Abstract
Fast pyrolysis bio-oils are feasible energy carriers and a potential source of chemicals. Detailed characterization of bio-oils is essential to further develop its potential use. In this study, quantitative 13C nuclear magnetic resonance (13C NMR) combined with comprehensive two-dimensional gas chromatography (GC × GC) was used to characterize fast pyrolysis bio-oils originated from pinewood, wheat straw, and rapeseed cake. The combination of both techniques provided new information on the chemical composition of bio-oils for further upgrading. 13C NMR analysis indicated that pinewood-based bio-oil contained mostly methoxy/hydroxyl (≈30%) and carbohydrate (≈27%) carbons; wheat straw bio-oil showed to have high amount of alkyl (≈35%) and aromatic (≈30%) carbons, while rapeseed cake-based bio-oil had great portions of alkyl carbons (≈82%). More than 200 compounds were identified and quantified using GC × GC coupled to a flame ionization detector (FID) and a time of flight mass spectrometer (TOF-MS). Nonaromatics were the most abundant and comprised about 50% of the total mass of compounds identified and quantified via GC × GC. In addition, this analytical approach allowed the quantification of high value-added phenolic compounds, as well as of low molecular weight carboxylic acids and aldehydes, which exacerbate the unstable and corrosive character of the bio-oil.
Keywords: Pyrolysis oil, Softwood, Agricultural residue, Bio-oil stability, Chemical shift, Compositional analysis, Light oxygenates, Aromatics
Short abstract
Detailed characterization of fast pyrolysis bio-oils originated from pinewood, wheat straw, and rapeseed cake was studied using a combination of quantitative 13C NMR and GC × GC-FID/TOF-MS.
Introduction
Lignocellulosic biomass has emerged as a potential alternative source of specialty chemicals, gaseous and liquid fuels, and thermal energy. Among different processing routes, the transformation of lignocellulosic biomass into liquid bio-oil through the fast pyrolysis process is receiving increased attention.1−4 Fast pyrolysis bio-oil exhibits key advantages: it is produced at high yields (up to 70 wt %), suitable for decentralized production, and practical for handling, transport, and storage and has a much higher energy density as compared to the parent biomass.5,6 However, fast pyrolysis bio-oils are complex mixtures of water and oxygenated compounds, including carbohydrates, heterocyclics, phenolics, carboxylic acids, aldehydes, ketones, esters, and alcohols.7,8 To fully realize the potential of the fast pyrolysis process, detailed knowledge on the chemical composition of the bio-oil is essential.7,9,10
Several analytical techniques have been developed to characterize pyrolysis bio-oil, and recent review articles summarize the different analytical strategies developed so far.7,11 A powerful technique for bio-oil characterization is nuclear magnetic resonance (NMR). NMR allows quantitative analysis of the complete bio-oil sample, rather than a fraction, and gives information on the type of chemical functional groups.12−14 In this way, NMR characterization of pyrolysis bio-oil can help to determine optimum operational conditions for the pyrolysis process and to find the suitable (alternative) feedstocks for producing the desired bio-oils, which are chemically stable over an extended time period.15 However, the majority of compounds present in bio-oils have very low concentrations (<0.2 wt %), and a detailed compositional analysis requires the combination of several techniques.7 Comprehensive two-dimensional gas chromatography (GC × GC) has proven to be very powerful for the quantitative analysis of different types of pyrolysis oils16−19 and complex hydrocarbon matrices,20,21 as it provides detailed information on the molecular composition of their volatile fraction. Overall high performance liquid chromatography (HPLC) and GC allow a primary qualitative and quantitative classification of the detectable components.22 Only a portion of the sample is identified and the higher MW components can be determined, e.g., by gel permeation chromatography (GPC) to be up 1000–2000 Da without any further information regarding their structure. Comprehensive LC also shows promise as demonstrated for the aqueous phase of a bio-oil by Tamasini et al.23 Nevertheless, NMR provides insight into the different structures and functionalities of the various components and, hence, assists the interpretation of the results of the other analytical methods.
In this work, three different bio-oils originating from pinewood, wheat straw and rapeseed cake are characterized by means of 13C NMR spectroscopy and GC × GC coupled to a flame ionization detector (FID) and a time-of-flight mass spectrometer (TOF-MS), and their composition is compared. 13C NMR is used to characterize the major functional groups, while GC × GC-FID/TOF-MS is applied to identify and quantify individual volatile compounds.
According to the Food and Agriculture Organization of the United Nations (FAO), the production of wood pellets, which are used as fuel, reached 26 million tons in 2014.24 This production was mainly driven by increasing consumption in Europe to meet renewable energy goals and represented a growth of 16% over the previous year. Europe and North America accounted for almost all global pellet production with respective shares of 60 and 33%. Pine is one of the most important softwoods for the production of pellets, contains low amounts of ash and nitrogen (≈1.0 and 0.2 wt % on a dry basis, respectively), and is adaptable to varied environmental conditions.25 The valorization of pinewood pellets, but also its industrial waste (e.g., sawdust, shavings, waste ships, etc.), beyond renewable heat and electric power requires advanced technologies like fast pyrolysis supported by detailed compositional characterization of the bio-oil. As a distinctive feature, pinewood contains less hemicellulose and extractives and more cellulose, lignin, and nitrogen as compared with fast-growing agricultural residues.26
FAO’s statistics rank wheat as the sixth among the world agricultural commodities with a harvested area of 218 million hectares and a total yield of 713 million tons of wheat flour in 2013.24 Wheat is one of the most important crops in Europe and Asia with respective shares of 8% and 14% of the global harvested area. Although the yield of wheat straw depends on specific varieties and is widely affected by agronomic and climatic factors, an average ratio of 1.3 kg of straw per kg of grain is found for the most common varieties.27 Assuming a grain-to-flour mass ratio of 1.3 results in an annual yield of ≈1200 million tons of wheat straw in 2013.27 These figures demonstrate the potential of wheat straw as a renewable source of fuels and chemicals via fast pyrolysis. In addition, wheat straw is also representative of residues from other fast-growing agricultural residues with high ash content (≈8 wt %) like barley, rice, and oats.26
According to the latest figures of the EU Vegetable Oil and Protein Meal Industry FEDIOL, the total annual production of rapeseed in 2014 was 69 million metric tons worldwide resulting in a yield of vegetable oil of 27 million metric tonnes.28 Around 90% of the total production of rapeseed is harvested in Europe. Rapeseed cake is the major byproduct in the production of vegetable oil by cold pressing of rapeseed. Together, cellulose, hemicellulose, and lignin account for less than one-fourth of the dry mass of rapeseed cake. The remaining portion consists of crude protein, triglycerides, other extractives, and ashes.29,30 Rapeseed cake is valorized as animal feed, because of the high content of proteins and triglycerides. However, the valorization of rapeseed cake via fast pyrolysis has been also considered,30 and detailed information on the chemical composition of the rapeseed cake bio-oil is thus essential. Among the starting materials of the studied bio-oils rapeseed cake contains the lowest amount of oxygen (≈35 wt %) and the highest amount of nitrogen and sulfur (≈5.0 and 1.0 wt %, respectively).29,30
In this work, we present a detailed characterization study of these three distinct bio-oils using a combination of quantitative 13C NMR and GC × GC-FID/TOF-MS. 13C NMR spectroscopy is used to characterize the major functional groups. The 13C NMR spectra are integrated over spectral regions to determine the percentages of carbons in functional groups based on chemical shift. The identified components from GC × GC analysis are grouped according to their organic carbon number and organic functionality, leading to remarkable differences in chemical composition and stability between the three types of bio-oils. This knowledge provides practical guidelines for improved bio-oil upgrading strategies.
Materials and Methods
Feedstocks
The bio-oils characterized in this study originated from pinewood (PW), wheat straw (WS), and rapeseed cake (RC). Bio-oils were produced in a rotating cone reactor (RCR) fast pyrolysis plant at different temperatures. PW bio-oil was produced at 500 °C, while WS and RC bio-oils were produced at 480 and 550 °C, respectively. Detailed descriptions of the RCR fast pyrolysis setup and the applied experimental conditions have been previously reported.31
Elemental Analysis
Elemental analysis was carried out with a Flash EA2000 elemental analyzer (Interscience, Belgium) equipped with a thermal conductivity detector (TCD). The elemental composition of each bio-oil was derived based on three repeat analyses. Uncertainties on the amount of carbon, hydrogen, oxygen, and nitrogen detected with this method were within the vendor specifications.
Nuclear Magnetic Resonance (NMR) Analysis
All bio-oil samples were freeze-dried for 12 h and kept under vacuum overnight. Then 30–50 mg of dried bio-oil was completely dissolved in 600 μL of deuterated DMSO-d6 (dimethyl sulfoxide-d6). The 13C NMR spectra were recorded on a Varian 400-MR spectrometer at a resonance frequency of 100.614 MHz using a 5 mm broadband probe. The solvent signal of 39.52 ppm was used as the internal reference. The 13C NMR spectra were acquired with 45° pulse angle, proton decoupling, sweep width of 25000 Hz, and corresponding acquisition time of 1.311 s. Acquisition of 10 000 transients using a 5 s pulse delay resulted in a good signal-to-noise ratio after 17.5 h of total time of measurement per sample. All experiments were performed at 25 °C. The spectra were processed using MestReNova to perform baseline corrections and integrations.
The accuracy of the NMR analysis depends upon several factors, such as signal-to-noise ratio, decoupling, relaxation delay, line shape consideration, and baseline correction. Measurements as accurate as ±5% can be achieved when the above-mentioned factors are optimized.12,32 For this purpose, reasonable signal-to-noise ratios were achieved to provide adequate recovery of the signal. In addition, proton decoupling was used to avoid nuclear Overhauser enhancement (NOE) of the 13C signal from attached protons.
Two-Dimensional Gas Chromatography (GC × GC) Analysis
Sample Preparation
Bio-oil samples were dissolved in tetrahydrofuran (THF). Dibutyl ether and fluoranthene were used as internal standards. Samples were stored in a refrigerator at temperatures in the range of 3–5 °C. The prepared samples were subsequently analyzed by both GC × GC-FID and GC × GC-TOF-MS.
GC × GC-FID/TOF-MS Setup
GC × GC analysis were carried out with a Thermo Scientific TRACE GC × GC (Interscience, Belgium). The columns (a nonpolar/medium polar column set) and the modulator (a two stage cryogenic modulator) were positioned together in a single oven. Both columns were connected to a piece of deactivated fused silica column (Rxi Guard, 0.1 m × 0.25 mm, Restek) by means of a SilTite metal ferrule from SGE. The columns combinations placed in the same oven are described in Table 1.
Table 1. Overview of the Columns Used for the GC × GC Analysis.
| combination | first column | second column |
|---|---|---|
| 1 | MXT-1a 60 m long × 0.25 mm I.D. × 0.25 μm | BPX-50b 2 m long × 0.15 mm I.D. × 0.15 μm df |
| 2 | RTX-1 PONAa 50 m long × 0.25 mm I.D. × 0.5 μm df | BPX-50 2 m long × 0.15 mm I.D. × 0.15 μm df |
Dimethyl polysiloxane (Restek).
50% phenyl polysilphenylene-siloxane (SGE).
Two different detectors mounted on different GC × GC setups were used. The detectors were a FID and a TOF-MS. Table 2 gives a summary of the GC × GC settings. Parameters changed depending on the used column combination as well as the bio-oil sample. The concentration of the internal standards was optimized to give peak highs comparable to those of the quantified compounds. Columns combination, split flow, and temperature program were tuned to obtain a compromise between enhanced resolution in the first dimension and minimization of wrap around in the second dimension.
Table 2. GC × GC Settings for FID Analysisa.
| detector | FID | TOF-MS, 25–500 amu | ||||
|---|---|---|---|---|---|---|
| origin of the bio-oil | pine wood | wheat straw | rapeseed cake | pine wood | wheat straw | rapeseed cake |
| column combination | 1 | 1 | 2 | 1 | 2 | 2 |
| injector, temperature [°C] | PTV, 350 | PTV, 350 | PTV, 300 | Split/Splitless, 300 | Split/Splitless, 300 | Split/Splitless, 300 |
| split flow [mL min–1] | 30 | 10 | 50 | 150 | 10 | 12 |
| carrier gas [mL min–1] | 2.1 | 2.1 | 2.1 | 3.5 | 2.9 | 2.9 |
| oven temperature program [°C] | –25→350 | –25→350 | –40 →300 | –40→300 | –25→350 | –40→300 |
| heating rate [°C min–1] | 3 °C min–1 | |||||
| modulation period [s] | 7 | |||||
| detector acquisition rate [Hz] | 100 | 100 | 100 | 30 | 30 | 30 |
Column combination 1 = MXT-1/BPX-50, column combination 2 = RTX-1 PONA/BPX-50.
GC × GC–FID/TOF-MS Data Acquisition and Quantification
Data acquisition and processing were carried out using Thermo Scientific’s Chrom-Card data system for the FID and Thermo Scientific’s XCalibur software for the TOF-MS. The raw GC × GC-FID data files were exported as CDF files and imported into GC Image software (Zoex Corporation, USA). With the aid of the GC Image software the contour plotting, retention time measurement, peak fitting and blob integration were performed. Each blob was tentatively identified based on both their chemical group and number of carbon atoms. The combined information from the pattern in the chromatogram obtained by the orthogonal separation of GC × GC-FID and the National Institute of Standards and Technology (NIST) library MS confirmation was used for the tentative identification of the peaks. The mass fraction wt %i of each compound was calculated using the mass fraction of the internal standard (3-chlorothiophene) wt %st, peak volumes obtained and the response factor relative to methane:
| 1 |
where fi is the relative response factor for compound i, Vi is the peak volume of compound i, fst is the relative response factor for the internal standard, and Vst is the peak volume of the internal standard. The response factor of each compound is calculated by means of eq 2 which is based on the effective carbon number approach:33,34
| 2 |
where Mi is the molar mass of compound i, MCH4 is the molar mass of methane (the chosen reference compound), and Ci,eff is the effective carbon number of compound i. The effective carbon number is approximately equal to the carbon number of the compound in the case of hydrocarbons. For oxygen containing compounds a correction method is applied. The effective carbon number is calculated as a function of both the carbon number of the compound and the type of oxygen containing functional groups. The effective carbon number is calculated by means of eq 3:35
| 3 |
where Ci is the carbon number of compound i and n is the correction value for functional groups (n = 1 for aldehydes, n = 1 for monoethers, n = 0.5 for primary alcohols, etc.). It has been demonstrated that the experimental response factors of oxygen compounds agree well with the ones calculated based on the effective carbon number approach.35 The agreement between the calculated and the experimental effective carbon number for oxygen containing compounds has also been experimentally validated elsewhere.16
Results and Discussion
Elemental Composition
As can be seen from Table 3 the contents of carbon and hydrogen, and consequently the molar H/C ratios of PW and WS bio-oils were comparable. However, the oxygen and nitrogen contents of PW and WS bio-oils differed notably. The higher nitrogen content of WS bio-oil is consistent with the typical nitrogen content of such a feedstock.36 RC bio-oil stood out because of its high carbon and hydrogen contents, and its remarkably low oxygen content which was less than half of the corresponding oxygen contents of the other investigated bio-oils. The relatively low molar O/C ratio for the RC bio-oil is consistent with the presence of significant amounts of triglycerides and high molecular weight triglyceride-derived pyrolysis products, e.g., fatty acids with molar O/C ratios of ≈0.1. In addition, RC bio-oil showed a high nitrogen content which was expected because crude protein is the major constituent of the starting material.30
Table 3. Elemental Composition of the Bio-Oilsa.
| origin of bio-oil | C (wt %) | H (wt %) | O (wt %) | N (wt %) | O/C molar ratio | H/C molar ratio |
|---|---|---|---|---|---|---|
| pinewood | 44.0 ± 0.45 | 7.46 ± 0.11 | 48.0 ± 0.17 | 0.092 ± 0.002 | 0.82 | 2.0 |
| wheat straw | 45.9 ± 2.48 | 7.69 ± 0.11 | 41.3 ± 2.37 | 2.19 ± 0.26 | 0.68 | 2.0 |
| rapeseed cake | 65.5 ± 0.21 | 9.79 ± 0.02 | 18.7 ± 0.01 | 5.62 ± 0.01 | 0.21 | 1.8 |
Each reported value along with its corresponding standard deviation corresponds to three repeated analyses.
The energy contents of PW and WS bio-oils, assessed by their higher heating value (HHV) according to the correlation in eq 4,37 were 19 and 21 MJ kg–1, respectively. This correlation was validated for a wide range of elemental compositions (C 0.00–92.3; H 0.43–25.2; S 0.00–94.1; O 0.00–50.0; N 0.00–5.60; ash 0.00–71.4 wt %).37 Additionally, this correlation has been commonly used to assess the HHV of bio-oils as can be seen from recent publications.22,38−40Table 3 shows that the elemental compositions of the studied bio-oils are within the limits of the correlation, except for the nitrogen content of rapeseed cake bio-oil (5.62 wt %) which slightly exceeds the upper limit of 5.60 wt % by 0.36%. The typical HHV range for bio-oil is 14–19 MJ kg–1.22 Based on their energy content, PW and WS bio-oils could replace heavy fuel oil. A high energy content is a distinctive feature of the bio-oils from the RCR fast pyrolysis plant.31 This high energy content is correlated to the moisture content in the feedstock, which is reduced to less than 10 wt %.
| 4 |
where, C, H, S, O, N, and A represents carbon, hydrogen, sulfur, nitrogen, and ash contents of bio-oils, respectively.
RC bio-oil showed a calculated HHV of 32 MJ kg–1. This remarkably high energy content has also been reported for several bio-oils obtained from the fast pyrolysis of rapeseed cake at temperatures exceeding 500 °C.29,30,41,42 At these operation conditions, the raw bio-oil from rapeseed cake separates spontaneously into an organic fraction and an aqueous fraction. The organic fraction, which is the one characterized here, is expected to contain significant amounts of triglycerides and fatty acids (cold pressing reduces the oil content of the rapeseed cake from ≈40–45 wt % to ≈14–15 wt %)28 along with products from the pyrolysis of other ethanol extractives (lipids, waxes, and resins). Based on its energy content and elemental composition, RC bio-oil appears to be a good candidate for hydroprocessing into transportation fuels. However, this application requires removing a considerable part of the nitrogen.
13C NMR Analysis
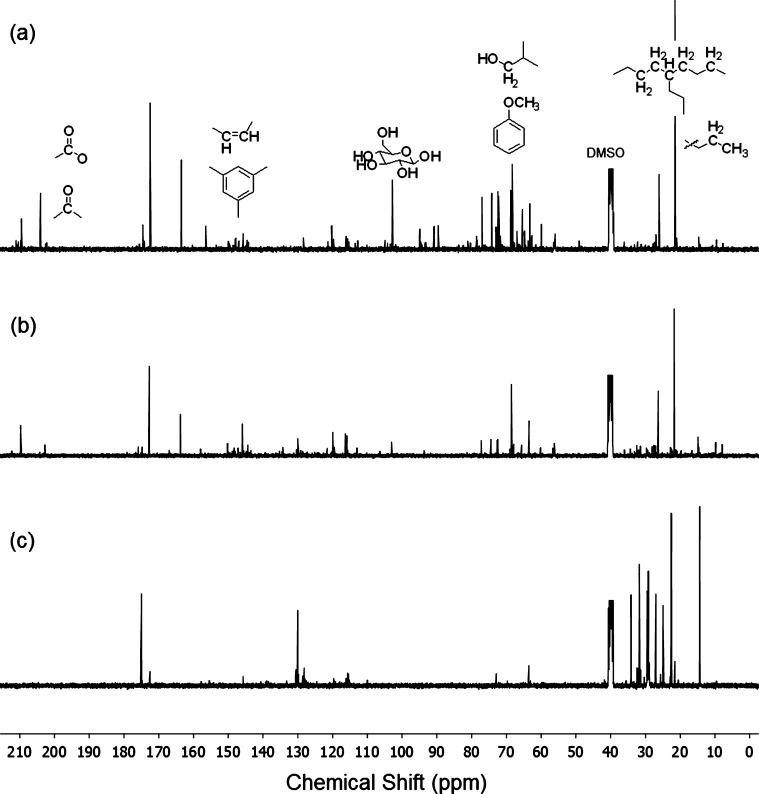
NMR analysis of complex mixtures, such as pyrolysis bio-oil, offers a reasonable trade-off between functional group identifications and analytical measurements. The advantage of NMR analysis is that the whole bio-oil sample can be dissolved in a suitable solvent and a quantitative assessment of the chemical functional groups can be determined by integration of the defined regions of spectra.12,15 The 13C NMR spectra of the bio-oils are depicted in Figure 1. An overview of the carbon content as a percentage within a given chemical shift range is summarized in Table 4. The assignments of the 13C NMR spectra are based on the works of Ingram et al.43 and Joseph et al.12 which provide information on the types of chemical functional groups in bio-oils. The 13C NMR spectra were divided into five chemical shift regions. The region 1–54 ppm, excluding the DMSO solvent, corresponds to alkyl carbons. The alkyl hydrocarbon region accounts for energy content, which is of primary interest when bio-oil is used as a fuel. This region can be further subdivided into primary carbons (6–24 ppm) and secondary/tertiary carbons (24–34 ppm).
Figure 1.
13C NMR spectra of the bio-oils obtained from (a) pinewood, (b) wheat straw, and (c) rapeseed cake.
Table 4. Quantitative Analysis of the 13C NMR Spectra of the Bio-Oils Derived from Pinewood, Wheat Straw, and Rapeseed Cake via Fast Pyrolysisa.
| carbon content in each spectrum (%) |
||||
|---|---|---|---|---|
| type of carbon | chemical shifts (ppm) | pinewood bio-oil | wheat straw bio-oil | rapeseed cake bio-oil |
| alkyl carbons (total) | 1–54 | 19.78 | 35.73 | 81.83 |
| primary alkyl carbons | 6–24 | 13.78 | 20.44 | 16.64 |
| secondary/tertiary alkyl carbons | 24–34 | 5.55 | 15.18 | 64.81 |
| methoxy/hydroxy | 54–70 | 29.46 | 14.73 | 1.27 |
| carbohydrate | 70–103 | 26.64 | 5.56 | 0.17 |
| aromatic (total) | 103–163 | 11.03 | 30.14 | 12.09 |
| aromatic (syringyl) | 110–112 | 0.27 | 0.68 | 0.19 |
| aromatic (guaiacyl) | 112–125 | 6.32 | 16.03 | 1.37 |
| aromatic (general) | 125–163 | 4.44 | 13.43 | 10.54 |
| carbonyl | 163-215 | 13.10 | 12.37 | 4.64 |
The different type of carbon compounds present in the bio-oils are grouped according to their chemical shift range. Spectra were obtained at 25 °C in DMSO-d6 at 100.614 MHz on Varian 400-MR spectrometer using inverse gated decoupling to avoid NOE effects. The strong DMSO solvent resonances at 39.5 ppm were excluded from this analysis.
The region between 54 and 70 ppm of the 13C NMR spectra corresponds to carbons adjacent to a heteroatom, mostly oxygen in ethers or alcohols as well as carbon adjacent to nitrogen. This region provides information on the oxygen or nitrogen functions present in bio-oils, e.g., lignin-derived hydroxyl- and methoxy-phenols. Carbons adjacent to oxygen in carbohydrates resonate in the 70–103 ppm region of the 13C NMR spectra. The next integrated region is between 103 and 163 ppm and represents aromatic, including heteroaromatic, e.g. furans, and alkene carbons in the bio-oils. The aromatics contents are important for synthetic modification of bio-oils. This region was further subdivided between 110 and 112 ppm (syringyl carbon) and 112–125 (guaiacyl carbon). The final portion of 13C NMR spectra is the downfield end between 163 and 215 ppm, which represents the carbonyl carbons. This spectral region is representative for acids, esters, ketones, and aldehydes.
The content of alkyl carbons of the studied bio-oils shows the following order: PW bio-oil < WS bio-oil < RC bio-oil. More than 80% of the total carbon in RC bio-oil was found in the alkyl region. The proportion of alkyl carbons in RC bio-oil was about twice that of alkyl carbons in PW bio-oil and approximately four times that of alkyl carbons in WS bio-oil. Primary, secondary, and tertiary carbons account for more than 98% of the alkyl carbons, thus the secondary/tertiary-to-primary alkyl carbon ratio provides an indication of the average size of the alkyl chain. The secondary/tertiary-to-primary alkyl carbon ratio for PW and WS bio-oils were respectively 0.43 and 0.74, while a ratio as high as 3.9 was found for RC bio-oil. These results indicate that alkyl carbons in PW and WS are mostly in methyl groups, while the alkyl carbons in RC are alkyl chains with C ≥ 5. The 13C NMR and the elemental analyses (see Table 3) can be combined to provide further evidence regarding the size of the alkyl chain in RC bio-oil. A hydrogen balance shows that an alkyl chain size of C = 4.4 would be required if all of the hydrogen quantified by elemental analysis is attached to alkyl carbons.
Approximately 30% of the total carbon in PW bio-oil and 15% of the total carbon in WS bio-oil were found to be in the methoxy/hydroxy region. In contrast, only around 1% of the total carbon in RC bio-oil was found in that region. These results are consistent with the higher lignin content of PW (≈25 wt % on a dry basis) when compared with the lignin content of WS (≈18 wt % on a dry basis) and that of RC (≈4 wt %, on a dry basis).26 However, the lignin content is most likely related to the methoxy groups attached to the aromatics. The difference between PW and WS in terms of cellulose and hemicellulose contents is less pronounced. Both, cellulose and hemicellulose pyrolysis products could substantially contribute to the presence of hydroxy carbons.
A pattern similar to the one for methoxy/hydroxy carbons was observed for carbohydrate carbons. Approximately 27% of the total carbon in PW bio-oil and 6% of the total carbon in WS bio-oil were found in the carbohydrate region. Notably, the content of carbohydrate carbons in RC bio-oil was found to be negligible. However, the mineral content of the feedstock has a considerable effect on the yield and composition of bio-oil.44 As soon as the pyrolysis products are formed, they can interact with catalytic minerals in the residual solid. The presence of metal cations leads to the increased formation of light oxygenates and pyrolytic water at the expense of levoglucosan formation.44 While PW typically contains less than 1.0 wt % of ashes, WS contains approximately 8 wt % (on a dry basis) which supports the consistency of the current 13C NMR analysis for carbohydrate carbons.26
Carbons corresponding to the aromatic/alkene region were more abundant in WS bio-oil, i.e., about 30% of the total carbon. Similar contents of aromatic/alkene carbons, i.e., around 10% of the total carbon, were found for the PW and RC derived bio-oils. The amount of syringyl carbons in PW bio-oil was low compared with its guaiacyl carbons content. This result is consistent with the respective proportions of syringyl and guaiacyl units in pinewood lignin.45 Similar amounts of syringyl and guaiacyl carbons would be expected in the WS bio-oil;46 however, results showed the same trend as for PW bio-oil. A plausible explanation for this result is the high reactivity of the methoxyl groups47 associated with syringyl carbons, which is further promoted by the minerals in WS. Yet, a significant amount of the carbon in this region was not identified either as syringyl or guaiacyl carbons (≈5% of the total carbon in PW bio-oil and 13% and 10% of the total carbon in WS bio-oil and RC bio-oil, respectively). The remaining part in PW bio-oil and WS bio-oil is most probably composed by carbons in heterocyclic aromatics such as furans. On the other hand, the content of both syringyl and guaiacyl carbons in RC bio-oil is low which is in line with the low lignin content of the originated feedstock. Most of the carbon corresponding to the aromatic/alkene region in RC bio-oil is probably composed by alkene carbons in the unsaturated fatty acids chains and their pyrolysis products. Rapeseed cake triglycerides are characterized by high contents of polyunsaturated fatty acids (mostly oleic, linoleic, and linolenic).48
One of the most important results of the 13C NMR analysis of bio-oils is the carbonyl carbon content. Aldehydes and ketones derived primarily from cellulose and hemicellulose, as well as acetic acids derived from the acetyl groups of the hemicellulose are expected in PW and WS bio-oils. On the other hand, esters from triglycerides and carboxylic acids are expected in RC bio-oil.42 The combination of phenolics and carboxylic acids leads to condensation reactions which are detrimental for the quality of the bio-oil upon storage.49 PW bio-oil and WS bio-oil showed comparable amounts of carbonyl carbons corresponding to ≈10% of their total carbon. Carbonyl carbons accounted for ≈5% of the total carbon in RC bio-oil. Again for this bio-oil 13C NMR and elemental analysis (see Table 3) results provide information on the water content of bio-oil. An oxygen balance showed that a maximum water content of 12 wt % would be present in this bio-oil if the remaining oxygen, i.e., oxygen not attached to the carbonyl group, is forming water. The actual water content is expected to be <12 wt % as has been experimentally found for bio-oil from the fast pyrolysis of rapeseed cake at 550 °C.30
Summarizing, 13C NMR revealed that PW bio-oil contains mostly methoxy/hydroxy, carbohydrate, and alkyl carbons. Aromatic carbon, mostly guaiacyl and heteroatomic, along with short-chain alkyl carbons are the major carbon types in WS bio-oil. RC bio-oil is very rich in long chain alkyl carbons.
GC × GC-FID/TOF-MS Analysis
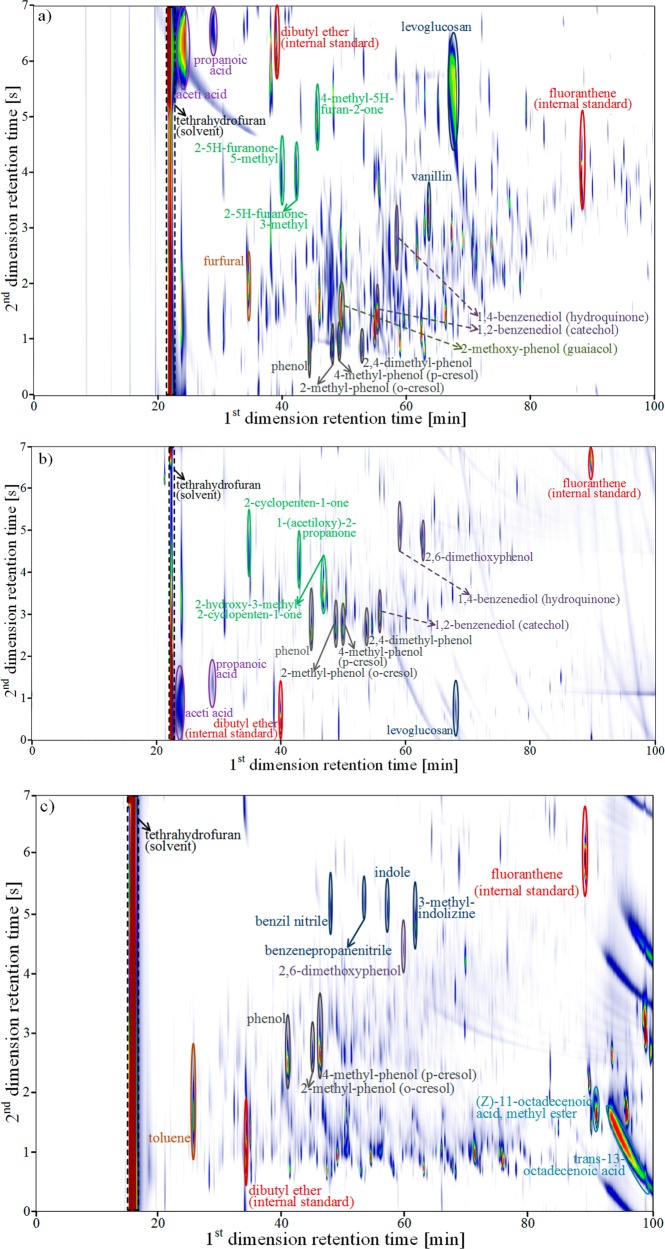
The employed GC × GC-FID/TOF-MS analysis provides complementary information to the elemental and 13C NMR analysis of the three investigated bio-oils. The obtained GC × GC-FID chromatograms are shown in Figure 2. It can be noted that different groups of chemical compounds were identified by using the orthogonal separation of the GC × GC method, while the internal standards, i.e., 3-chlorothiophene and fluoranthene, were adequately separated from the other compounds. The quality of the separation between peaks was assessed by the two-dimensional resolution (RS2D) which has been defined for a pair of compounds A and B in terms of the peak width along each dimension (ωA1, ωA2, and ωB1, ωB2) and the difference in retention times for each dimension (Δtr1 and Δtr2) as follows:50
The separation between peaks is considered acceptable if the two-dimensional resolution is higher than 1 and good if its value is higher than 1.5. For the three studied bio-oils, two-dimensional resolutions higher than 1.5 were calculated from the GC × GC chromatograms.
Figure 2.
GC × GC-FID chromatograms for (a) pinewood-based bio-oil, (b) wheat straw-based bio-oil, and (c) rapeseed cake-based bio-oil. Some representative compounds are highlighted. The complete list of plausibly identified compounds, classified according to their organic functionality, is presented in Table S1 of the Supporting Information.
The total number of identified peaks that were subsequently quantified in the three different bio-oil samples amounted to 256. Those 256 peaks corresponded to 209 compounds as some of these compounds were present either in two or the three studied bio-oils. From those peaks, 74 corresponded to PW bio-oil, 112 to WS bio-oil, and 70 to RC bio-oil. The identified compounds accounted for 22.6 wt % of PW bio-oil, 17.2 wt % of the WS bio-oil, and 12.3 wt % of the RC bio-oil. As can be seen from Table 5, most of the identified compounds in PW bio-oil exhibited carbon numbers from C2 to C9; 32% of that fraction was C2, while 38% was C6. The identified fraction in WS bio-oil had carbon numbers from C2 to C20. C2, C3, and C6 compounds were the most abundant in WS bio-oil with 30%, 21%, and 18% of the identified fraction, respectively. The identified fraction in RC bio-oils presented carbon numbers from C4 to C19, with C4 and C18 as the most abundant ones, i.e., 17% and 11% of the identified fraction, respectively.
Table 5. Detailed Composition of the Bio-Oils (wt %) by Group Type and Carbon Number Obtained via GC × GC-FID/TOF-MS for Pinewood-Derived Bio-Oil (PW), Wheat Straw-Derived Bio-Oil (WS), and Rapeseed Cake-Derived Bio-Oil (RS)a.
| carbon number |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| group type | bio-oil | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| NA OX | PW | 7.18 | 2.14 | 0.36 | 0.29 | 0.43 | 0.13 | 0.03 | 0.03 | |||||||||||
| WS | 5.09 | 3.58 | 1.16 | 0.39 | 0.82 | 0.33 | 0.04 | 0.003 | 0.01 | 0.03 | 0.07 | 0.05 | 0.04 | 0.02 | 0.004 | |||||
| RS | 0.88 | 0.05 | 0.30 | 0.14 | 0.07 | 1.34 | ||||||||||||||
| HTC OX | PW | 0.62 | 0.64 | 0.54 | 0.15 | |||||||||||||||
| WS | 0.40 | 0.39 | 0.03 | 0.01 | ||||||||||||||||
| RS | 0.46 | I | ||||||||||||||||||
| CBHD | PW | 7.05 | ||||||||||||||||||
| WS | 1.24 | 0.03 | ||||||||||||||||||
| RS | ||||||||||||||||||||
| NA HC | PW | |||||||||||||||||||
| WS | 0.003 | 0.02 | 0.005 | 0.003 | 0.01 | |||||||||||||||
| RS | 0.12 | 0.31 | 0.23 | 0.24 | 0.46 | 0.43 | 0.16 | 0.31 | 0.15 | 0.55 | 0.13 | 0.39 | 0.95 | |||||||
| NA NIT | PW | |||||||||||||||||||
| WS | ||||||||||||||||||||
| RS | 0.09 | 1.65 | ||||||||||||||||||
| A OX | PW | 0.50 | 0.76 | 1.19 | 0.57 | |||||||||||||||
| WS | 0.58 | 1.23 | 0.71 | 0.34 | 0.44 | 0.12 | 0.01 | 0.03 | ||||||||||||
| RS | 0.50 | 0.66 | 0.22 | - | 0.31 | |||||||||||||||
| A HC | PW | |||||||||||||||||||
| WS | ||||||||||||||||||||
| RS | 0.39 | 0.26 | 0.11 | |||||||||||||||||
| A NIT | PW | |||||||||||||||||||
| WS | ||||||||||||||||||||
| RS | 0.17 | 0.21 | 0.13 | |||||||||||||||||
| total | PW | 7.18 | 2.14 | 0.98 | 0.93 | 8.53 | 0.89 | 1.36 | 0.57 | 0.03 | ||||||||||
| WS | 5.09 | 3.58 | 1.16 | 0.79 | 3.03 | 1.57 | 0.78 | 0.36 | 0.45 | 0.12 | 0.03 | 0.01 | 0.05 | 0.07 | 0.07 | 0.04 | 0.02 | 0.034 | ||
| RS | 1.34 | 0.55 | 1.16 | 0.97 | 0.43 | 0.68 | 0.57 | 0.73 | 0.16 | 0.53 | 0.15 | 0.62 | 0.13 | 2.04 | 2.29 | |||||
NA OX nonaromatic oxygenates (i.e., alcohols, carboxylic acids, aldehydes, and ketones); HTC OX heterocyclic oxygenates (i.e., furans, pyrans, and others); CBHD carbohydrates; NA HC nonaromatic hydrocarbons; NA NIT nonaromatic nitrogenates; A OX aromatic oxygenates; A HC aromatic hydrocarbons; A NIT aromatic nitrogenates.
The total mass fractions of bio-oil quantified in the present study compare favorably with those reported in literature (5–25 wt %).16,51 Most of the published studies on GC × GC analysis of bio-oils report concentrations in terms of relative peak area percentage and a direct comparison with our results is not possible.52,53 Additionally, the total mass fractions reported in our study correspond to peaks annotated with names of plausible compounds. A reasonable water content of 25 wt % can be assigned to the bio-oil from PW based on published studies in which bio-oil produced from the same feedstock and by the same company has been used.54,55 Noticeably, the elemental composition reported for those PW bio-oils closely matches the elemental composition of the PW bio-oil from our study with differences in mass percentages for C, H, and O of less than 5%. Water and the quantified fraction account for around 50 wt % of the bio-oil from PW. A water content of 28.4 ± 0.45 wt % was measured using Karl Fischer titration for the bio-oil from WS. A mass fraction of 46 wt % was then quantified for this bio-oil. Finally, hydrogen and oxygen balances based on the elemental composition combined with results from the 13C NMR analysis, show that the water content of RC bio-oil is around 10 wt %. This water content is consistent with that reported for bio-oil from the same feedstock and produced at similar pyrolysis temperature.30 When water and the quantified mass fraction are combined, around 20 wt % of this bio-oil was quantified, i.e., less than half in comparison to the other studied bio-oils.
PW bio-oil and WS bio-oil contained relatively high and comparable amounts of acetic acid. In addition, these bio-oils also contained significant amounts of glycolaldehyde and 1-hydroxy-2-propanone. PW bio-oil stood out due to its high content of levoglucosan (≈6 wt %), which was the most abundantly present compound in the current investigation. This high content of levoglucosan is in agreement with the 13C NMR results for this bio-oil. The nitrogenated compound (Z)-9-octadecenamide was the most abundant one in RC bio-oil.
Of the identified compounds, nonaromatic ones were present in the highest concentrations. In PW bio-oil, these nonaromatic compounds were followed in abundance by the carbohydrates, aromatics and heterocyclic compounds. A different order was observed for WS bio-oil in which aromatics were detected in an amount exceeding that of the carbohydrates. In RC bio-oil no carbohydrates were detected, while the aromatics were found to be present in a higher amount than the detected heterocyclic compounds. Despite the limited fraction that was detected and quantified by GC × GC-FID/TOF-MS, the general trends observed in the main groups of compounds are in line with the results from the 13C NMR analysis.
Our results for the main groups in PW bio-oil are in agreement with those for the PW bio-oil from Cheng et al.56 who reported carbohydrates as the major class of the identified compounds. Cheng et al.56 also reported significant amounts of nonaromatics, ketones, and carboxylic acids in PW bio-oil, which is also in agreement with the current results. Compounds that were found in PW bio-oil at concentrations exceeding 1.0 wt % in our analysis, i.e., acetic acid, levoglucosan, and glycolaldehyde, were also found in PW bio-oil at concentrations higher than 1.0 wt % by Djokic et al.16 Our results for the main groups in WS bio-oil match those obtained by Charon et al.10 The current results are also in agreement with those of Charon et al.10 for individual nonaromatic compounds that were found at high concentrations in WS bio-oil: i.e., acetic acid, glycolaldehyde, and 1-hydroxy-2-propanone. The significant amount of these light oxygenates in WS bio-oil is consistent with the high amounts of alkali metals in wheat straw, which can act as catalyst for secondary cracking reactions.10,57
Smets et al. reported the presence of free fatty acids in RC bio-oil, which are expected to be degradation products of triglycerides.30 Additionally, in the same study, gel permeation chromatography (GPC) revealed the presence of fatty acids and triglycerides in RC bio-oil. Finally, the peak of triglycerides increased with the pyrolysis temperature. The fatty acids identified by Smets et al. in RC bio-oil via GC-MS analysis were 9-octadecenoic acid, 9,12-octadecadienoic acid and hexadecanoic acid.30 Fatty acids were also found in RC bio-oil in the present study, as specifically tetradecanoic acid and hexadecanoic acid have been identified. In addition, significant amounts of 11-octadecenoic acid, methyl ester and trans-13-octadecenoic acid, methyl ester were also found in RC bio-oil. Comparable amounts (≈3 wt %) of aromatic compounds were identified in PW bio-oil and RC bio-oil, while the identified phenols were found in higher concentrations in WS bio-oil and RC bio-oil (i.e., ≈1 wt %). Finally, methoxy- and dimethoxyphenol derivatives were detected in relatively high amounts in PW bio-oil (1.5 wt %).
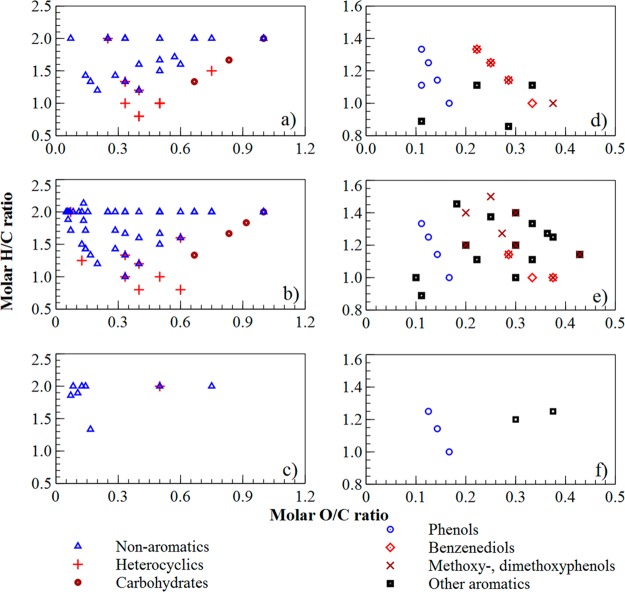
To have a more detailed overview of the chemical composition of the different bio-oils under study Van Krevelen (VK) diagrams for the aromatic and nonaromatic fractions (hydrocarbons and nitrogenated compounds are excluded) have been constructed; see Figure 3. VK diagrams for the nonaromatic oxygenates show that ketones and carboxylic acids, which are the most abundant among the identified compounds, are broadly distributed in terms of O/C and H/C ratios. Carbohydrates are well separated at the right-hand side of the VK diagram due to its high O/C molar ratios, which vary from 0.67 to 1. Heterocyclic compounds exhibit the lowest H/C ratios with a maximum value of 1.6.
Figure 3.
Van Krevelen diagrams from the detailed chemical characterization of bio-oil by GC × GC-FID/TOF-MS. Nonaromatic and aromatic oxygenates by group type identified in pinewood-based bio-oil (a, d), wheat straw-based bio-oil (b, e), and rapeseed cake-based bio-oil (c, f).
The group of compounds at the top of the left-hand side of the VK diagrams for nonaromatic oxygenates (i.e., molar O/C ratios of 0.05–0.15; molar H/C ratios of 1.8–2.0) exhibits carbon numbers from C15 to C20 and probably originated from extractives for the case of PW and WS bio-oil, and from extractives and triglycerides for the case of RC bio-oil. On the other hand, aromatic compounds are concentrated between O/C ratios from 0.1 to 0.43 and H/C ratios from 0.86 to 1.45. Besides, phenols appeared well separated from benzenediols and methoxy-dimethoxy phenol derivatives. Other aromatic oxygenates were more distributed and comprise indenes, substituted benzenediols, aromatic carboxylic acids, and aromatic ketones among others.
Chemical Application of Bio-Oils
Phenolic compounds in bio-oils have attracted attention because of its potential uses as fuel additives and chemical precursors.7,58 Some phenols and phenol derivatives can be used after separation as food antioxidants, transportation fuel additives, precursors for chemical products (pesticides, dyes, pharmaceutical products), and in the resin industry.58 In addition, formaldehyde could be suitable for the production of phenol-formaldehyde resins in the polymer industry. Thirty-nine different phenolic monomers, e.g., phenols, benzenediols, and methoxy- and dimethoxyphenol derivatives, etc., were identified and quantified in this work using GC × GC-FID/TOF-MS. Phenolic compounds detected in amounts ≥0.5 wt % were phenol in RC bio-oil, and 2-methoxy-phenol, also denoted as guaiacol, and 4-methylguaiacol in PW bio-oil. Feedstock screening and optimization of fast pyrolysis operating conditions for the enrichment of phenolics can be carried out based on the quantitative information provided by the current combined GC × GC-FID/TOF-MS approach.
Levoglucosan as major compound found in PW bio-oil could be used for the manufacturing of pharmaceuticals, surfactants, and biodegradable polymers. Low molecular carbonyl compounds such as acetaldehyde, glycolaldehyde (hydroxyacetaldehyde), and 2-furaldehyde (furfural) and volatile carboxylic acids are very reactive at ambient conditions with negative impact on bio-oil quality and storage infrastructure. Within this context, GC × GC-FID/TOF-MS can provide quantitative results, which can assist in the selection of additives for stabilization.
The acidity of fast pyrolysis bio-oils is caused mainly by volatile carboxylic acids.59 The carboxylic acids with molecular weight below 100 detected using GC × GC are acetic acid, propionic acid, 3-butenoic acid, butyric acid, and 2-methyl-propanoic (isobutyric) acid. Taking the concentrations of low molecular weight carboxylic acids as an indicator of acidity, the PW and WS bio-oils showed similar results (4.9 and 4.3 wt %, respectively). In contrast, the RC bio-oil showed a rather low value (0.14 wt %). A similar trend for acidity is also observed based on total nonaromatic carboxylic acids (4.9 and 4.9 vs 1.0 wt %) and on total nonaromatic carbonyl (including carboxylic acids, aldehydes, ketones, and esters, 10 and 11 vs 2.4 wt %). The typical pH range for bio-oils from lignocellulosic materials is 2–3,22 while the reported pH for RC bio-oil is notably much higher (≈7).30 These results confirm the application of GC × GC for assessing acidity and corrosivity of bio-oils.
In addition to the presented chromatographic techniques, which are usually employed for the identification of individual components, spectroscopic methods assist in the chemical group analysis. NMR analyses give strategic information on the type and relative percentage of chemical functionalities present in bio-oils. As already mentioned, alkyl hydrocarbon groups contribute significantly to the energy content of bio-oils. About 66% of gasoline and 80% of diesel fuels have alkyl hydrocarbons.60 The content of alkyl hydrocarbons and consequently the overall energy value of the studied bio-oils follows the trend of rapeseed cake > wheat straw > pinewood. The aromatic contents are important for synthetic modifications when considering the solubility of a feedstock for downstream processing or end products derivatives. The aromatic contents of bio-oils follow the following trend: wheat straw > rapeseed cake ≈ pinewood. On the other hand, the carbonyl contents, such as aldehyde or ketones, provide useful information for modification or further improvements of bio-oil.15 Finally, NMR analysis can also provide an insight into the stability of bio-oil. The methoxy/hydroxy, carbohydrate, and carbonyl carbon contents of bio-oil can be seen as an stability indicator.61 In our case, as expected, the stability order is RC bio-oil with 5% of unstable carbon followed by WS bio-oil with 30% of unstable carbon and finally PW bio-oil with 70% of unstable carbon. These results indicate PW bio-oil is prone to phase separation upon storage and separating its carbohydrate fraction could be desirable.
Conclusions
A detailed chemical analysis of bio-oils produced from the fast pyrolysis of pinewood, rapeseed, and wheat straw was performed using a combination of quantitative 13C NMR spectroscopy and GC × GC-FID/TOF-MS, providing new insights for the development of bio-oil upgrading strategies. 13C NMR analysis showed that pinewood bio-oil was rich in methoxy/hydroxyl groups and carbohydrates, while rapeseed cake bio-oil was rich in alkyl hydrocarbons. On the other hand, wheat straw bio-oil contained high amount of aromatics and alkyl hydrocarbons. The alkyl hydrocarbon content of the bio-oils as energy value index showed the following trend: rapeseed cake > wheat straw > pinewood. Using a GC × GC-FID/TOF-MS analytical approach, more than 200 individual compounds have been identified and quantified. Nonaromatic oxygenates were the most abundant compounds in all investigated bio-oils. Pinewood and wheat straw bio-oils contained significant amounts of volatile carboxylic acids. Pinewood in particular contained significant amounts of low molecular weight aldehydes, which rises concern regarding its stability and suitability for long-term storage before being used in biorefinery operations. The results indicate that rapeseed cake bio-oil is chemically more stable and relatively noncorrosive when compared with the two other bio-oils. In addition, valuable phenolic compounds were identified and quantified in all bio-oils.
Acknowledgments
This work is financially supported by the CAPITA-WAVES: WAste biofeedstocks hydro-Valorisation processES, CAPITA-13-6 Project and the European Research Council under the European Union’s Seventh Framework Programme FP7/2007-2013/ERC grant agreement no. 290793. Dr. Pieter Bruijnincx and Dr. Sandra Constant, both from Utrecht University, are greatly acknowledged for the scientific discussions of the NMR data.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.6b01329.
List of identified compounds and detailed composition of the bio-oils obtained via a GC × GC-FID/TOF-MS analytical approach (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Venderbosch R. H.; Prins W. Fast pyrolysis technology development. Biofuels, Bioprod. Biorefin. 2010, 4 (2), 178–208. 10.1002/bbb.205. [DOI] [Google Scholar]
- Bridgwater A. V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. 10.1016/j.biombioe.2011.01.048. [DOI] [Google Scholar]
- Howe D.; Westover T.; Carpenter D.; Santosa D.; Emerson R.; Deutch S.; Starace A.; Kutnyakov I.; Lukins C. Field-to-Fuel Performance Testing of Lignocellulosic Feedstocks: An Integrated Study of the Fast Pyrolysis–Hydrotreating Pathway. Energy Fuels 2015, 29 (5), 3188–3197. 10.1021/acs.energyfuels.5b00304. [DOI] [Google Scholar]
- Shen D.; Jin W.; Hu J.; Xiao R.; Luo K. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: Structures, pathways and interactions. Renewable Sustainable Energy Rev. 2015, 51, 761–774. 10.1016/j.rser.2015.06.054. [DOI] [Google Scholar]
- Kan T.; Strezov V.; Evans T. J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renewable Sustainable Energy Rev. 2016, 57, 1126–1140. 10.1016/j.rser.2015.12.185. [DOI] [Google Scholar]
- Manganaro J.; Chen B.; Adeosun J.; Lakhapatri S.; Favetta D.; Lawal A.; Farrauto R.; Dorazio L.; Rosse D. J. Conversion of Residual Biomass into Liquid Transportation Fuel: An Energy Analysis. Energy Fuels 2011, 25 (6), 2711–2720. 10.1021/ef200327e. [DOI] [Google Scholar]
- Staš M.; Kubička D.; Chudoba J.; Pospíšil M. Overview of analytical methods used for chemical characterization of pyrolysis bio-oil. Energy Fuels 2014, 28 (1), 385–402. 10.1021/ef402047y. [DOI] [Google Scholar]
- Anca-couce A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. 10.1016/j.pecs.2015.10.002. [DOI] [Google Scholar]
- Kanaujia P. K.; Sharma Y. K.; Agrawal U. C.; Garg M. O. Analytical approaches to characterizing pyrolysis oil from biomass. TrAC, Trends Anal. Chem. 2013, 42, 125–136. 10.1016/j.trac.2012.09.009. [DOI] [Google Scholar]
- Charon N.; Ponthus J.; Espinat D.; Broust F.; Volle G.; Valette J.; Meier D. Multi-technique characterization of fast pyrolysis oils. J. Anal. Appl. Pyrolysis 2015, 116, 18–26. 10.1016/j.jaap.2015.10.012. [DOI] [Google Scholar]
- Michailof C. M.; Kalogiannis K. G.; Sfetsas T.; Patiaka D. T.; Lappas A. A. Advanced analytical techniques for bio-oil characterization. Wiley Interdiscip. Rev.: Energy Environ. 2016, 10.1002/wene.208. [DOI] [Google Scholar]
- Joseph J.; Baker C.; Mukkamala S.; Beis S. H.; Wheeler M. C.; DeSisto W. J.; Jensen B. L.; Frederick B. G. Chemical shifts and lifetimes for nuclear magnetic resonance (NMR) analysis of biofuels. Energy Fuels 2010, 24 (9), 5153–5162. 10.1021/ef100504d. [DOI] [Google Scholar]
- Landucci L. L. Quantitative 13C NMR characterization of lignin 1. A methodology for high precision. Holzforschung 1985, 39 (6), 355–360. 10.1515/hfsg.1985.39.6.355. [DOI] [Google Scholar]
- Sudasinghe N.; Cort J. R.; Hallen R.; Olarte M.; Schmidt A.; Schaub T. Hydrothermal liquefaction oil and hydrotreated product from pine feedstock characterized by heteronuclear two-dimensional NMR spectroscopy and FT-ICR mass spectrometry. Fuel 2014, 137, 60–69. 10.1016/j.fuel.2014.07.069. [DOI] [Google Scholar]
- Mullen C. A.; Strahan G. D.; Boateng A. A. Characterization of various fast-pyrolysis bio-oils by NMR spectroscopy. Energy Fuels 2009, 23 (5), 2707–2718. 10.1021/ef801048b. [DOI] [Google Scholar]
- Djokic M. R.; Dijkmans T.; Yildiz G.; Prins W.; van Geem K. M. Quantitative analysis of crude and stabilized bio-oils by comprehensive two-dimensional gas-chromatography. J. Chromatogr. A 2012, 1257, 131–140. 10.1016/j.chroma.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Toraman H. E.; Dijkmans T.; Djokic M. R.; Van Geem K. M.; Marin G. B. Detailed compositional characterization of plastic waste pyrolysis oil by comprehensive two-dimensional gas-chromatography coupled to multiple detectors. J. Chromatogr. A 2014, 1359, 237–246. 10.1016/j.chroma.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Marsman J. H.; Wildschut J.; Mahfud F.; Heeres H. J. Identification of components in fast pyrolysis oil and upgraded products by comprehensive two-dimensional gas chromatography and flame ionisation detection. J. Chromatogr. A 2007, 1150 (1), 21–27. 10.1016/j.chroma.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Kloekhorst A.; Wildschut J.; Heeres H. J. Catalytic hydrotreatment of pyrolytic lignins to give alkylphenolics and aromatics using a supported Ru catalyst. Catal. Sci. Technol. 2014, 4 (8), 2367–2377. 10.1039/C4CY00242C. [DOI] [Google Scholar]
- Dijkmans T.; Van Geem K. M.; Djokic M. R.; Marin G. B. Combined comprehensive two-dimensional gas chromatography analysis of polyaromatic hydrocarbons/polyaromatic sulfur-containing hydrocarbons (PAH/PASH) in complex matrices. Ind. Eng. Chem. Res. 2014, 53 (40), 15436–15446. 10.1021/ie5000888. [DOI] [Google Scholar]
- Dijkmans T.; Djokic M. R.; Van Geem K. M.; Marin G. B. Comprehensive compositional analysis of sulfur and nitrogen containing compounds in shale oil using GC x GC – FID/SCD/NCD/TOF-MS. Fuel 2015, 140, 398–406. 10.1016/j.fuel.2014.09.055. [DOI] [Google Scholar]
- Oasmaa A.; van de Beld B.; Saari P.; Elliott D. C.; Solantausta Y. Norms, Standards, and Legislation for Fast Pyrolysis Bio-oils from Lignocellulosic Biomass. Energy Fuels 2015, 29 (4), 2471–2484. 10.1021/acs.energyfuels.5b00026. [DOI] [Google Scholar]
- Tomasini D.; Cacciola F.; Rigano F.; Sciarrone D.; Donato P.; Beccaria M.; Caramão E. B.; Dugo P.; Mondello L. Complementary Analytical Liquid Chromatography Methods for the Characterization of Aqueous Phase from Pyrolysis of Lignocellulosic Biomasses. Anal. Chem. 2014, 86 (22), 11255–11262. 10.1021/ac5038957. [DOI] [PubMed] [Google Scholar]
- FAO Food and Agricultural Organization of the United Nations. http://www.fao.org (July 14, 2016).
- Wolde B.; Lal P.; Alavalapati J.; Burli P.; Munsell J. Factors affecting forestland owners’ allocation of non-forested land to pine plantation for bioenergy in Virginia. Biomass Bioenergy 2016, 85, 69–75. 10.1016/j.biombioe.2015.12.007. [DOI] [Google Scholar]
- ECN Phyllis2, Database for biomass and waste. https://www.ecn.nl/phyllis2/ (June 23, 2016).
- Ruiz H. A.; Ruzene D. S.; Silva D. P.; da Silva F. F. M.; Vicente A. A.; Teixeira J. A. Development and characterization of an environmentally friendly process sequence (autohydrolysis and organosolv) for wheat straw delignification. Appl. Biochem. Biotechnol. 2011, 164 (5), 629–641. 10.1007/s12010-011-9163-9. [DOI] [PubMed] [Google Scholar]
- FEDIOL The EU Vegetable Oil and Proteinmeal Industry. http://www.fediol.be/ (July 14, 2016).
- Özçimen D.; Karaosmanoğlu F. Production and characterization of bio-oil and biochar from rapeseed cake. Renewable Energy 2004, 29 (5), 779–787. 10.1016/j.renene.2003.09.006. [DOI] [Google Scholar]
- Smets K.; Adriaensens P.; Reggers G.; Schreurs S.; Carleer R.; Yperman J. Flash pyrolysis of rapeseed cake: Influence of temperature on the yield and the characteristics of the pyrolysis liquid. J. Anal. Appl. Pyrolysis 2011, 90 (2), 118–125. 10.1016/j.jaap.2010.11.002. [DOI] [Google Scholar]
- Venderbosch R.; Prins W. Fast pyrolysis of biomass for energy and chemicals: technologies at various scales. Sustainable Dev. Process Ind. 2010, 109. 10.1002/9780470586099.ch7. [DOI] [Google Scholar]
- Ben H.; Ragauskas A. J. NMR characterization of pyrolysis oils from kraft lignin. Energy Fuels 2011, 25 (5), 2322–2332. 10.1021/ef2001162. [DOI] [Google Scholar]
- Scanlon J. T.; Willis D. E. Calculation of flame ionization detector relative response factors using the effective carbon number concept. J. Chromatogr. Sci. 1985, 23 (8), 333–340. 10.1093/chromsci/23.8.333. [DOI] [Google Scholar]
- Beens J.; Boelens H.; Tijssen R.; Blomberg J. Quantitative aspects of comprehensive two-dimensional gas chromatography (GC x GC). J. High Resolut. Chromatogr. 1998, 21 (1), 47–54. . [DOI] [Google Scholar]
- Schofield K. The enigmatic mechanism of the flame ionization detector: Its overlooked implications for fossil fuel combustion modeling. Prog. Energy Combust. Sci. 2008, 34 (3), 330–350. 10.1016/j.pecs.2007.08.001. [DOI] [Google Scholar]
- Vassilev S. V.; Baxter D.; Andersen L. K.; Vassileva C. G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. 10.1016/j.fuel.2009.10.022. [DOI] [Google Scholar]
- Channiwala S. A.; Parikh P. P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81 (8), 1051–1063. 10.1016/S0016-2361(01)00131-4. [DOI] [Google Scholar]
- Zhou G.; Jensen P. A.; Le D. M.; Knudsen N. O.; Jensen A. D. Direct upgrading of fast pyrolysis lignin vapor over the HZSM-5 catalyst. Green Chem. 2016, 18 (7), 1965–1975. 10.1039/C5GC01976A. [DOI] [Google Scholar]
- Mahmood R.; Parshetti G. K.; Balasubramanian R. Energy, exergy and techno-economic analyses of hydrothermal oxidation of food waste to produce hydro-char and bio-oil. Energy 2016, 102, 187–198. 10.1016/j.energy.2016.02.042. [DOI] [Google Scholar]
- Zaafouri K.; Trabelsi A. B. H.; Krichah S.; Ouerghi A.; Aydi A.; Claumann C. A.; Wüst Z. A.; Naoui S.; Bergaoui L.; Hamdi M. Enhancement of biofuels production by means of co-pyrolysis of Posidonia oceanica (L.) and frying oil wastes: Experimental study and process modeling. Bioresour. Technol. 2016, 207, 387–398. 10.1016/j.biortech.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Ucar S.; Ozkan A. R. Characterization of products from the pyrolysis of rapeseed oil cake. Bioresour. Technol. 2008, 99 (18), 8771–8776. 10.1016/j.biortech.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Pstrowska K.; Walendziewski J.; Stolarski M. Hydrorefining of oil from rapeseed cake pyrolysis over NiMo/Al2O3 catalyst. Fuel Process. Technol. 2014, 128, 191–198. 10.1016/j.fuproc.2014.07.035. [DOI] [Google Scholar]
- Ingram L.; Mohan D.; Bricka M.; Steele P.; Strobel D.; Crocker D.; Mitchell B.; Mohammad J.; Cantrell K.; Pittman C. U. Jr Pyrolysis of wood and bark in an auger reactor: physical properties and chemical analysis of the produced bio-oils. Energy Fuels 2008, 22 (1), 614–625. 10.1021/ef700335k. [DOI] [Google Scholar]
- Lin F.; Waters C. L.; Mallinson R. G.; Lobban L. L.; Bartley L. E. Relationships between Biomass Composition and Liquid Products Formed via Pyrolysis. Front. Energy Res. 2015, 3, 45. 10.3389/fenrg.2015.00045. [DOI] [Google Scholar]
- Torri I. D. V.; Paasikallio V.; Faccini C. S.; Huff R.; Caramão E. B.; Sacon V.; Oasmaa A.; Zini C. A. Bio-oil production of softwood and hardwood forest industry residues through fast and intermediate pyrolysis and its chromatographic characterization. Bioresour. Technol. 2016, 200, 680–690. 10.1016/j.biortech.2015.10.086. [DOI] [PubMed] [Google Scholar]
- Ghaffar S. H.; Fan M. Lignin in straw and its applications as an adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. 10.1016/j.ijadhadh.2013.09.001. [DOI] [Google Scholar]
- Ben H.; Ragauskas A. J. In situ NMR characterization of pyrolysis oil during accelerated aging. ChemSusChem 2012, 5 (9), 1687–1693. 10.1002/cssc.201200429. [DOI] [PubMed] [Google Scholar]
- Stedile T.; Ender L.; Meier H. F.; Simionatto E. L.; Wiggers V. R. Comparison between physical properties and chemical composition of bio-oils derived from lignocellulose and triglyceride sources. Renewable Sustainable Energy Rev. 2015, 50, 92–108. 10.1016/j.rser.2015.04.080. [DOI] [Google Scholar]
- Black S. K.; Ferrell J. R. III Determination of Carbonyl Groups in Pyrolysis Bio-oils using Potentiometric Titration: Review and Comparison of Methods. Energy Fuels 2016, 10.1021/acs.energyfuels.5b02511. [DOI] [Google Scholar]
- Dutriez T.; Courtiade M.; Thiébaut D.; Dulot H.; Bertoncini F.; Vial J.; Hennion M. High-temperature two-dimensional gas chromatography of hydrocarbons up to nC 60 for analysis of vacuum gas oils. J. Chromatogr. A 2009, 1216 (14), 2905–2912. 10.1016/j.chroma.2008.11.065. [DOI] [PubMed] [Google Scholar]
- Michailof C.; Sfetsas T.; Stefanidis S.; Kalogiannis K.; Theodoridis G.; Lappas A. Quantitative and qualitative analysis of hemicellulose, cellulose and lignin bio-oils by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1369, 147–160. 10.1016/j.chroma.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Tessarolo N. S.; dos Santos L. R. M.; Silva R. S. F.; Azevedo D. A. Chemical characterization of bio-oils using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1279, 68–75. 10.1016/j.chroma.2012.12.052. [DOI] [PubMed] [Google Scholar]
- Lazzari E.; Schena T.; Primaz C. T.; da Silva Maciel G. P.; Machado M. E.; Cardoso C. A.; Jacques R. A.; Caramão E. B. Production and chromatographic characterization of bio-oil from the pyrolysis of mango seed waste. Ind. Crops Prod. 2016, 83, 529. 10.1016/j.indcrop.2015.12.073. [DOI] [Google Scholar]
- Staš M.; Chudoba J.; Kubička D.; Pospíšil M. Chemical characterization of pyrolysis bio-oil: application of Orbitrap mass spectrometry. Energy Fuels 2015, 29 (5), 3233–3240. 10.1021/acs.energyfuels.5b00407. [DOI] [Google Scholar]
- Leijenhorst E. J.; Wolters W.; Van de Beld L.; Prins W. Autothermal catalytic reforming of pine wood derived fast pyrolysis-oil in a 1.5 kg/h pilot installation: Aspects of adiabatic operation. Fuel Process. Technol. 2013, 115, 164–173. 10.1016/j.fuproc.2013.05.015. [DOI] [Google Scholar]
- Cheng T.; Han Y.; Zhang Y.; Xu C. Molecular composition of oxygenated compounds in fast pyrolysis bio-oil and its supercritical fluid extracts. Fuel 2016, 172, 49–57. 10.1016/j.fuel.2015.12.075. [DOI] [Google Scholar]
- Carpenter D.; Westover T. L.; Czernik S.; Jablonski W. Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16 (2), 384–406. 10.1039/C3GC41631C. [DOI] [Google Scholar]
- Toraman H. E.; Vanholme R.; Borén E.; Vanwonterghem Y.; Djokic M. R.; Yildiz G.; Ronsse F.; Prins W.; Boerjan W.; Van Geem K. M.; Marin G. B. Potential of genetically engineered hybrid poplar for pyrolytic production of bio-based phenolic compounds. Bioresour. Technol. 2016, 207, 229–236. 10.1016/j.biortech.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Oasmaa A.; Peacocke C.. Properties and fuel use of biomass-derived fast pyrolysis liquids; VTT Publications: Finland, 2010; Vol. 731, p 79. [Google Scholar]
- Strahan G. D.; Mullen C. A.; Boateng A. A. Characterizing biomass fast pyrolysis oils by 13C NMR and chemometric analysis. Energy Fuels 2011, 25 (11), 5452–5461. 10.1021/ef2013166. [DOI] [Google Scholar]
- Meng J.; Moore A.; Tilotta D. C.; Kelley S. S.; Adhikari S.; Park S. Thermal and Storage Stability of Bio-Oil from Pyrolysis of Torrefied Wood. Energy Fuels 2015, 29 (8), 5117–5126. 10.1021/acs.energyfuels.5b00929. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.