Abstract
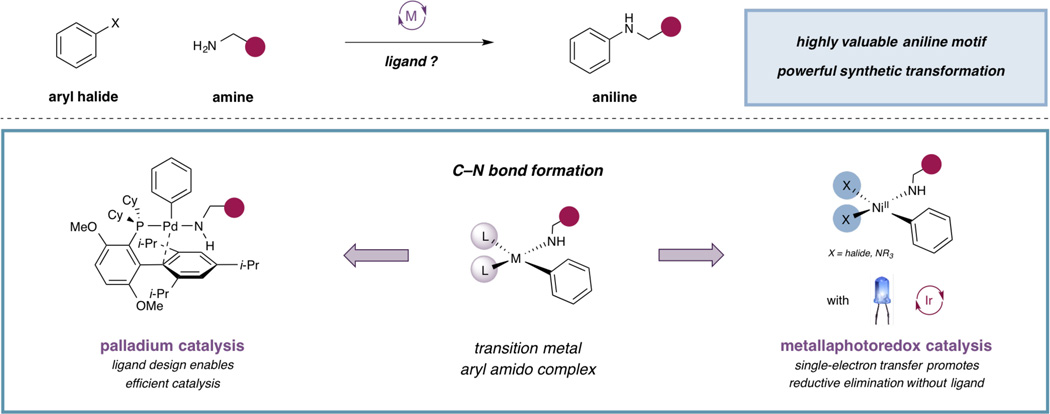
Over the past two decades, there have been major developments in transition metal-catalyzed aminations of aryl halides to form anilines, a common structure found in drug agents, natural product isolates, and fine chemicals. Many of these approaches have enabled highly efficient and selective coupling through the design of specialized ligands, which facilitate reductive elimination from a destabilized metal center. We postulated that a general and complementary method for C–N bond formation could be developed through the destabilization of a metal amido complex via photoredox catalysis, thus providing an alternative approach to the use of structurally complex ligand systems. Herein, we report the development of a distinct mechanistic paradigm for aryl amination using ligand-free nickel(II) salts, in which facile reductive elimination from the nickel metal center is induced via a photoredox-catalyzed electron-transfer event.
Transition metal-catalyzed cross-coupling reactions are fundamental methods for constructing complex molecular architectures. Key among these reactions has been the formation of carbon–nitrogen bonds to access anilines, a common structure found in medicinal agents and other complex molecules, through the coupling of amines with aryl halides or pseudohalides (1–6). Over the past two decades, multiple generations of ligands have been designed that have elevated palladium-catalyzed aryl amination to an essential transformation with both predictable reactivity and practical significance. The ligand frameworks that have been developed for palladium catalysis often employ a few key design elements (i.e., sterically encumbered architecture, hemilabile coordinating groups, electronically-tuned substituents) to enact high catalytic efficiency (7). One essential feature in ligand design is the capacity for destabilization of the palladium(II) amido complex, which induces reductive elimination resulting in C–N bond formation (8–10).
The broad success of ligated-palladium catalysis has hinged on the ability to selectively design, synthesize, and implement ligand classes to enable specific coupling reactions in high levels of efficiency and selectivity. Inspired by the effectiveness of structurally diverse ligand systems on palladium reductive elimination, we recognized that a general and complementary approach for productive C–N bond formation could be developed through an alternative mechanistic approach. In particular, we envisioned that the destabilization of a metal amido species could occur through an electron transfer pathway via photoredox catalysis, thus inducing facile carbon–nitrogen bond formation analogous to the role of ligand in traditional palladium couplings (Figure 1).
Figure 1. Proposed approach to C–N cross-coupling – promotion of reductive elimination via single-electron transfer.
We proposed that nickel could be an ideal transition metal for this catalytic platform due to its known proclivity to participate in one-electron processes (11–13). Furthermore, this ligand-free mechanism could potentially broaden the applicability of nickel catalysis in aryl amination, (14–18) a system that is often plagued with the use of harsh reductants and air-sensitive nickel(0) complexes. Nickel(II) amido complexes, as detailed by Hillhouse and others, tend not to undergo reductive elimination at ambient temperature (19–21). Computations suggest/reveal that, C–N reductive elimination from nickel(II) alkyl amido complexes is thermodynamically disfavored, in contrast to the exothermic reductive elimination from analogous palladium(II) alkyl amido complexes (22). Exposure of nickel(II) amido complexes to various oxidants results in facile reductive elimination, presumably through single-electron oxidation of the nickel(II) amido complex to the respective nickel(III) complex, lending support to our design strategy (19, 20).
In recent years, the merging of photoredox catalysis with nickel catalysis has enabled both C–O (23) and C–S (24, 25) bond formation through the generation of reactive radical intermediates and/or the single-electron modulation of nickel oxidation states (26, 27). The feasibility of these reactions arises from the capacity of photocatalysts to act as both strong single-electron oxidants and reductants upon irradiation with visible light (28). Critical to the success of our design plan, the photoredox catalyst would be involved in two key aspects of our postulated mechanism: i) reduction of a nickel(II) salt to the active Ni(0) catalyst through the use of a mild sacrificial reductant and ii) electron transfer to destabilize the nickel(II) amido complex towards reductive elimination in the absence of an exogenous ligand.
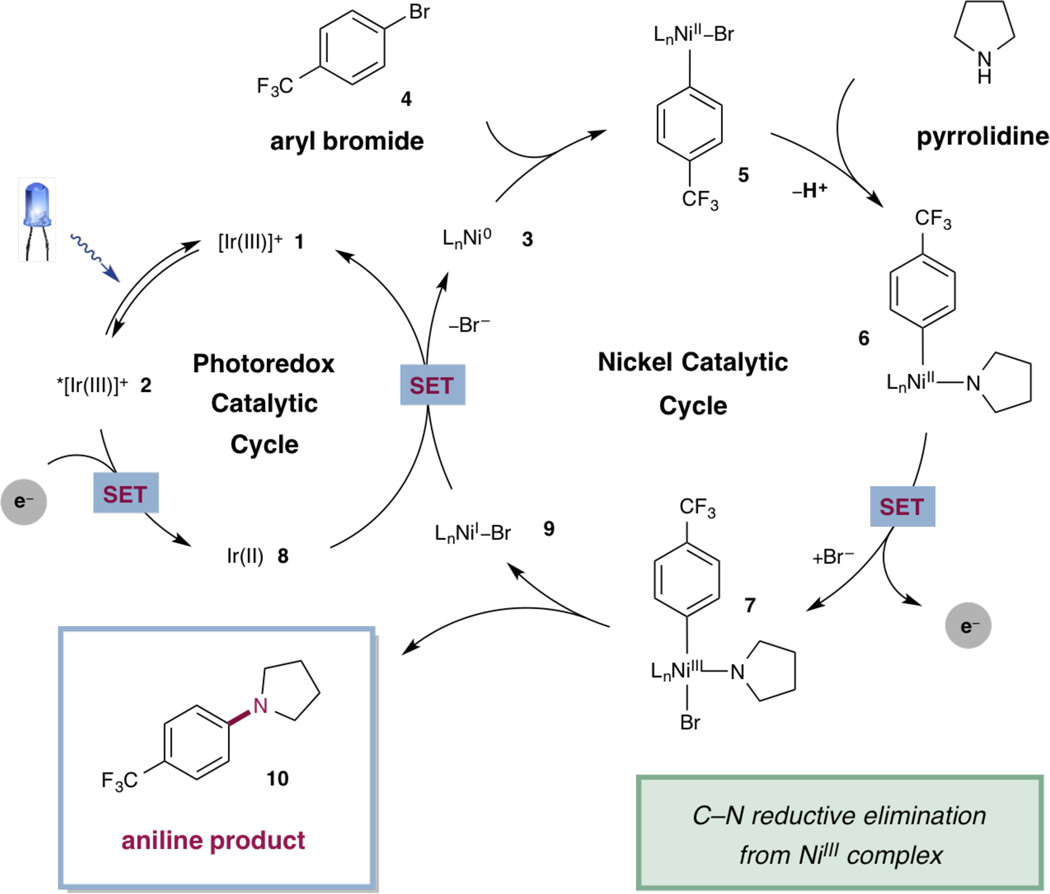
In our proposed mechanism (Fig. 2), irradiation of heteroleptic iridium(III) photocatalyst Ir[dF(CF)3ppy]2 (dtbbpy)PF6 [dF(CF3)ppy = 2-(2,4--difluorophenyl)-5-(trifluoromethyl)pyr-idine; dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine] (1) with visible light would produce long-lived triplet photoexcited state *IrIII 2 (τ = 2.3 µs) (29). Concomitantly, nickel(0) complex 3 would oxidatively insert into bromoarene 4 to yield the corresponding nickel(II) aryl bromide complex 5. The nickel(II) complex would then undergo ligand exchange and subsequent deprotonation to arrive at Ni(II)-aryl amido complex 6. This nickel(II) complex could then participate in a single-electron transfer (SET) event with the photoexcited *IrIII catalyst (E1/2red [*IrIII/IrII] = + 1.21 V versus saturated calomel electrode (SCE) in CH3CN) to afford Ni(III) complex 7 and the reduced iridium(II) photocatalyst (8) (29). Reductive elimination of the resultant nickel(III) complex would yield nickel(I) bromide species 9 and desired aniline product 10. Both the photoredox and nickel catalytic cycles are closed upon a SET event between Ni(I)Br species 9 and Ir(II) (E1/2red [IrIII/IrII] = −1.37 V versus SCE in CH3CN) in which nickel(0) catalyst 3 and ground state iridium(III) catalyst 1 are regenerated simultaneously (29). Importantly, the homogeneous nickel species is presumably coordinated to solvent, base, and/or amine throughout the process (denoted as Ln in Fig. 2), but would not require a designed ligand for efficient reactivity.
Figure 2. Proposed mechanism for the metallaphotoredox-catalyzed amination reaction.
L, DABCO or amine; SET, single-electron transfer.
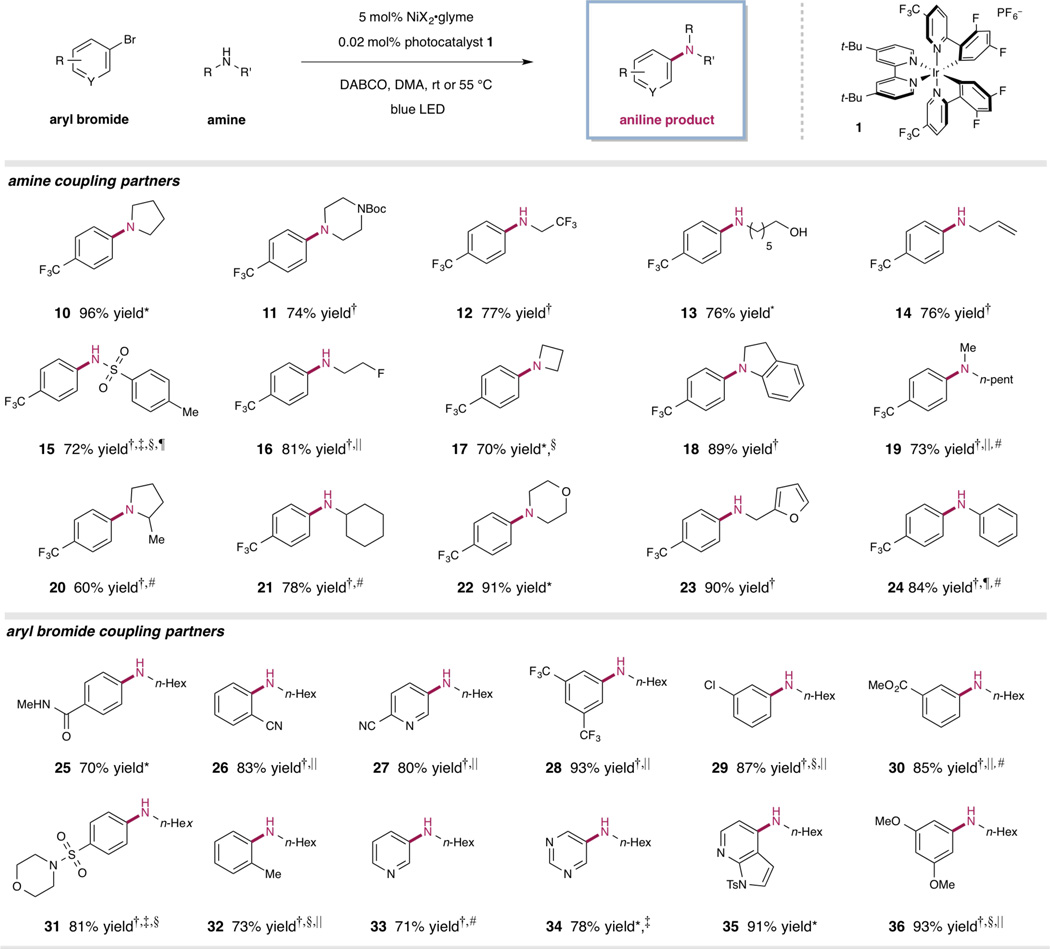
With this mechanistic hypothesis in hand, we first examined the proposed cross-coupling reaction with 4-bromobenzotrifluoride, pyrrolidine, nickel(II) bromide glyme, and a variety of ligands, bases, and solvents (see Supplementary Materials for details). The employment of photocatalyst 1, 1,4-diazobicyclo[2.2.2] nonane (DABCO) as a base, and di-tert-butyl-2,2′-bipyridine as a supporting ligand provided the respective aniline product 10 in 72% yield in the presence of blue light. Control experiments established the importance of both nickel and photocatalyst, as no formation of the desired cross-coupled product was observed in the absence of either nickel, photocatalyst, or light. We sought to investigate the effectiveness of this protocol in the absence of ligand to confirm our initial mechanistic hypothesis. The cross-coupling reaction proceeded with enhanced reactivity in the absence of di-tert-butyl-2,2′-bipyridine, delivering the desired aniline product in 96% yield. This result supports our proposed mechanistic pathway in Fig. 2 and the capacity of visible light and a photocatalyst to initiate reductive elimination in the absence of ligand.
With the optimal conditions in hand, we sought to demonstrate the generality of this ligand-free amination. As highlighted in Table 3, a wide variety of functional groups were tolerated on the amine coupling partner, including protected nitrogen atoms, trifluoromethyl groups, alcohols, alkenes, and sulfonamides (11–15, 72–77% yield). The high efficiency of the photoredox catalytic cycle is evidenced by the low photocatalyst loadings (≤ 0.02 mol%) required to effect the desired cross-coupling reactions. Monofluorinated amines (16) coupled cleanly under these conditions in 81% yield. Coupling of amine hydrogen chloride salts was accomplished in good yield by utilizing MTBD (7-methyl-1,5,7-diazabicyclo-[4.4.0]deca-5-ene) as the base (16 and 17, 81% and 70% yield); although the expense of MTBD often limits its practicality, synthetically useful yields were also obtained with DABCO in most cases (see Supplementary Materials for details). Chemoselective N-arylation in the presence of alcohols was also achieved, as no C–O coupling was observed (13, 76% yield, single regioisomer).
We found that α-substitution on the amine partner was tolerated in moderate yields (20 and 22, 60% and 78% yield, respectively), but hindered amines, e.g., tert-butylamine, did not productively couple. Although nucleophilic amines, such as pyrrolidine and morpholine, coupled in good efficiencies (10 and 22, 96% and 91% yield, respectively) at ambient temperature after only a few hours, less nucleophilic substrates, such as trifluoroethylamine (12, 77% yield), allylamine (14, 76% yield), and furfurylamine (23, 90% yield), required longer reaction times (>24 h). Amines lacking α-hydrogens failed to react under these reaction conditions. We hypothesized that an initial β-hydride elimination event is prerequisite for accessing the active Ni (0) catalyst in this reaction. Indeed, the addition of a substoichiometric amount of amine possessing α-hydrogens, such as pyrrolidine, to the reaction resulted in good yields (72% and 84% yield) of cross-coupled products 15 and 24, respectively, with less than 10% of the pyrrolidine-coupled product being observed. For a number of substrates, product formation was observed in high yield utilizing Ru(bpy)3(PF6)2 as the photocatalyst (see Fig. S8). In cases where the yield is lower, protodehalogenation, along with trace phenol, aryl ether, or aryl chloride formation, can account for the mass balance of the reaction.
A variety of aryl bromides containing diverse functional groups, such as nitriles, amides, trifluoromethyl groups, halides, esters, and sulfonamides, performed well under the reaction conditions (25–31, 70–93% yield), with substitution at the ortho position being tolerated (26 and 32, 83% and 73% yield, respectively) (Figure 3). Six-membered heteroaromatic substrates were effectively coupled in good yields (27 and 33–35, 71–91% yield). Unfortunately, a series of aryl bromides, e.g. select heterocycles and a 2,6-disubstituted arene, failed to couple under the current reaction conditions (see Fig. S8). For electron-rich arenes (32 and 36), lowering the photocatalyst loading (0.002 mol%) increased reaction efficiency and inhibited competitive protodehalogenation, as did the utilization of MTBD as base. Additionally, the initial evaluation of this C–N cross-coupling platform in flow chemistry has shown high efficiency in decreased reaction times (see Supplementary Materials).
Figure 3. Metallaphotoredox-catalyzed amination: amine and arene scope.
For each entry number (in boldface), data are reported as percent isolated yield. R, H, alkyl, or aryl substrate; Y, C, CH, or N; X, CI or Br; DABCO, 1,4-diazabicyclo[2.2.2]octane; MTBD, 7-methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene; LED, light-emitting diode; Me, methyl; n-pent, n-pentyl; Boc, tert-butoxycarbonyl. *Run at ambient temperature. †Reaction heated to 55 °C. ‡DMSO used as solvent. §MTBD used as base. ‖0.002 mol% 1 used. ¶10 mol% pyrrolidine included. #See Supplementary Materials for details.
To investigate the role of the photocatalyst in switching on nickel-catalyzed aryl amination, Ni(cod)2 was employed. In the absence of light, the formation of aniline 10 was severely diminished (10% yield), while standard reaction conditions yielded 10 in 91% yield. This result supports our hypothesis that the cross-coupling is light-mediated and the effect of the photoredox catalyst is not limited to the reduction of a Ni(II) salt to the active Ni(0) species.
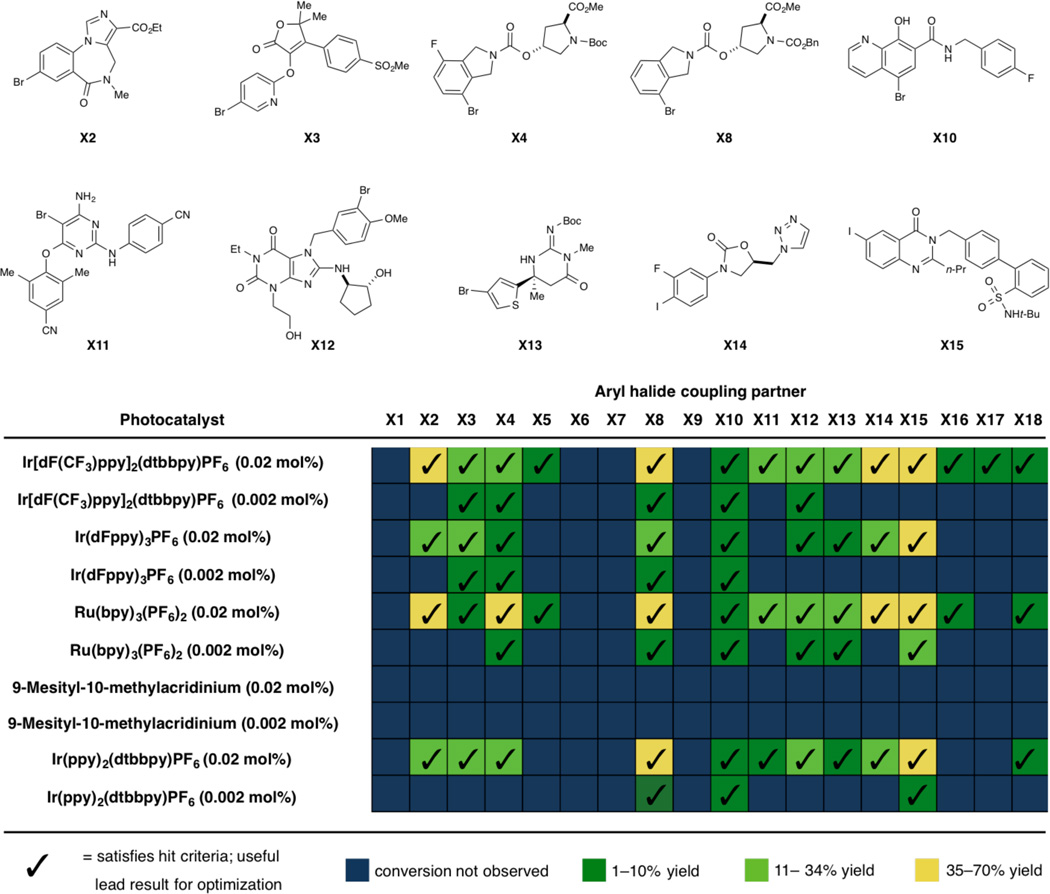
Lastly, this C–N cross-coupling platform was evaluated in a high throughput fashion using a microscale informer plate as developed by Merck laboratories. A chemistry informer library of eighteen complex, drug-like aryl halides was subjected to Ni-photoredox based amination conditions with piperidine. Remarkably, 78% of substrates underwent successful cross-coupling and provided “reaction hits” that were suitable for optimization (Fig. 4) (30). Benchmarked against other protocols that have been evaluated with this informer series, this study represents one of the most successful (31), generic catalysis platforms for aryl amination that has been evaluated within Merck. Based on these results, we are confident that this new, complementary method will find broad applicability in the synthesis of structurally diverse, drug-like compounds.
Figure 4. High throughput metallaphotoredox-catalyzed aryl amination using a chemistry informer library.
For each compound numbered in bold, the cross-coupling was performed with piperidine using DABCO as a base. Ac, acetyl; Bn, benzyl; Boc, tert-butoxycarbonyl; Et, ethyl; Me, methyl; n-Pr, n-propyl; t-Bu, tert-butyl. Conversion was quantified by ultra pressure liquid chromatography/mass spectrometry (UPLC/MS) using an internal standard. See Supplementary Materials for exact yields and conditions.
Through the merger of photoredox and nickel catalysis, challenging C–N reductive elimination can be switched on through the use of visible light and a photocatalyst via the intermediacy of a Ni(III) oxidation state (32–34). This strategy represents a complementary approach to traditional ligated-palladium catalysis through the use of a distinct mechanistic pathway for reductive elimination, which will likely be broadly applicable across a range of substrate classes.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award numbers GM58160 and RO1-GM078201-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M. T. P. thanks the National Institutes of Health for a postdoctoral fellowship (GM113311).
Footnotes
Supplementary Materials
Materials and Methods
Supplementary Text
Fig. S1 to S6
References (34–48)
NMR Spectra
References
- 1.Ullmann F. Chem. Ber. 1903;36:2382. [Google Scholar]
- 2.Goldberg I. Chem. Ber. 1906;39:1691. [Google Scholar]
- 3.Kosugi M, Kameyama M, Migita T. Chem. Lett. 1983:927. [Google Scholar]
- 4.Driver MS, Hartwig JF. J Am. Chem. Soc. 1996;118:7217. [Google Scholar]
- 5.Chan D. Tetrahedron Lett. 1996;37:9013. [Google Scholar]
- 6.Guram AS, Buchwald SL. J Am. Chem. Soc. 1994;116:7901. [Google Scholar]
- 7.Surry DS, Buchwald SL. Chem. Sci. 2011;2:27. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J Am. Chem. Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartwig JF. Synlett. 1997:329. [Google Scholar]
- 10.Brusoe AT, Hartwig JF. J Am. Chem. Soc. 2015;137:8460. doi: 10.1021/jacs.5b02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsou TT, Kochi JK. J Am. Chem. Soc. 1979;101:6319. [Google Scholar]
- 12.There is a report regarding the use of ligated nickel and photoredox catalysis for C–N bond formation (13).
- 13.Tasker SZ, Jamison TF. J Am. Chem. Soc. 2015;137:9531. doi: 10.1021/jacs.5b05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe JP, Buchwald SL. J Am. Chem. Soc. 1997;119:6054. [Google Scholar]
- 15.Gao C-Y, Yang L-M. J Org. Chem. 2008;73:1624. doi: 10.1021/jo7022558. [DOI] [PubMed] [Google Scholar]
- 16.Park NH, Teverovskiy G, Buchwald SL. Org. Lett. 2014;16:220. doi: 10.1021/ol403209k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge SG, Green RA, Hartwig JF. J Am. Chem. Soc. 2014;136:1617. doi: 10.1021/ja411911s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimasahi T, Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2010;49:2929. doi: 10.1002/anie.200907287. [DOI] [PubMed] [Google Scholar]
- 19.Koo K, Hillhouse GL. Organometallics. 1995;14:4421. [Google Scholar]
- 20.Lin BL, Clough CR, Hillhouse GL. J Am. Chem. Soc. 2002;124:2890. doi: 10.1021/ja017652n. [DOI] [PubMed] [Google Scholar]
- 21.Ilies L, Matsubara T, Nakamura E. Org. Lett. 2012;14:5570. doi: 10.1021/ol302688u. [DOI] [PubMed] [Google Scholar]
- 22.MacGregor SA, Neave GW, Smith C. Faraday Discuss. 2003;124:111. doi: 10.1039/b212309f. [DOI] [PubMed] [Google Scholar]
- 23.Terrett JA, Cuthbertson JD, Shurtleff VW, MacMillan DWC. Nature. 2015;524:330. doi: 10.1038/nature14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oderinde MS, Frenette M, Robbins DW, Aquila B, Johannes JW. J Am. Chem. Soc. 2016;138:1760. doi: 10.1021/jacs.5b11244. [DOI] [PubMed] [Google Scholar]
- 25.Jouffroy M, Kelly CB, Molander GA. Org. Lett. 2016;18:876. doi: 10.1021/acs.orglett.6b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry MS, Goldsmith JI, Slinker JD, Rohl R, Pascal RA, Jr, Malliaras GG, Bern hard S. Chem. Mater. 2005;17:5712. [Google Scholar]
- 30.Previous studies leveraging high throughput experimentation at Merck validated 5% yield as a good screening hit and 20% yield as a robust hit.
- 31.Kutchukian PS, Dropinski JF, Dykstra KD, Li B, DiRocco DA, Streckfuss EC, Campeau L-C, Cernak T, Vachal P, Davies IW, Krsksa SW, Dreher SD. Chem. Sci. 2016;7:2604. doi: 10.1039/c5sc04751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.There has been a report detailing NiCl2 as a heterogeneous catalyst for the N-arylation of aryl iodides under microwave conditions in the absence of an exogenous ligand (33).
- 33.Gupta AK, Rao GT, Singh KN. Tetrahedron Lett. 2012;53:2218. [Google Scholar]
- 34.While we favor the mechanism outlined in Figure 2, we cannot rule out the possibility of energy transfer or direct excitation of nickel (II) in the presence of visible light.
- 35.Brenner E, Schneider R, Fort Y. Tetrahedron. 1999;55:12829. [Google Scholar]
- 36.Manolikakes G, Gavryushin A, Knochel P. J Org. Chem. 2008;73:1429. doi: 10.1021/jo702219f. [DOI] [PubMed] [Google Scholar]
- 37.Luo H, Wu G, Zhang Y, Wang J. Angew. Chem. Int. Ed. 2015;54:14503. doi: 10.1002/anie.201507219. [DOI] [PubMed] [Google Scholar]
- 38.Kita Y, Sakaguchi H, Hoshimoto Y, Nakau chi D, Nakahara Y, Carpentier J-F, Ogoshi S, Mashima K. Chem. Eur. J. 2015;21:14571. doi: 10.1002/chem.201502329. [DOI] [PubMed] [Google Scholar]
- 39.Liwosz TW, Chemler SR. Chem. Eur. J. 2013;19:12771. doi: 10.1002/chem.201301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riedmüller S, Nachtsheim BJ. Synlett. 2015;26:651. [Google Scholar]
- 41.Barker TJ, Jarvo ER. Angew. Chem. Int. Ed. 2011;50:8325. doi: 10.1002/anie.201103700. [DOI] [PubMed] [Google Scholar]
- 42.Martin AR, Nelson DJ, Meiries S, Slawin AMZ, Nolan SP. Eur. J. Org. Chem. 2014;15:3127. [Google Scholar]
- 43.Dhayalan V, Sämann C, Knochel P. Chem. Commun. 2015;51:3239. doi: 10.1039/c4cc08846h. [DOI] [PubMed] [Google Scholar]
- 44.DeAngelis A, Wang D-H, Buchwald SL. Angew. Chem. Int. Ed. 2013;52:3434. doi: 10.1002/anie.201208544. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 46.Fors BP, Watson DA, Biscoe MR, Buchwald SL SL. J Am. Chem. Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagaw S, Buchwald SL. J Org. Chem. 1996;61:7240. doi: 10.1021/jo9612739. [DOI] [PubMed] [Google Scholar]
- 48.Kampmann SS, Skelton BW, Wild DA, Koutsantonis GA, Stewart SG. Eur. J. Org. Chem. 2015:5995. doi: 10.1107/S2053229615001680. [DOI] [PubMed] [Google Scholar]
- 49.Halperin SD, Kwon D, Holmes M, Regala do E, Campeau L-C, DiRocco DA, Britton R. Org. Lett. 2015;17:5200. doi: 10.1021/acs.orglett.5b02532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.