial photosynthesis. Chem Sci (Camb) 2013;4(11):4127–4136. [Google Scholar]
- 51.Moin ST, Hofer TS, Sattar R, Ul-Haq Z. Molecular dynamics simulation of mammalian 15S-lipoxygenase with AMBER force field. Eur Biophys J. 2011;40(6):715–726. doi: 10.1007/s00249-011-0684-5. [DOI] [PubMed] [Google Scholar]
- 52.Spiegel K, De Grado WF, Klein ML. Structural and dynamical properties of manganese catalase and the synthetic protein DF1 and their implication for reactivity from classical molecular dynamics calculations. Proteins. 2006;65(2):317–330. doi: 10.1002/prot.21113. [DOI] [PubMed] [Google Scholar]
- 53.Moradi M, Sagui C, Roland C. Investigating rare events with nonequilibrium work measurements. II. Transition and reaction rates. J Chem Phys. 2014;140(3):034115. doi: 10.1063/1.4861056. [DOI] [PubMed] [Google Scholar]