Abstract
Background: the prevalence of sarcopenia increases with age. Physical activity might slow the rate of muscle loss and therewith the incidence of sarcopenia.
Objective: to examine the association of physical activity with incident sarcopenia over a 5-year period.
Design: data from the population-based Age, Gene/Environment, Susceptibility–Reykjavik Study were used.
Setting: people residing in the Reykjavik area at the start of the study.
Subjects: the study included people aged 66–93 years (n = 2309).
Methods: the amount of moderate–vigorous physical activity (MVPA) was assessed by a self-reported questionnaire. Sarcopenia was identified using the European Working Group on Sarcopenia in Older People algorithm, including muscle mass (computed tomography imaging), grip strength (computerised dynamometer) and gait speed (6 m).
Results: mean age of the participants was 74.9 ± 4.7 years. The prevalence of sarcopenia was 7.3% at baseline and 16.8% at follow-up. The incidence proportion of sarcopenia over 5 years was 14.8% in the least-active individuals and 9.0% in the most-active individuals. Compared with the least-active participants, those reporting a moderate–high amount of MVPA had a significantly lower likelihood of incident sarcopenia (OR = 0.64, 95% CI 0.45–0.91). Participants with a high amount of MVPA had higher baseline levels of muscle mass, strength and walking speed, but baseline MVPA was not associated with the rate of muscle loss.
Conclusion: a higher amount of MVPA seems to contribute to counteracting the development of sarcopenia. To delay the onset of sarcopenia and its potential adverse outcomes, attention should be paid to increasing physical activity levels in older adults.
Keywords: older people, sarcopenia, incidence proportion, physical activity, EWGSOP
Introduction
Sarcopenia, defined as the loss of muscle mass and function, affects quality of life and increases the risk of physical limitations and disability in older adults [1, 2]. Depending on the definition used, the prevalence of sarcopenia in community-dwelling older adults ranges from 1 to 50%, with higher prevalence rates in older age groups [3, 4]. Although the loss of muscle mass appears to be an inevitable part of the aging process, the rate of muscle loss is modifiable [5]. For instance, resistance training interventions have shown to be effective in reversing losses of skeletal muscle mass and function [6]. Aiming to delay the onset of disability and progression of chronic diseases and to gain other health benefits, current recommendations for physical activity are set at 150 min per week for moderate-intensity aerobic activity and 2 or more days per week for muscle-strengthening activities [7, 8]. Moderate-intensity activity noticeably accelerates the heart rate and includes activities like brisk walking or dancing [8]. In industrialised countries, physical activity levels in older adults are low, with 40–60% of the older adults not meeting the recommendations for physical activity [8, 9]. Although exercise has been proved to be effective in reversing losses of muscle mass [6, 10], studies investigating the effect of general physical activity on the prevention of sarcopenia show inconsistent results [11]. For example, Ryu et al., using data from a cross-sectional Korea National Health and Nutrition Examination Survey, report that moderate–vigorous physical activity (MVPA) is associated with a reduced risk of sarcopenia [12]. Also, Foong et al. [13] found that a higher level of (accelerometer-determined) MVPA was associated with greater lean mass percentage and lower limb strength. On the contrary, Volpato et al. [14] did not find an association between light/moderate/vigorous physical activity and sarcopenia. Raguso et al. [15] performed a 3-year longitudinal study and found that leisure time physical activity (light/moderate/vigorous) did not seem to prevent the loss of muscle mass.
In addition to the issue of inconsistent findings of the effect of general physical activity on sarcopenia, only a few studies have examined the incidence proportion of sarcopenia [16–18], of which only one study looked at sarcopenia incidence in relation to physical activity [18]. All these studies used dual-energy X-ray absorptiometry (DXA) or bio-electrical impedance analysis to assess muscle mass; no studies were found estimating muscle mass by computed tomography (CT). The aim of this study, then, was to examine the association of physical activity with the incidence of sarcopenia over a 5-year period in a large population-based cohort study of older adults, the Age, Gene/Environment, Susceptibility (AGES)–Reykjavik study [19]. To identify people with sarcopenia, the algorithm of the European Working Group on Sarcopenia in Older People (EWGSOP) was used [20]. This algorithm includes measurements of muscle mass (CT of the mid-thigh), isometric muscle strength of the hand (computerised dynamometer) and gait speed (6 m walk).
Methods
Design and study population
This paper describes a secondary data analysis using data of the AGES–Reykjavik study [19]. AGES–Reykjavik is a population-based study undertaken in survivors of the Reykjavik study [19, 21, 22]. The Reykjavik Study, established in 1967 and followed by the Icelandic Heart Association, aimed to prospectively study cardiovascular disease in people born between 1907 and 1935 and residing in Reykjavik [19, 21, 22]. Between 2002 and 2006, the AGES–Reykjavik study re-examined 5764 survivors of the original cohort who had participated in the Reykjavik study (T1). The second examination (T2) took place between 2007 and 2011 (n = 3316). All participants signed informed consent. The National Bioethics Committee in Iceland and the National Institute on Aging Intramural Institutional Review Board in Bethesda, USA, approved the study (approval number VSN-00-063).
Measurements
The baseline examination consisted of three clinic visits within 4–6 weeks [19]. It included, among others, vascular, neurocognitive and musculoskeletal components and questionnaires on physical, psychological and social health. An overview of all examinations included in the AGES–Reykjavik study has been previously published [19]. For this paper, relevant measurements are described below. The included measurements were performed at both baseline (T1) and follow-up (T2).
Identification of sarcopenia
Sarcopenia was identified using the algorithm of the EWGSOP [20]. According to this algorithm, sarcopenia is present in persons with low muscle mass in combination with poor muscle strength and/or performance. Muscle mass was assessed by CT imaging, using a four-detector CT system (Sensation, Siemens Medical Systems, Erlangen, Germany) [23]. Average thigh total muscle cross-sectional area (cm2) was obtained from a single axial 10-mm-thick section in both legs [24]. To our knowledge, this is the first study to apply the EWGSOP definition using a CT image-based measure for muscle mass [3]. The EWGSOP does not provide CT cut-points for low muscle mass; therefore, the lowest gender-specific 20th percentile of the thigh total muscle cross-sectional area (<83.2 cm2 in females, <116.5 cm2 in males) was used in the main analyses and the lowest gender-specific 10th percentile (<78.2 cm2 in females, <108.2 cm2 in males) in the sensitivity analyses. The 20th percentile method has been used before in sarcopenia research using DXA [25, 26]. Maximum grip strength of the dominant hand was measured by a computerised dynamometer affixed to an adjustable special chair (Good Strength software, Metitur, Finland), with the elbow flexed at 90° and armrests adjusted for height so that the shoulders were relaxed [27]. Participants performed three trials, each lasting 4–5 s, and after each exam they rested for half a minute. Participants were provided with standardised verbal encouragement throughout the testing protocol. EWGSOP cut-points for poor grip strength are <20 kg (women) and <30 kg (men) [20]. Usual walking speed (m/s) was assessed over a 6 m track [28]. The EWGSOP cut-point for slow gait speed is ≤0.8 m/s [20].
Physical activity assessment
Physical activity was assessed by a self-reported questionnaire. Participants were asked, among others, how many hours per week they participated in moderate–vigorous intensity physical activity (MVPA) in the past 12 months (one question). Provided examples of MVPA were badminton, golf, biking, swimming, heavy gardening, weight lifting, hiking/mountain climbing, fast walking/heavy housework, rowing, aerobics, jogging and running. Predefined answer categories were never, rarely, occasionally (weekly but <1 h), moderate (1–3 h per week) and high (>4 h per week). In the final analyses, the MVPA categories were combined into 1. Never, 2. Rarely–occasionally and 3. Moderate–high.
Covariates
Age, sex, education (primary, secondary, college, university), marital status (married/living together, widow/widower, divorced, single), smoking status (never, previous, current) and >5 kg weight loss in the past 12 months were assessed by a questionnaire. BMI was calculated by dividing body weight in kilograms by height in metres squared. The total number of comorbidities was obtained by self-report, medication assessment and clinical assessment, and included cancer, chronic lung disease, asthma, dementia, diabetes, heart attack, congestive heart failure, hypertension, rheumatic disorder and stroke. Depressive symptoms were assessed by the validated 15-item Geriatric Depression Scale (GDS) [29, 30]. The total score of the GDS ranges from 0 (no depressive symptoms) to 15 (high number of depressive symptoms), with 6 or more depressive symptoms as a cut-point for depression [30]. Cognitive function was assessed by the Mini-Mental State Examination, with scores ranging from 0 to 30, where higher scores indicate better cognitive function [31].
Statistical analysis
To compare baseline characteristics of people with and without sarcopenia, χ2 tests (categorical variables) and t-tests (continuous variables) were used. One-way ANOVA was performed to compare baseline characteristics of participants according to MVPA category. Multinomial regression was used to examine differences in the amount of MVPA between participants with and without sarcopenia at T1. Model 1 was adjusted for age, sex, education and marital status. Model 2 further included BMI, smoking status, total number of comorbidities, depressive symptoms, weight loss and cognitive function.
To assess the association between baseline physical activity and incidence of sarcopenia, logistic regression was used. For this analysis, only people without sarcopenia at baseline were included (n = 2140). As above, Model 1 was adjusted for age, sex, education and marital status. Model 2 additionally included BMI, smoking status, total number of comorbidities, depressive symptoms, weight loss and cognitive function. As the EWGSOP did not provide cut-points for low muscle mass assessed by CT, the 20th percentile was chosen for the main analyses as this cut-point has been used in previous studies [25, 26] and the 10th percentile for the sensitivity analyses.
The association between baseline physical activity and the individual sarcopenia parameters (muscle mass, grip strength and walking speed) was explored by multivariate linear regression analyses, using the covariates of Models 1 and 2, as described above. First, the association between MVPA and muscle mass, grip strength and walking speed at baseline was studied (cross-sectional analysis). Second, the association between baseline MVPA and the loss of muscle mass, grip strength and walking speed over 5-year time was examined.
Results
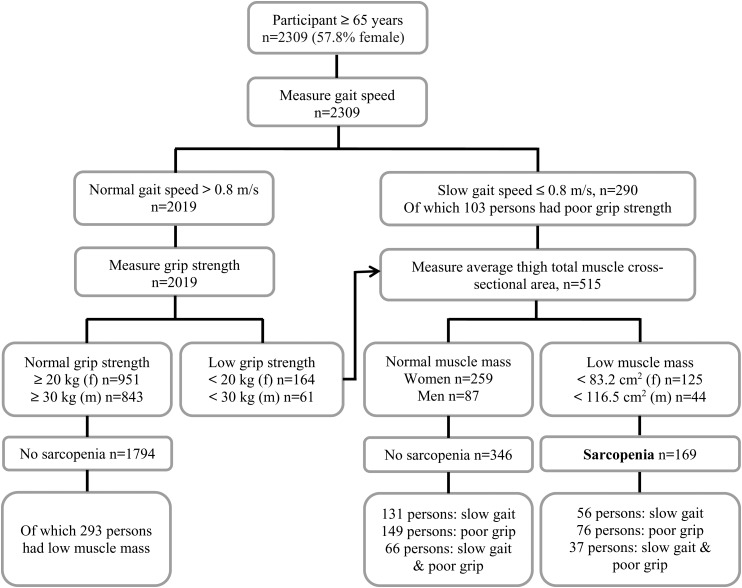
Between baseline (n = 5764) and follow-up (mean follow-up 5.2 ± 0.3 years, range 4.2–8.2 years), 1039 participants died and 1409 were lost to follow-up or refused to participate, leaving a total sample at follow-up of 3316 participants (see the Supplementary data, Figure in Appendix 1, available in Age and Ageing online). Of these 3316 participants, 1007 were excluded due to missing data on muscle parameters (n = 670), physical activity (n = 66) or baseline covariates (n = 271), leaving a total analytical sample of 2309 participants. Characteristics of participants who participated at baseline only and characteristics of participants excluded because of missing data are shown in Supplementary data, Table S1, available in Age and Ageing online. Participants who dropped out between T1 and T2 were at baseline significantly older, had a lower BMI, more comorbidities, a lower educational level, were more often living alone, were less active and were more often sarcopenic compared with participants who did not drop out. Characteristics of the 2309 included participants are shown in Table 1. The mean age of the participants was 74.9 years at baseline, and the majority were female (57.8%), which reflects the gender distribution of this age group in the general population. The prevalence of sarcopenia was 7.3% (n = 169) at baseline (see Figure 1) and 16.8% (n = 389) at follow-up. At baseline, significant differences between sarcopenic and non-sarcopenic older adults were found for all characteristics, except for smoking status and weight loss. At baseline, 38.5% of the participants did not engage in MVPA, which was 47.7% at follow-up.
Table 1.
Characteristics of the study population at baseline (T1) and follow-up (T2)
| Variable | T1 |
T2 |
||||
|---|---|---|---|---|---|---|
| Total (n = 2309) | No sarcopenia (n = 2140) | Sarcopenia (n = 169) | Total (n = 2309) | No sarcopenia (n = 1920) | Sarcopenia (n = 389) | |
| Age, mean year (SD) | 74.7 (4.7)* | 74.3 (4.5) | 79.1 (5.1) | 79.9 (4.7)* | 79.1 (4.3) | 83.7 (4.8) |
| Women, n (%) | 1335 (57.8)* | 1210 (56.5) | 125 (74.0) | 1335 (57.8) | 1094 (57.0) | 241 (62.0) |
| Education, n (%) | * | |||||
| Primary | 464 (20.1) | 417 (19.5) | 47 (27.8) | 464 (20.1) | 37 (19.3) | 93 (23.9) |
| Secondary | 1187 (51.4) | 1107 (51.7) | 80 (47.3) | 1187 (51.4) | 998 (52.0) | 189 (48.6) |
| College/university | 658 (28.5) | 616 (28.8) | 42 (24.9) | 658 (28.5) | 551 (28.7) | 107 (27.5) |
| Marital status, n (%) | * | * | ||||
| Married/living together | 1509 (65.4) | 1435 (67.1) | 74 (43.8) | 1298 (56.2) | 1140 (59.4) | 158 (40.6) |
| Widow or widower | 551 (23.9) | 475 (22.2) | 76 (45.0) | 757 (32.8) | 574 (9.9) | 183 (47.0) |
| Divorced | 126 (5.5) | 118 (5.5) | 8 (4.7) | 118 (5.1) | 101 (5.3) | 17 (4.4) |
| Single | 123 (5.3) | 112 (5.2) | 11 (6.5) | 136 (5.9) | 105 (5.5) | 31 (8.0) |
| BMI, mean kg/m2 (SD) | 27.2 (4.1)* | 27.4 (4.0) | 24.4 (3.7) | 26.8 (4.3)* | 27.3 (4.1) | 24.1 (3.7) |
| Weight loss > 5 kg, n (%) | 235 (10.2) | 219 (10.2) | 16 (9.5) | 315 (13.6)* | 287 (13.4) | 28 (16.6) |
| Smoking status, n (%) | ||||||
| Never | 992 (43.0) | 906 (42.3) | 86 (50.9) | 959 (41.5)# | 789 (41.1) | 170 (43.7) |
| Previous | 1069 (46.3) | 1004 (46.9) | 65 (38.5) | 1138 (49.3) | 955 (49.7) | 183 (47.0) |
| Current | 248 (10.7) | 230 (10.7) | 18 (10.7) | 180 (7.8) | 148 (7.7) | 32 (8.2) |
| Comorbidities, mean (SD) | 1.9 (1.2)* | 1.8 (1.2) | 2.1 (1.1) | 2.3 (1.2)* | 2.2 (1.2) | 2.5 (1.2) |
| Cognitive function, mean MMSE score (SD) | 27.4 (2.2)* | 27.4 (2.2) | 26.6 (2.7) | 26.1 (3.7)* | 26.3 (3.5) | 24.8 (4.4) |
| Depression, n (%) | 103 (4.5)* | 89 (4.2) | 14 (8.3) | 141 (6.1)* | 102 (5.3) | 39 (10.0) |
| Thigh muscle mass, mean cm2 (SD) | ||||||
| Men (n = 974) | 132.5 (19.0)* | 133.9 (18.2) | 104.0 (12.3) | 125.0 (20.2)* | 129.5 (18.0) | 99.9 (11.4) |
| Women (n = 1335) | 95.9 (14.5)* | 98.0 (13.4) | 75.6 (5.9) | 91.2 (14.5)* | 95.0 (12.8) | 73.9 (7.0) |
| Grip strength, mean kg (SD) | ||||||
| Men (n = 974) | 41.9 (8.6)* | 42.5 (8.3) | 30.4 (7.5) | 37.6 (9.4)* | 39.4 (8.7) | 27.7 (6.9) |
| Women (n = 1335) | 24.9 (5.7)* | 25.5 (5.5) | 18.9 (4.5) | 22.5 (6.2)* | 23.6 (5.9) | 17.2 (4.9) |
| 6 m walk, mean m/s (SD) | 1.0 (0.2)* | 1.0 (0.2) | 0.8 (0.2) | 0.9 (0.2)* | 1.0 (0.2) | 0.8 (0.2) |
| Current amount of MVPA, n (%) | * | * | ||||
| Never | 890 (38.5) | 799 (37.3) | 91 (53.8) | 1101 (47.7) | 834 (43.4) | 267 (68.1) |
| Rarely–occasionally | 570 (24.7) | 527 (24.6) | 43 (25.4) | 238 (10.3) | 206 (10.7) | 32 (8.2) |
| Moderate–high | 849 (36.8) | 814 (38.0) | 35 (20.7) | 970 (42.0) | 880 (45.8) | 90 (23.1) |
BMI, body mass index; MMSE, mini-mental state examination; MVPA, moderate–vigorous physical activity. Depression score is based on GDS score, ranging from 0 (no depressive symptoms) to 15 (high number of depressive symptoms).
*Significant difference (P < 0.05) between sarcopenic and non-sarcopenic older adults.
#Depression at follow-up n = 2249 (60 missings); smoking status at follow-up, n = 2277 (32 missings).
Figure 1.
Identification of sarcopenia at baseline using the EWGSOP algorithm [20].
Baseline characteristics of participants according to MVPA category are shown in Supplementary data, Table S2, available in Age and Ageing online. A significant difference (P < 0.05) between MVPA categories was found for all variables, except for weight loss.
Physical activity in people with and without sarcopenia
Multinomial regression indicated that sarcopenic older adults engaged in significantly less MVPA (not tabulated). People with sarcopenia at baseline had a lower likelihood (OR = 0.49, 95% CI 0.32–0.76) of having a moderate–high amount of MVPA compared with participants without sarcopenia. People with sarcopenia at baseline also tended to have a lower likelihood (OR = 0.89, 95% CI 0.59–1.34) of engaging rarely–occasionally in MVPA compared with participants without sarcopenia, but this was not statistically significant.
Incidence of sarcopenia
As shown in Table 2, the incidence proportion of sarcopenia in participants who never engaged in MVPA, rarely–occasionally engaged in MVPA and participants with a moderate–high amount of MVPA was 14.8% (118 out of 799), 10.4% (55 out of 527) and 9.0% (74 out of 814), respectively. Participants who reported a moderate–high amount of MVPA at baseline had a significantly decreased likelihood of incident sarcopenia compared with those who reported never to participate in MVPA (OR = 0.68, 95% CI 0.49–0.94; see Table 2, Model 1). Participants who reported to rarely–occasionally perform MVPA also tended to have a lower likelihood (OR = 0.79, 95% CI 0.54–1.14) of incident sarcopenia compared with those who reported never to participate in MVPA, but this was not statistically significant. Additionally, in Model 1, older age was significantly associated with the incidence of sarcopenia. In Model 2, next to older age, lower BMI and worse cognitive function were significantly associated with the incidence of sarcopenia.
Table 2.
Association of physical activity with the sarcopenia incidence proportion over a 5-year period
| Sarcopenia incidence (%) | Unadjusted model OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | |
|---|---|---|---|---|
| Amount of MVPA at baseline | ||||
| Never (n = 799) | 14.8 | Ref | Ref | Ref |
| Rarely–occasionally (n = 527) | 10.4 | 0.67 (0.48–0.95) | 0.78 (0.54–1.12) | 0.79 (0.54–1.14) |
| Moderate–high (n = 814) | 9.0 | 0.58 (0.42–0.79) | 0.68 (0.49–0.94) | 0.64 (0.45–0.91) |
Ref, reference group. Model 1 is adjusted for age, sex, education and marital status. Model 2 further included BMI, smoking status, total number of comorbidities, depressive symptoms, weight loss and cognitive function.
Sensitivity analyses using the lowest gender-specific 10th percentile for muscle mass showed the same trend, a significant difference was found in the incidence of sarcopenia between participants who never participated in MVPA compared with participants with a moderate–high amount of MVPA (OR = 0.64, 95% CI 0.45–0.90; not tabulated).
Physical activity and loss of muscle mass, grip strength and walking speed
Multivariate linear regression analyses (Models 1 and 2) showed an association between baseline MVPA and muscle mass (both sexes), grip strength (in women only) and walking speed (both sexes). Between baseline and follow-up, participants lost on average 5.9 cm2 (5.2%) muscle mass, 3.2 kg (8.7%) grip strength and 0.07 m/s (6.4%) walking speed. Baseline MVPA was not associated with the rate of loss of muscle mass, grip strength or walking speed (Supplementary data, Table S3, available in Age and Ageing online).
Discussion
This study showed that older adults with sarcopenia engaged significantly less in MVPA than their non-sarcopenic peers. Furthermore, the incidence proportion of sarcopenia was significantly lower in the highly active participants, compared with the least-active participants. Baseline MVPA was not associated with the rate of loss of muscle mass, grip strength or walking speed.
The incidence proportion found in this study (9.0–14.8%) is roughly comparable with the incidence proportion found in two other recent studies performed in community-dwelling older adults [16, 18]. Both studies used the EWGSOP algorithm to define sarcopenia, though slightly different cut-points were applied [16, 18]. Kim et al. [16] found a 4-year sarcopenia incidence proportion of 15.8% in a community-dwelling population of women aged 75 years and older. Yu et al. [18] reported a 4-year incidence proportion of sarcopenia of 7.8% in a population of 65 years and older, recruited in three Australian cohort studies. Yu et al. [18] also found that lower physical activity levels (assessed by the Physical Activity Scale for the Elderly; no distinction in PA intensities reported) were associated with a higher incidence of sarcopenia, though physical activity was not associated with reversibility of sarcopenia. However, in an intervention study by Liu et al. [32], in which sarcopenia was defined as low appendicular lean muscle mass, it was found that a physical activity intervention (including strength, balance and flexibility exercises) improved physical performance in both sarcopenic and non-sarcopenic older adults. Foong et al. [13] found that a higher level of (accelerometer-determined) MVPA was associated with greater lean mass percentage and lower limb strength. Murphy et al. [33] showed that in the Health ABC study people with more physical activity (assessed as kilocalorie spent per week walking or exercising in the prior week) were less likely to transition to sarcopenia. A longitudinal study using objectively measured physical activity data, by accelerometer, showed that a greater habitual physical activity (assessed as the number of steps taken per day and the daily duration of moderate–vigorous intensity exercise) decreased the risk of developing sarcopenia [34]. Our results support these findings [13, 33, 34] but additionally show that the decreased risk of developing sarcopenia in people with a moderate–high amount of MVPA may be explained by their higher baseline values of muscle mass, strength and walking speed, and not because MVPA delays the rate of muscle loss. In contrast, in a cross-sectional study by Volpato et al. [14], no association was found between light/moderate/vigorous physical activity and sarcopenia. This may be explained by the method used to assess muscle mass, i.e. bioelectrical impedance, which might have led to an overestimation of muscle mass [14].
Our study supports the idea that physical activity delays the onset of sarcopenia. In 2011, Pillard et al. [35] discussed the idea of prescribing physical activity as a countermeasure for sarcopenia. The paper describes several steps that a medical practitioner can take to encourage physical activity as a medicine, including how to define the physical activity dose [35]. Both Shephard et al. [34] and the European Society for Clinical Nutrition and Metabolism (ESPEN) expert group [36] recommend daily physical activity for older adults; 15–20 min of at least a moderate intensity. The expert group also advises combining physical activity with a diet including 1.0–1.2 g protein/kg body weight/day [36]. These are first steps in sarcopenia prevention and control.
Some limitations should be addressed. The prevalence of sarcopenia found in this study is comparable with other studies using the EWGSOP definition in community-dwelling and long-term care populations [3]. However, the ‘real’ baseline prevalence of sarcopenia (12.2%, n = 4833) was higher than in the analytical sample because sarcopenic participants were more likely to become lost-to-follow-up. Also, the people who dropped out between the first (T1) and second (T2) examinations had on average more comorbidities and more than half never performed MVPA (differential attrition). Since these factors are both likely to increase the risk of developing sarcopenia, the actual incidence of sarcopenia is likely to be higher than shown in this study, and the association between MVPA and the development of sarcopenia might have been stronger when attrition would have not occurred. CT imaging is seen as one of the gold standards to assess muscle mass; however, no official cut-points for low muscle mass were available, and using other cut-points might affect the outcome [3, 37]. Physical activity was assessed by self-report. This might have led to an overestimation of physical activity levels [38]. Objective measurement of physical activity could improve the reliability of physical activity data. However, we do believe that self-report gives a fair indication of whether a person is not active at all or highly active. During the 5-year follow-up, no interim evaluation of physical activity and other measures was performed. It could be possible that events (such as the development of disease or hospitalisation) that occurred within these 5 years have confounded the relationship between incident sarcopenia and physical activity. Although physical activity and exercise are often used interchangeably, in theory they are different concepts [7]. For this study, one single question was included with regard to physical activity, including both general physical activity and exercise; data on reliability and validity of the physical activity questionnaire are unknown. Furthermore, no conclusions can be drawn with regard to the type of physical activity or exercise that contributed mostly to the incidence of sarcopenia. Future studies using objective data that also take into account the intensity of activity are needed.
To conclude, a moderate–high amount (>1 h per week) of MVPA was shown to delay the onset of sarcopenia but did not affect the rate of loss of muscle mass and function. Attention should be paid to increasing physical activity levels in older adults since this might decrease the incidence of sarcopenia and therefore might prevent the onset of poor health outcomes.
Key points.
This study aimed to examine the association of physical activity with incident sarcopenia over a 5-year period.
The incidence proportion of sarcopenia over the 5 years was 9.0–14.8% in, respectively, the most- and least-active subjects.
Baseline physical activity was not associated with the rate of loss of muscle mass, grip strength or walking speed.
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
Conflicts of interest
None declared.
Funding
This work was supported by National Institutes of Health, National Institute on Aging (N01-AG-1-2100), the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association) and the Althingi (the Icelandic Parliament).
Supplementary Material
Acknowledgements
The authors would like to thank all the participants of the AGES–Reykjavik study. We would specially like to thank Melissa E. Garcia for her support with the data handling.
References
Only the most important references are listed here and are represented by bold type throughout the text. The full list of references is available as supplementary data at Age and Ageing online.
- 1.Hairi NN, Cumming RG, Naganathan V et al. . Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc 2010; 58: 2055–62. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli R, Reginster JY, Arnal JF et al. . Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013; 93: 101–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Landi F, Schneider SM et al. . Prevalence of and interventions for sarcopenia in ageing adults: a systematic review: report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014; 43: 748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fielding RA, Vellas B, Evans WJ et al. . Sarcopenia: an undiagnosed condition in older adults: current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Fernández N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013; 49: 131–43. [PubMed] [Google Scholar]
- 6.Landi F, Marzetti E, Martone AM et al. . Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care 2014; 17: 25–31. [DOI] [PubMed] [Google Scholar]
- 7.Chodzko-Zajko W, Proctor DN, Fiatarone Singh MA et al. . Exercise and physical activity for older adults. Med Sci Sports Exercise 2009; 41: 1510–30. [DOI] [PubMed] [Google Scholar]
- 8.Taylor D. Physical activity is medicine for older adults. Postgrad Med J 2014; 90: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallal PC, Andersen LB, Bull FC et al. . Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012; 380: 247–57. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Suzuki T, Saito K, Kojima N, Hosoi E, Yoshida H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: a 4-year follow-up study. Geriatr Gerontol Int. 2015; 16: 175–81. [DOI] [PubMed] [Google Scholar]
- 11.Scott D, Blizzard L, Fell J et al. . The epidemiology of sarcopenia in community living older adults: what role does lifestyle play. J Cachexia Sarcopenia Muscle 2011; 2: 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu M, Jo J, Lee Y et al. . Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013; 42: 734–40. [DOI] [PubMed] [Google Scholar]
- 13.Foong YC, Chherawala N, Aitken D, Scott D, Winzenberg T, Jones G. Accelerometer-determined physical activity, muscle mass, and leg strength in community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016; doi:10.1002/jcsm.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpato S, Bianchi L, Cherubini A et al. . Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci 2014; 69: 438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raguso CA, Kyle U, Kossovsky MP et al. . A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr 2006; 25: 573–80. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Suzuki T, Kim M et al. . Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc 2015; 16: 85 e81–88. [DOI] [PubMed] [Google Scholar]
- 17.Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G. Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int 2014; 25: 187–93. [DOI] [PubMed] [Google Scholar]
- 18.Yu R, Wong M, Leung J et al. . Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr Gerontol Int 2014; 14: 15–28. [DOI] [PubMed] [Google Scholar]
- 19.Harris TB, Launer LJ, Eiriksdottir G et al. . Age, gene/environment susceptibility: Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu S, Appleton S, Adams R et al. . The impact of low muscle mass definition on the prevalence of sarcopenia in older Australians. Biomed Res Int 2014; doi:10.1155/2014/361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagotto V, Silveira EA. Methods, diagnostic criteria, cutoff points, and prevalence of sarcopenia among older people. ScientificWorldJournal 2014; doi:10.1155/2014/231312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Era P, Rantanen T, Avlund K et al. . Maximal isometric muscle strength and anthropometry in 75-year-old men and women in three Nordic localities. Scand J Med Sci Sports 1994; 4: 26–31. [Google Scholar]
- 28.Ostir GV, Markides KS, Black SA et al. . Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci 1998; 53: M491–5. [DOI] [PubMed] [Google Scholar]
- 32.Liu CK, Leng X, Hsu FC et al. . The impact of sarcopenia on a physical activity intervention: the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P). J Nutr Health Aging 2014; 18: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy RA, Ip EH, Zhang Q et al. . Transition to sarcopenia and determinants of transitions in older adults: a population-based study. J Gerontol A Biol Sci Med Sci 2014; 69: 751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shephard RJ, Park H, Park S et al. . Objectively measured physical activity and progressive loss of lean tissue in older Japanese adults: longitudinal data from the Nakanojo study. J Am Geriatr Soc 2013; 61: 1887–93. [DOI] [PubMed] [Google Scholar]
- 35.Pillard F, Laoudj-Chenivesse D, Carnac G et al. . Physical activity and sarcopenia. Clin Geriatr Med 2011; 27: 449–70. [DOI] [PubMed] [Google Scholar]
- 36.Deutz NE, Bauer JM, Barazzoni R et al. . Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014; 33: 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakicic JM, King WC, Gibbs BB et al. . Objective versus self-reported physical activity in overweight and obese young adults. J Phys Act Health 2015; 12: 1394–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.