Abstract
Objective
to examine whether psychomotor speed predicts individual and combined disorders in cognition, mobility and mood and if white matter hyperintensities explain these associations.
Design and setting
longitudinal; Cardiovascular Health Study.
Subjects
5,888 participants (57.6% women, 15.7% black, 75.1 (5.5), mean years (SD)).
Methods
psychomotor speed (Digit Symbol Substitution Test (DSST)) and small vessel disease (white matter hyperintensities (WMH)) were measured in 1992–94. Global cognition (Modified Mini-Mental State (3MS) examination), mobility (gait speed (GS)) and mood (Center for Epidemiologic Studies Depression (CES-D) scale) were measured annually over 5 years and classified as clinical, subclinical or no disorders based on established values (3MS: 80 and 85 points; GS: 0.6 and 1.0 m/s; CES-D: 10 and 5 points). Analyses were adjusted for demographics, baseline status, education, diabetes, hypertension, ankle–arm index.
Results
among those with no disorder in cognition, mobility and mood (N = 619) in 1992–94, being in the lowest DSST quartile compared to the highest was associated with nearly twice the odds of developing 1+ clinical or subclinical disorders (N = 413) during follow-up. Associations were stronger for incident clinical disorders in cognition (OR: 8.44, p < 0.01) or mobility (OR: 9.09, p < 0.05) than for mood (OR: 1.88, p < 0.10). Results were similar after adjustment for WMH.
Conclusions
slower psychomotor speed may serve as a biomarker of risk of clinical disorders of cognition, mobility and mood. While in part attributable to vascular brain disease, other potentially modifiable contributors may be present. Further studying the causes of psychomotor slowing with ageing might provide novel insights into age-related brain disorders.
Keywords: older people processing speed, cognition, mobility, mood
Introduction
Clinical disorders of cognition, mobility and mood are common in later life and are major causes of disability and high healthcare costs [1–3]. Even subclinical disorders in these domains are associated with increased risk for progression to clinical states, impaired independence and future incident disability [2, 4, 5]. Although frequently diagnosed, treated and studied separately, these disorders co-exist more frequently than expected by chance alone [6–8]. This co-occurrence implies a potential for a shared aetiology.
There is consistent evidence that disorders in these domains are related to vascular risk factors, cerebral small vessel disease and to psychomotor slowing [9]. Psychomotor speed reflects overall efficiency of operations [10] and rapidly declines with older age [11], and it is associated with small vessel disease [9, 12]. Psychomotor slowing has been shown to precede and longitudinally predict faster rates of gait speed decline in some studies but not others [13–15], and to anticipate the onset of mild cognitive impairment and dementia [16–18]. We found no studies reporting psychomotor slowing prior to the onset of mood disorders, although older adults with depression are known to have psychomotor slowing [19].
Given the cross-sectional evidence for the association of psychomotor slowing with disorders of cognition, mobility and mood, and for shared vascular pathology, we hypothesize that slower psychomotor speed increases the future risk of developing clinically significant or subclinical disorders of cognition, mobility and mood. If slower psychomotor speed precedes and predicts these disorders rich new avenues could be opened to novel approaches to prevention and treatment of these major sources of later life disability. If, as we hypothesize, processing speed provides valid prediction of these outcomes, perhaps it might be used to detect persons at high risk and to initiate special efforts at evaluation and management of these disorders.
Methods
Study population
In 1992–94, 5,888 individuals (57.6% women, 15.7% black, and age 75.1 (5.5) mean years (SD) old) participated to the baseline in-person visit of the Cardiovascular Health Study [20] and were seen annually in the clinic or at home through 1998–99. Participants were queried twice per year by phone for new diagnoses, hospitalizations and procedures. All-cause mortality was ascertained via adjudicated events. All participants provided written informed consent. The institutional review boards at each site approved the protocol.
Dependent variables
Modified mini-mental state examination (3MS)
The 3MS includes components for orientation, concentration, language, praxis, and immediate and delayed memory [21]. Scores range from 0 to 100, with higher scores indicating higher cognitive function. Clinical (<80 points) and subclinical (80–85 points) disorders, and normal status (>85 points) were identified using cut-off values associated with greater risk of having dementia and mild cognitive impairment, respectively [22]. Baseline 3MS was missing in 550 participants who were generally older and less educated compared to those with a score.
Center for epidemiologic studies depression scale (CES-D)
The CES-D is a well-established 20-item self-report questionnaire of mood-related symptoms. Clinical (≥11) and subclinical (5–10) disorders and normal status (<5) were identified using cut-off values known to be associated with greater risk of having clinical or sub-syndromal depression [23]. Baseline CES-D score was missing in 1,198 participants who were more likely to be males and have less than a high school education compared to those with a score.
Gait speed (GS)
Usual GS is a reliable and valid measure of mobility measured as time to walk 15 feet beginning from a standing position, recorded using a stopwatch (timed to 0.1 s) [24]. Clinical (<0.6 m/s) and subclinical (0.6–0.1 m/s) disorders, and normal status (>1.0 m/s) were identified using cut-off values known to be associated with high risk of developing health-related adverse events [25]. Baseline GS was missing in 657 participants who were generally older and less likely to have a high school education compared to those with a score.
Independent variables
Digit Symbol Substitution Test (DSST)
The DSST is a pencil and paper test of psychomotor performance [26] in which the subject is given a key grid of numbers and matching symbols and a test section with numbers and empty boxes. The test consists of filling as many empty boxes as possible with a symbol matching each number. The score is the number of correct number-symbol matches achieved in 90 s. This test has high test-retest reliability [27]. We used the DSST both as a continuous variable and as a 4-level categorical variable based on quartiles (≤29/30–39/40–48/49+). Baseline DSST was missing for 699 participants who were more likely to be older (mean age 80 vs 75) male (47% vs 42%), lack a high school diploma (48% vs 27%), have hypertension (52% vs 41%) and have a lower ankle–arm index (0.93 vs 1.08; all p < 0.01).
White Matter Hyperintensity (WMH)
Brain images were acquired in 1992–94 using a 1.5 T Signa scanner (GE Medical Systems) with high performance gradients (4 G/cm and 150 T/m-s) [28]. A volumetric Spoiled Gradient Recalled Acquisition (SPGR) sequence with parameters optimized for maximal contrast among grey matter, white matter and cerebrospinal fluid was acquired in the coronal plane (TE/TR = 5/25, flip angle = 40°, NEX = 1, slice thickness = 5 mm/0 mm inter-slice gap). Neuroradiologists were trained in a standardized protocol according to an atlas of predefined visual standards and interpreted all scans at a central MRI Reading Center. WMH were quantified as the presence of signal abnormalities or hyperintensities form the white matter of the periventricular and subcortical regions on standardized axial spin-density/T2-weighted images. WMH burden was graded from 0 (lowest) to 9 (highest) and dichotomized based on a cut-off between grades 2 and 3. Of the 3,660 participants with WMH scores, 3,525 had concurrent DSST and were included in the analysis. Those excluded from this analysis were more likely to be older (mean age 76 vs 75), white (84% vs 83%), lack a high school diploma (36% vs 25%), diabetic (14% vs 10%) and have a lower ankle–arm index (1.06 vs 1.09; all p < 0.05).
Covariates
In addition to age, race, gender and education, the following risk factors and conditions were included because of their known association with the independent and dependent variables although not considered to be a direct step in the causal pathway: hypertension, diabetes, smoking, physical activity and ankle–arm index. Hypertension was defined as a previous diagnosis of hypertension, taking hypertensive medication, or having a current systolic blood pressure of ≥140 mmHg or a diastolic blood pressure ≥90 mmHg. Persons were considered diabetic if they had a validated medical diagnosis of diabetes or a fasting glucose level ≥126 mg/dl. Smoking habit was recorded by self-report as ever/never smoker. Regular leisure-time physical activity level (kcal) from the last 12 month was recorded by self-report. Ankle–arm index < 0.9 (AAI) was used as a surrogate measure of peripheral arterial disease, based on previously published cut-off values [29].
Statistical analysis
DSST was considered both as a continuous and as a categorical variable in separate models, with any missing value imputed with those from the previous year along with covariates if available. 3MS, GS and CES-D were considered both as continuous or categorical indicating whether normal, subclinical, clinically significant or indeterminate due to missing data. For the longitudinal analyses of incident categorical outcomes, we included only participants without any disorder at baseline. We included ‘indeterminate’ as a level in categorical outcomes in our models due to pervasive informative censoring, and to demonstrate similarities in findings for indeterminate and subclinical or clinical outcomes, suggestive of data not missing at random in longitudinal studies of the elderly. However, we also performed sensitivity analyses for all presented tables using a multiple imputation approach for the indeterminate category [30]. The results were similar and thus data not shown in the manuscript. Longitudinal outcome status was determined as: (1) using both continuous and categorical approaches at one point after five years (1997–99); (2) cumulative incident categorical status accumulated over 5 years defined as clinical or subclinical at any wave, or always normal, or ‘indeterminate’ due to no evidence of a clinical or subclinical status in the presence of missing data for at least one follow-up visit; and (3) same as approach (2) except that participants with missing data between years 1 and 5 were classified as normal if they were never classified as having a clinically significant or subclinical status but were classified as normal at the 5-year follow up. The results were not meaningfully different among the three approaches so only the results for (3) are presented. Outcomes combining concurrent disorders in mobility, mood and memory were based on counts of the number of clinical and subclinical disorders, with adjacent categories combined in models to obtain sufficiently large numbers in each cell for model fitting.
We used general linear models (SAS® GLM procedure) for outcomes as continuous variables and multinomial polytomous logistic regression with a generalized logit link (SAS® LOGISTIC procedure) for outcomes as categorical variables. The generalized logit link allowed estimation of odds ratios for different outcomes (subclinical, clinical and indeterminate) independently in the same model without assumptions of proportionality of odds. Analyses were performed (a) without covariates, (b) with baseline values of the three domains as covariates in longitudinal analyses and (c) with a general set of risk factors (age, gender, race, diabetes, hypertension, smoking, physical activity, AAI and education) in addition to (b) in longitudinal analyses. The resulting regression coefficients with the rate of change, means difference and odds ratio interpretations and their statistical significances were used to draw the main conclusions. We repeated statistical modeling in a subsample of participants with baseline brain imaging, to examine white matter grade as a predictor. To assess the bias that could result from examining survivors alone, we inspected the relationship between death rates and DSST. SAS® 9.3 (SAS institute, Inc., Cary, North Carolina) was used for all statistical analyses.
Results
Baseline prevalence of a clinical disorder was 10.7% for cognition (629/5,888), 10.9% for mobility (644/5,888) and 13.3% for mood (783/5,888). Prevalence of subclinical disorders was higher and more common in mobility (53.7% (3,164/5,888)) and mood domains (29.9% (1,761/5,888)) than in cognition (9.8% (579/5,888), Table 1). Baseline prevalence of ≥2 clinical disorders was 8.6% (509/5,888, Supplementary data, Appendix 1, available in Age and Ageing online). Compared to participants free from disorders in cognition, mobility or mood at baseline, those with either subclinical or clinical disorders were more likely to be older, black, have less education and be more likely to have hypertension, diabetes and worse AAI. Women were more likely than men to have disorders in mobility and mood and less likely than men to have disorders in cognition.
Table 1.
Demographic characteristics in 1992–93 for participants in the Cardiovascular Health study, stratified by presence of disorders in cognition (Modified Mini-Mental State examination, 3MS), mobility (gait speed, GS) and mood (Center for Epidemiological Studies–Depression, CES-D). Mean ± standard deviation or N (%) are reported
| All cohort | Cognitive disorders (3MS, points) | Mobility disorders (GS, m/s) | Mood disorders (CES-D, points) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None (> 85) | Subclinical (80–85) | Clinical (< 80) | Missing 3MS | None (> 1.0) | Subclinical (0.6–1.0) | Clinical(< 0.6) | Missing GS | None (< 5) | Subclinical (5–10) | Clinical (≥ 11) | Missing CES-D | ||
| Number | 5,888 | 4,130 | 579 | 629 | 550 | 1,423 | 3,164 | 644 | 657 | 2,146 | 1,761 | 783 | 1,198 |
| DSST, points | 38.1 ± 13.9 | 41.7 ± 12.1 | 28.3 ± 11.0 | 19.2 ± 9.9 | – | 43.8 ± 12.6 | 37.5 ± 13.5 | 28.2 ± 12.8 | 30.7 ± 14.1 | 39.9 ± 13.4 | 36.7 ± 14.1 | 33.8 ± 14.3 | 40.4 ± 13.1 |
| Age, years | 75.1 ± 5.5 | 74.3 ± 4.9 | 76.1 ± 5.9 | 78.4 ± 6.8 | 78.9 ± 7.4 | 73.2 ± 4.3 | 75.0 ± 5.2 | 78.4 ± 6.4 | 78.7 ± 7.0 | 74.6 ± 5.1 | 75.4 ± 5.6 | 75.6 ± 5.8 | 75.2 ± 6.0 |
| Race: White | 4,925 (83.6) | 3,649 (88.4) | 406 (70.1) | 368 (58.5) | 502 (91.3) | 1,251 (87.9) | 2,610 (82.5) | 479 (74.4) | 585 (89.0) | 1,840 (85.7) | 1,437 (81.6) | 607 (77.5) | 1,041 (86.9) |
| Gender: Female | 3,393 (57.6) | 2,443 (59.2) | 327 (56.5) | 338 (53.7) | 285 (51.8) | 711 (50.0) | 1,868 (59.1) | 451 (70.0) | 363 (55.3) | 1,179 (54.9) | 1,100 (62.5) | 533 (68.1) | 581 (48.5) |
| Education: <HS | 1,732 (29.5) | 851 (20.6) | 257 (44.6) | 384 (61.6) | 240 (43.8) | 250 (17.6) | 930 (29.5) | 274 (42.8) | 278 (42.5) | 507 (23.7) | 555 (31.6) | 291 (37.3) | 379 (31.7) |
Please note that a full version of this table is available as supplementary data, Appendix 3, available in Age and Ageing online.
HS: high school or post-secondary.
In cross-sectional analyses, lower DSST score (either as continuous or quartile) was associated with increased odds of clinical and subclinical disorders of cognition, mobility and mood, in both unadjusted and adjusted analyses (Supplementary data, Appendix 2, available in Age and Ageing online). Lower DSST score was also associated with greater mortality rates (not shown).
Among the 619 with no clinical or subclinical disorder at baseline, the risk of developing one or more incident disorder in the three domains (N = 413) was two times higher for participants in the lowest compared to the highest quartile of DSST, independent of covariates (Supplementary data, Appendix 4, available in Age and Ageing online).
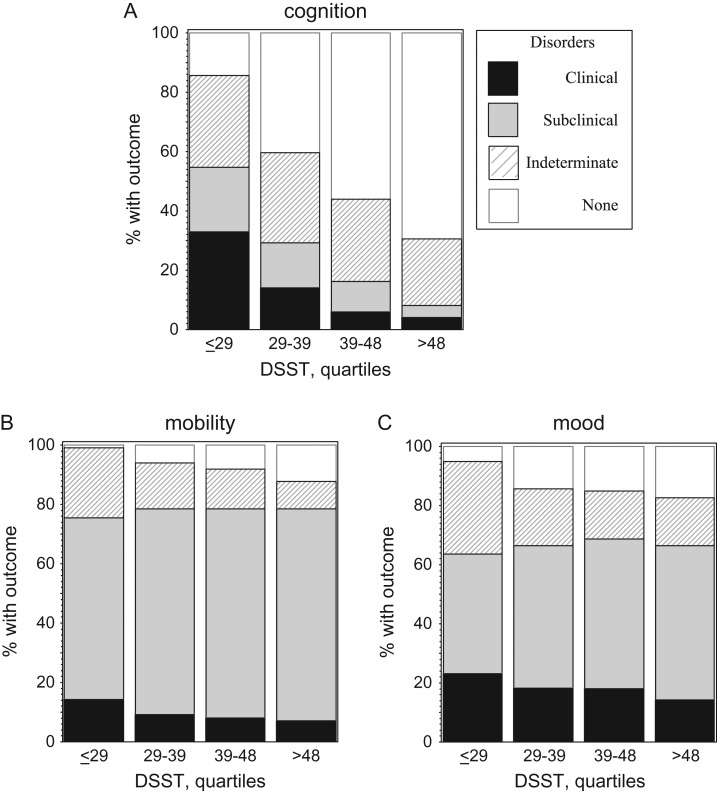
The percentage of participants developing a clinical disorder in cognition, mobility or mood decreased for increasing DSST quartiles (Figure 1, unadjusted). In adjusted models, the risk of developing a clinical or subclinical disorder of cognition or mobility decreased as the DSST score increased, while the risk of developing a clinical or subclinical disorder of mood disappeared in adjusted analyses (Supplementary data, Appendix 5, available in Age and Ageing online, Model 3). When DSST was coded in quartiles, associations were similar, with especially high levels of risk for the lowest compared to the highest DSST quartile in cognition and mobility and a marginal effect for mood (Model 3, Supplementary data, Appendix 5, available in Age and Ageing online). Associations with mood were marginally significant in parsimonious models adjusted for demographics and vascular risk factors only (AAI, diabetes and hypertension). The risk of having an indeterminate outcome due to missing outcome data was associated with lower baseline DSST score and was of similar magnitude to the risk of developing a disorder.
Figure 1.
Incidence of disorders in cognition (A), mobility (B) and mood (C) expressed as percentage of individuals devleoping the outcome during follow-up.
Results were similar in the 3,660 participants with MRI in 1992–94 (Table 2). Associations of either DSST or white matter grade ≥ 3 with the outcomes were both significant and independent of other factors and of each other, although associations were less strong for mood. The association of white matter grade ≥ 3 with mobility and cognition outcomes was substantially attenuated after adjustment for DSST, and the attenuating effect of DSST was independent of covariates. In contrast, the association of DSST with mobility and cognition outcomes changed minimally after adjustment for white matter grade. This effect was not present for mood outcomes.
Table 2.
Longitudinal association of Digit Symbol Substitution Test Score (DSST) and white matter hyperintensity (WMH, grade ≥ 3) in 1992–94 with cumulative incident outcomes through 1999 in cognition (Modified Minimental Status score, 3MS), mobility (gait speed, GS) and mood (Center for Epidemiological Studies- Depression score, CES-D). Subgroup with magnetic resonance imaging (N = 3630). Only those without a baseline disorder in that domain are included in the analysis for each outcome. Odds ratio (95% confidence intervals) and significance level
| Cognitive disorders (3MS, points) Analyzed N = 2,851 | Mobility disorders (GS, m/s) Analyzed N = 1,019 | Mood disorders (CES-D, points) Analyzed N = 1,422 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical (80–85) | Clinical (<80) | Indeterminate Outcome | Subclinical (0.6–1.0) | Clinical (<0.6) | Indeterminate Outcome | Subclinical (5–10) | Clinical (≥11) | Indeterminate Outcome | |||
| N = 484 | N = 510 | N = 1,131 | N = 973 | N = 130 | N = 197 | N = 1,010 | N = 408 | N = 445 | |||
| DSST, per point | Model 1 | 0.91*** | 0.89*** | 0.96*** | 0.97*** | 0.95*** | 0.95*** | 0.99** | 0.96*** | 0.97*** | |
| 0.90–0.93 | 0.88–0.90 | 0.95–0.97 | 0.95–0.99 | 0.92–0.97 | 0.92–0.97 | 0.97–1.00 | 0.95–0.98 | 0.95–0.98 | |||
| Model 2 | 0.94*** | 0.92*** | 0.97*** | 0.98* | 0.95*** | 0.95*** | 1.00 | 0.99 | 0.98* | ||
| 0.93–0.96 | 0.91–0.93 | 0.96–0.98 | 0.95–1.00 | 0.92–0.98 | 0.92–0.98 | 0.99–1.02 | 0.97–1.01 | 0.97–1.00 | |||
| Model 3 | 0.96*** | 0.93*** | 0.97*** | 0.98 | 0.96** | 0.95** | 1.00 | 1.00 | 1.00 | ||
| 0.94–0.97 | 0.92–0.95 | 0.96–0.99 | 0.95–1.01 | 0.93–1.00 | 0.92–0.99 | 0.99–1.02 | 0.98–1.02 | 0.97–1.02 | |||
| WMH ≥ 3 | Model 1 | 1.55*** | 2.29*** | 1.65*** | 2.89*** | 5.72*** | 3.01*** | 1.72*** | 2.30*** | 1.94*** | |
| 2.20–1.99 | 1.82–2.90 | 1.35–2.01 | 1.55–5.38 | 2.79–11.7 | 1.38–6.55 | 1.21–2.45 | 1.56–3.40 | 1.28–2.94 | |||
| Model 2 | 1.39** | 1.95*** | 1.60*** | 2.09** | 4.23*** | 2.61** | 1.54** | 1.79*** | 1.60** | ||
| 1.03–1.86 | 1.47–2.58 | 1.28–2.00 | 1.07–4.09 | 1.94–9.22 | 1.14–5.96 | 1.08–2.21 | 1.19–2.70 | 1.05–2.46 | |||
| Model 3 | 1.13 | 1.52*** | 1.31** | 1.59 | 2.80** | 2.19* | 1.50** | 1.55** | 1.40 | ||
| 0.82–1.55 | 1.12–2.07 | 1.03–1.68 | 0.79–3.24 | 1.22–6.34 | 0.91–5.29 | 1.03–2.19 | 1.01–2.40 | 0.89–2.20 | |||
| DSST and WMH ≥ 3 | Model 1 | DSST | 0.91*** | 0.89*** | 0.96*** | 0.97*** | 0.95*** | 0.95*** | 0.99** | 0.97*** | 0.97*** |
| 0.90–0.93 | 0.88–0.90 | 0.95–0.97 | 0.96–0.99 | 0.93–0.97 | 0.92–0.97 | 0.98–1.00 | 0.95–0.98 | 0.96–0.99 | |||
| WMH | 1.25* | 1.76*** | 1.48*** | 2.69*** | 5.12*** | 2.64** | 1.64*** | 1.90*** | 1.66** | ||
| 0.96–1.63 | 1.36–2.28 | 1.20–1.82 | 1.44–5.04 | 2.48–10.6 | 1.21–5.79 | 1.15–2.34 | 1.27–2.85 | 1.08–2.55 | |||
| Model 2 | DSST | 0.95*** | 0.92*** | 0.97*** | 0.98* | 0.96*** | 0.95*** | 1.00 | 0.99 | 0.99 | |
| 0.93–0.96 | 0.91–0.93 | 0.96–0.98 | 0.96–1.00 | 0.93–0.99 | 0.92–0.98 | 0.99–1.02 | 0.97–1.00 | 0.97–1.00 | |||
| WMH | 1.22 | 1.67*** | 1.49*** | 1.97* | 3.81*** | 2.26* | 1.56** | 1.76*** | 1.60** | ||
| 0.91–1.65 | 1.24–2.23 | 1.19–1.86 | 1.00–3.87 | 1.73–8.36 | 0.98–5.21 | 1.09–2.24 | 1.16–2.66 | 1.04–2.46 | |||
| Model 3 | DSST | 0.96*** | 0.93*** | 0.98*** | 0.98 | 0.97* | 0.96** | 1.01 | 1.00 | 1.00 | |
| 0.94–0.97 | 0.92–0.95 | 0.96–0.99 | 0.95–1.01 | 0.93–1.00 | 0.92–0.99 | 0.99–1.02 | 0.98–1.02 | 0.98–1.02 | |||
| WMH | 1.05 | 1.38** | 1.26* | 1.54 | 2.56** | 1.94 | 1.52** | 1.56** | 1.43 | ||
| 0.76–1.44 | 1.00–1.90 | 0.99–1.61 | 0.75–3.13 | 1.12–5.90 | 0.80–4.73 | 1.04–2.22 | 1.01–2.42 | 0.91–2.26 | |||
MODEL 1: unadjusted.
MODEL 2: Adjusted for Modified Mini-Mental State examination, gait speed and Center for Epidemiological Studies- Depression in 1992–94.
MODEL 3: Further adjusted for age, gender, race, diabetes, hypertension, smoking, physical activity, ankle–arm index and education in 1992–94.
Indeterminate status is assigned if there is missing data on the outcomes from interval annual visits and final status was not normal.
*p < 0.10; **p < 0.05; ***p < 0.01.
Discussion
Lower DSST score predicted future clinical and subclinical disorders of cognition and mobility, and to a marginal extent, mood. These risks were only partially explained by WMH. If confirmed by others, lower DSST might serve as a biomarker of risk of developing these disorders.
The effects of DSST on mood were less than expected. It is possible that other factors, and especially vascular factors, contribute more than DSST to mood disorders, since adding vascular factors to the models predicting mood completely eliminated the association between DSST and mood outcomes, whereas it only marginally modified the association between DSST and cognition or mobility outcomes. Similarly, in the analyses restricted to the group with MRI measures, the associations of WMH with subclinical or clinical disorders in mood were robust to adjustment for covariates, whereas the associations of DSST were not. Future studies should investigate whether psychomotor slowing is predominantly a manifestation of ischaemic brain pathology or is also a consequence of other alterations in the ageing brain.
Why would psychomotor speed, as measured by DSST, be a predictor? Digit Symbol Substitution is a classic indicator of psychomotor speed used by Salthouse [10] and many others to explore issues related to cognition and ageing [31, 32]. Psychomotor slowing reflects fundamental aspects of brain function which, like in a computer, reflect overall efficiency of operations [10, 33], which in turn may play a pivotal role in maintaining function. Generalized slowing with ageing is such an obvious phenomenon that it has been described scientifically since the nineteenth century [34]. We are not aware of studies that have prospectively examined the role of psychomotor slowing in the development of later-life disorders. Hajjar et al. used cluster analysis of cross-sectional data to define a group with executive dysfunction as measured by the Trail Making Test part B, slow gait and decreased mood and showed longitudinal associations of the triad with disability and risk from hypertension but did not predict the risk of onset of disorders [35].
Strengths of our study include a large sample followed longitudinally with multiple relevant measures including neuroimaging. Given the inevitability of informative censoring in cohort studies of ageing, we carefully evaluated the potential influence of missing data on our findings through analyses of the effect of ‘indeterminate’ status, and note that there were strong relationships between baseline DSST and developing missing outcomes. This informative censoring suggests that true effects may be even stronger than those found in our analyses. Moreover, we inspected the possibility that survivorship could have biased our findings toward a greater effect than was truly present. We find that death rates are higher among the group with low DSST. Thus, bias due to differential mortality rates among DSST groups is more likely to have led to underestimation than overestimation of the associations. We based our outcomes on generally well accepted categories of performance on widely used tests, but did not apply professionally adjudicated diagnoses.
The study has limitations. We did not examine other aspects of processing speed or executive function. We used only a baseline measure of DSST in these analyses, whereas repeated measures might have contributed to further insights. Neuroimaging techniques have advanced tremendously ever since these brain images were obtained 15–20 years ago. Our estimates of white matter disruption based on an ordinal scale of overall severity are primitive compared to modern capacity to localize white matter disease and assess microstructural integrity using diffusion tensor imaging. Thus, our interpretations regarding the potential vascular independence of psychomotor slowing to outcomes requires evaluation using more modern imaging techniques.
Since prior studies have suggested that differences in psychomotor speed are detectable in childhood and persist through adulthood [11], future research should further explore the role of psychomotor speed across the lifespan in the development of later life disorders in cognition, movement and mood. If early life psychomotor speed is an important contributor to later life risk, perhaps, like general intelligence, it is one source of resilience or brain reserve to the adverse effects of brain pathology. If so, preventive interventions must start during development and maturation. Aspects of psychomotor speed appear to be trainable, as demonstrated by the ACTIVE study and numerous other intervention strategies including videogames [36, 37]. While some intervention studies based on speed of processing training have examined effects on cognition and mood, as well as daily functioning, we have not found any reports to date of effects on mobility. Other strategies to treat slowing might include existing or novel pharmacological agents or aerobic exercise. If simple tests like the DSST are confirmed to provide valid prediction, perhaps clinical cut-points such as the threshold for the lowest quartile detected here might be used clinically to detect persons at high risk and to initiate special efforts at evaluation and management.
Conclusions
Disorders of cognition, mobility and mood appear to share a common indicator of slowed processing. Slower processing speed may represent an important indicator of future risk of these common disorders of ageing. If nothing else, clinical and research inquiry into any one of these important conditions of ageing should attend to the potential co-existence of disorders in the others.
Key points.
Disorders of cognition, mobility and mood co-occur and all appear to share a common indicator of slowed processing.
Clinical and research inquiry into any one of these important conditions of ageing should attend to the potential co-existence of disorders in the others.
Clinical cut-points of DSST, such as the threshold for the lowest quartile detected here might be used clinically to detect persons at high risk and to initiate special efforts at evaluation and management.
Since psychomotor speed appears to be trainable, it is possible that by promoting psychomotor speed, we could ameliorate these common disorders of ageing.
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Conflicts of interest
None declared.
Funding
Intramural Research Program of the National Institute on Aging, Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827-06).
References
- 1.Alexopoulos GS. Depression in the elderly. Lancet 2005; 365: 1961–70. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility-giving mobility clinical visibility: a mobility working group recommendation. JAMA 2014; 311: 2061–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demakis GJ. Disability in Alzheimer's disease: causes, consequences, and economic considerations. J Health Hum Serv Adm 2007; 30: 292–305. [PubMed] [Google Scholar]
- 4.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2: 15–21. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatr 2012; 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butters MA, Young JB, Lopez O et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialog Clin Neurosci 2008; 10: 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielke MM, Roberts RO, Savica R et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2013; 68: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA 1998; 279: 1720–6. [DOI] [PubMed] [Google Scholar]
- 9.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link. J Gerontol A Biol Sci Med Sci 2004; 59: 818–26. [DOI] [PubMed] [Google Scholar]
- 10.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103: 403–28. [DOI] [PubMed] [Google Scholar]
- 11.Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging. Psychol Aging 2010; 25: 219–28. [DOI] [PubMed] [Google Scholar]
- 12.de Groot JC, de Leeuw FE, Oudkerk M et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000; 47: 145–51. [DOI] [PubMed] [Google Scholar]
- 13.Watson NL, Rosano C, Boudreau RM et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci 2010; 65: 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population-based longitudinal study. J Gerontol A Biol Sci Med Sci 2014; 69: 1503–10. [DOI] [PubMed] [Google Scholar]
- 15.Rosano C, Simonsick EM, Harris TB et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology 2005; 24: 8–14. [DOI] [PubMed] [Google Scholar]
- 16.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ.. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology 2005; 19: 520–31. [DOI] [PubMed] [Google Scholar]
- 17.Saxton J, Lopez OL, Ratcliff G et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 2004; 63: 2341–7. [DOI] [PubMed] [Google Scholar]
- 18.Rapp MA, Reischies FM.. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry 2005; 13: 134–41. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann LL, Goodwin GM, Ebmeier KP.. The cognitive neuropsychology of depression in the elderly. Psychol Med 2007; 37: 1693–702. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Borhani NO, Enright P et al. The cardiovascular health study: design and rationale. Ann Epidemiol 1991; 1: 263–76. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC.. The modified mini-mental state (3MS) examination. J Clin Psychiatry 1987; 48: 314–8. [PubMed] [Google Scholar]
- 22.Espeland MA, Rapp SR, Robertson J et al. Benchmarks for designing two-stage studies using modified mini-mental state examinations: experience from the Women's Health Initiative Memory Study. Clin Trials 2006; 3: 99–106. [DOI] [PubMed] [Google Scholar]
- 23.Irwin M, Artin KH, Oxman MN.. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med 1999; 159: 1701–4. [DOI] [PubMed] [Google Scholar]
- 24.Studenski S, Perera S, Wallace D et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51: 314–22. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000; 55: M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wechsler, D. Adult Intelligence Scale- revised. New York: Psychological Corporation, 1981. [Google Scholar]
- 27.Matarazzo JD, Herman D. Base rate data for the WAIS-R: test-retest stability and VIQ-PIQ differences. J Clin Neuropsychol 1984; 6: 351–66. [DOI] [PubMed] [Google Scholar]
- 28.Bryan RN, Manolio TA, Schertz LD et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. Am J Neuroradiol 1994; 15: 1625–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Newman AB, Shemanski L, Manolio TA et al. Ankle–arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol 1999; 19: 538–45. [DOI] [PubMed] [Google Scholar]
- 30.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, 1987. [Google Scholar]
- 31.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH.. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc 2008; 56: 1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and digit symbol substitution performance: a meta-analysis. Psychol Aging 2004; 19: 211–4. [DOI] [PubMed] [Google Scholar]
- 33.Birren JE, Fisher LM. Aging and speed of behavior: possible consequences for psychological functioning. Annu Rev Psychol 1995; 46: 329–53. [DOI] [PubMed] [Google Scholar]
- 34.Galton F. On the anthropometric laboratory at the late International Health Exhibition. J Anthropol Inst 1885; 14: 205–21, 275–87. [Google Scholar]
- 35.Hajjar I, Yang F, Sorond F et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci 2009; 64: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi H, Kawashima R.. Effects of processing speed training on cognitive functions and neural systems. Rev Neurosci 2012; 23: 289–301. [DOI] [PubMed] [Google Scholar]
- 37.Wolinsky FD, Vander Weg MW, Martin R et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci 2009; 64: 468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.