Abstract
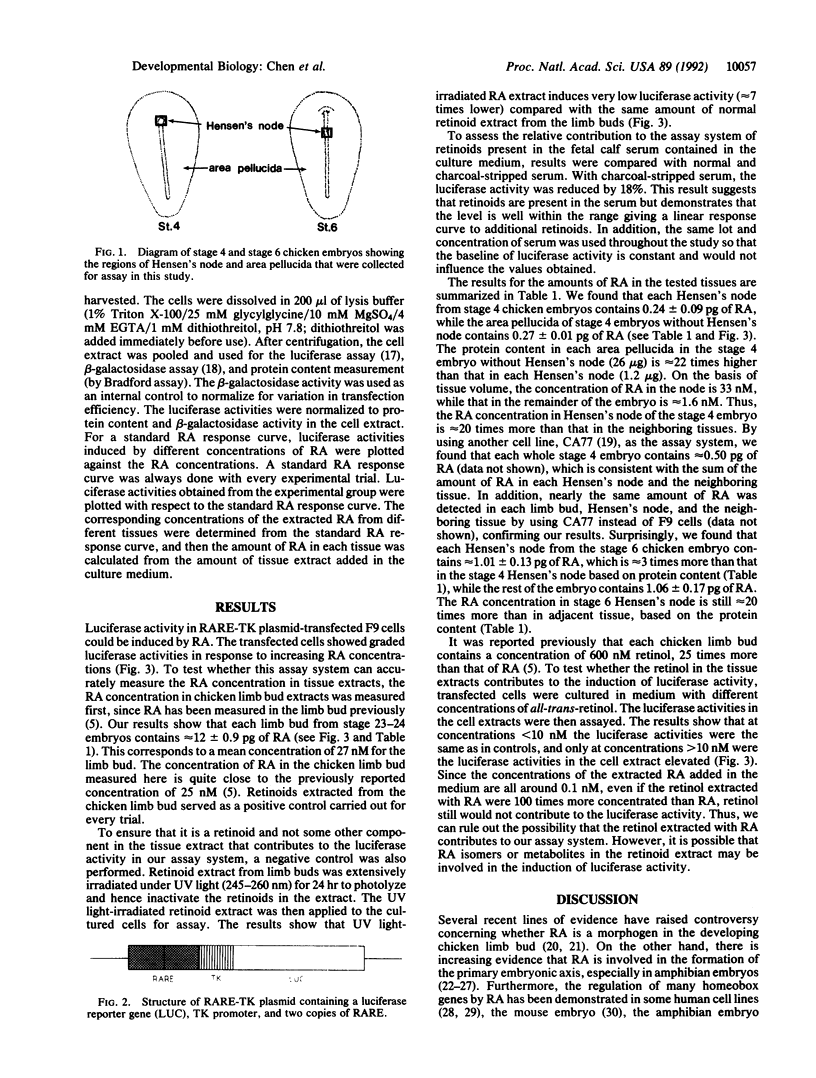
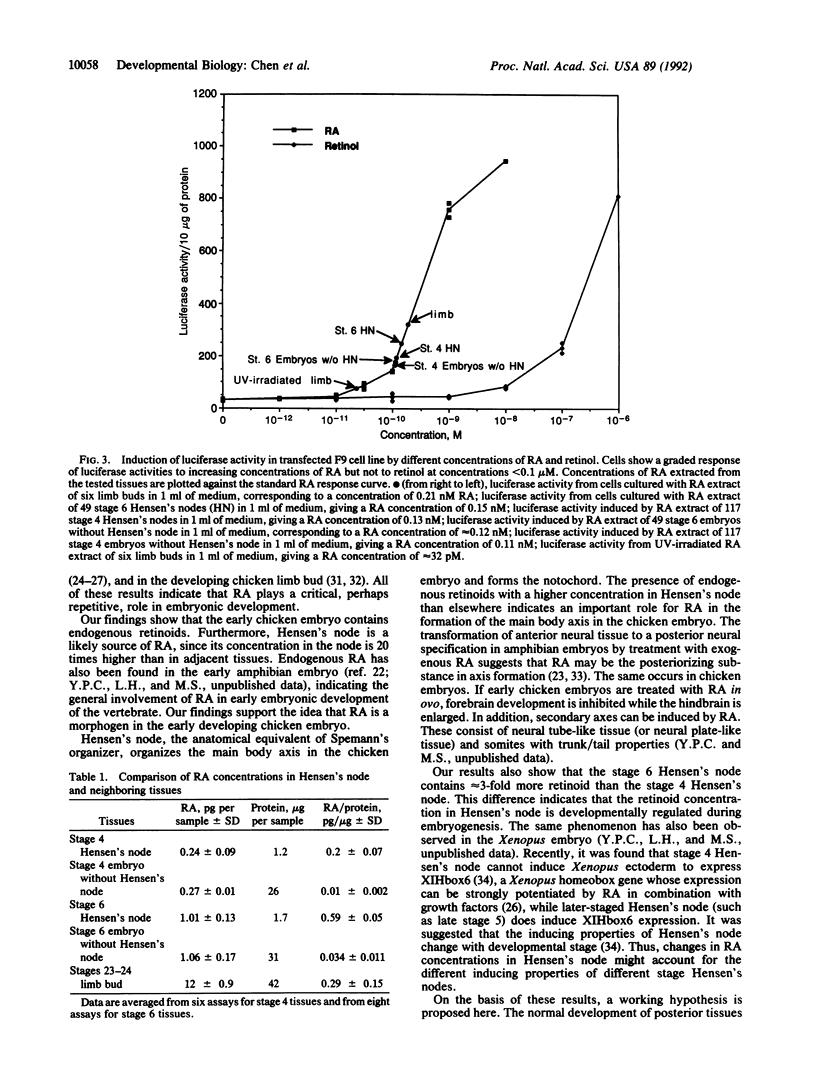
Retinoic acid (RA) has been considered as a potential morphogen in the chicken limb and has also been suggested to be involved in early embryonic development. On the basis of biological activity, previous reports suggest that Hensen's node, the anatomical equivalent in the chicken of the Spemann's organizer, may contain RA. Here, by using a molecular assay system, we demonstrate that Hensen's node contains retinoids in a concentration approximately 20 times more than that in the neighboring tissues. Furthermore, stage 6 Hensen's node contains approximately 3 times more retinoid than that of stage 4 embryos. These endogenous retinoids may establish a concentration gradient from Hensen's node to adjacent tissues and play a role in establishing the primary embryonic axis in the vertebrate. The results also suggest that the retinoid concentration in Hensen's node is developmentally regulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasier A. R., Tate J. E., Habener J. F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989 Nov-Dec;7(10):1116–1122. [PubMed] [Google Scholar]
- Cho K. W., De Robertis E. M. Differential activation of Xenopus homeo box genes by mesoderm-inducing growth factors and retinoic acid. Genes Dev. 1990 Nov;4(11):1910–1916. doi: 10.1101/gad.4.11.1910. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Eichele G., Tickle C., Alberts B. M. Studies on the mechanism of retinoid-induced pattern duplications in the early chick limb bud: temporal and spatial aspects. J Cell Biol. 1985 Nov;101(5 Pt 1):1913–1920. doi: 10.1083/jcb.101.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Green J. B. Retinoic acid: the morphogen of the main body axis? Bioessays. 1990 Sep;12(9):437–439. doi: 10.1002/bies.950120907. [DOI] [PubMed] [Google Scholar]
- Hornbruch A., Wolpert L. Positional signalling by Hensen's node when grafted to the chick limb bud. J Embryol Exp Morphol. 1986 Jun;94:257–265. [PubMed] [Google Scholar]
- Izpisúa-Belmonte J. C., Tickle C., Dollé P., Wolpert L., Duboule D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature. 1991 Apr 18;350(6319):585–589. doi: 10.1038/350585a0. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Dodd J. Hensen's node induces neural tissue in Xenopus ectoderm. Implications for the action of the organizer in neural induction. Development. 1991 Dec;113(4):1495–1505. doi: 10.1242/dev.113.4.1495. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay G. M., Murphy P., Hill R. E., Davidson D. R. Effects of retinoic acid excess on expression of Hox-2.9 and Krox-20 and on morphological segmentation in the hindbrain of mouse embryos. EMBO J. 1991 Oct;10(10):2985–2995. doi: 10.1002/j.1460-2075.1991.tb07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno T., Noji S., Koyama E., Ohyama K., Myokai F., Kuroiwa A., Saito T., Taniguchi S. Involvement of the Chox-4 chicken homeobox genes in determination of anteroposterior axial polarity during limb development. Cell. 1991 Mar 22;64(6):1197–1205. doi: 10.1016/0092-8674(91)90274-3. [DOI] [PubMed] [Google Scholar]
- Noji S., Nohno T., Koyama E., Muto K., Ohyama K., Aoki Y., Tamura K., Ohsugi K., Ide H., Taniguchi S. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature. 1991 Mar 7;350(6313):83–86. doi: 10.1038/350083a0. [DOI] [PubMed] [Google Scholar]
- Roberts C., Platt N., Streit A., Schachner M., Stern C. D. The L5 epitope: an early marker for neural induction in the chick embryo and its involvement in inductive interactions. Development. 1991 Aug;112(4):959–970. doi: 10.1242/dev.112.4.959. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. M. Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development. 1991 Aug;112(4):945–958. doi: 10.1242/dev.112.4.945. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. Retinoic acid modifies mesodermal patterning in early Xenopus embryos. Genes Dev. 1991 Feb;5(2):175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Axial patterning and the establishment of polarity in the frog embryo. Trends Genet. 1990 Feb;6(2):57–64. doi: 10.1016/0168-9525(90)90075-h. [DOI] [PubMed] [Google Scholar]
- Russo A. F., Lanigan T. M., Sullivan B. E. Neuronal properties of a thyroid C-cell line: partial repression by dexamethasone and retinoic acid. Mol Endocrinol. 1992 Feb;6(2):207–218. doi: 10.1210/mend.6.2.1569964. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Cheng P. F. Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 1991 Aug;5(8):1321–1332. doi: 10.1101/gad.5.8.1321. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Embryology: we have a morphogen! Nature. 1987 Jun 18;327(6123):553–554. doi: 10.1038/327553a0. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Pang K., Sundin O., Wedden S. E., Thaller C., Eichele G. Molecular approaches to vertebrate limb morphogenesis. Development. 1989;107 (Suppl):121–131. doi: 10.1242/dev.107.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Stornaiuolo A., Acampora D., Pannese M., D'Esposito M., Morelli F., Migliaccio E., Rambaldi M., Faiella A., Nigro V., Simeone A. Human HOX genes are differentially activated by retinoic acid in embryonal carcinoma cells according to their position within the four loci. Cell Differ Dev. 1990 Aug;31(2):119–127. doi: 10.1016/0922-3371(90)90015-o. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Summerbell D. The effect of local application of retinoic acid to the anterior margin of the developing chick limb. J Embryol Exp Morphol. 1983 Dec;78:269–289. [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Lee J., Eichele G. A quantitative analysis of the effect of all-trans-retinoic acid on the pattern of chick wing development. Dev Biol. 1985 May;109(1):82–95. doi: 10.1016/0012-1606(85)90348-3. [DOI] [PubMed] [Google Scholar]
- Wanek N., Gardiner D. M., Muneoka K., Bryant S. V. Conversion by retinoic acid of anterior cells into ZPA cells in the chick wing bud. Nature. 1991 Mar 7;350(6313):81–83. doi: 10.1038/350081a0. [DOI] [PubMed] [Google Scholar]




