Abstract
The epidemiology of vector-borne pathogens is determined by mechanisms and interactions at different scales of biological organization, from individual-level cellular processes to community interactions between species and with the environment. Most research, however, focuses on one scale or level with little integration between scales or levels within scales. Understanding the interactions between levels and how they influence our perception of vector-borne pathogens is critical. Here two examples of biological scales (pathogen transmission and mosquito mortality) are presented to illustrate some of the issues of scale and to explore how processes on different levels may interact to influence mosquito-borne pathogen transmission cycles. Individual variation in survival, vector competence, and other traits affect population abundance, transmission potential, and community structure. Community structure affects interactions between individuals such as competition and predation, and thus influences the individual-level dynamics and transmission potential. Modeling is a valuable tool to assess interactions between scales and how processes at different levels can affect transmission dynamics. We expand an existing model to illustrate the types of studies needed, showing that individual-level variation in viral dose acquired or needed for infection can influence the number of infectious vectors. It is critical that interactions within and among biological scales and levels of biological organization are understood for greater understanding of pathogen transmission with the ultimate goal of improving control of vector-borne pathogens.
Keywords: mosquito-borne pathogen, vector, scale, transmission
In the classic children’s book Horton Hears a Who (Seuss 1954), Horton can hear the Whos living on a dust speck. However, others cannot hear them and are convinced nothing can exist on such a small scale. In the end, they become aware that all scales are important. In the case of vector-borne pathogens, a better understanding of how processes at different scales ultimately determine patterns of transmission and disease is needed to improve risk assessment.
Issues of scale and connections between scales have been topics of interest in biology and ecology since Darwin’s entangled bank (Darwin 1859). The concept of scale is difficult to define precisely and is best understood by example (Table 1). Spatial scales can be measured absolutely from microns through kilometers, or be defined by the biology of the system under study. In that sense, spatial scales range from micro scales, local to the organism (e.g., specific feeding locations, mating sites, etc.), through regional spaces supporting communities, to macro scales (e.g., continental or global). Likewise, temporal scales can use conventional measurements such as days, years, centuries, or system-relevant concepts such as the duration of an epidemic, seasonal changes, or projections for climate change. Both spatial and temporal scales have been extensively studied in ecology and epidemiology (e.g., Wiens 1989, Levin 1992, Tilman and Kareiva 1997, Ostfeld et al. 2005, Riley 2007, Reisen 2010, Hastings et al. 2011, Paull et al. 2012, Salje et al. 2012).
Table 1.
Examples of different types of scales, points along those axes, and some key processes that occur at those points for vector-borne pathogen systems
| Level | Scale axis |
||
|---|---|---|---|
| Spatial | Temporal | Biological organization | |
| Micro | Local | Days–months | Within individual |
| Adult microhabitat choice | Epidemics | Immunology | |
| Gene expression | |||
| Vector competence | |||
| Intermediate | Regional | Multi-year | Populations |
| Niche modeling | Seasonality, endemic cycles | Vectorial capacity | |
| Watershed dynamics | Invasion biology | ||
| Transmission dynamics | |||
| Macro | Global | Decadal and longer | Community/Ecosystem |
| Comparative epidemiology | Effects of climate change | Food webs | |
| Eco-epidemiology | |||
The scales are continuous and we denote specific points along them for convenience in discussion; many processes occur at multiple levels. Interactions between the scale axes and between the points along the scales are common and an integral part of understanding how issues of scale impact vector-borne disease and study design and interpretation.
A less obvious scale is biological organization, which has had only limited attention (e.g., Blair 2004, Tompkins et al. 2011). This scale spans processes and interactions from within the individual (e.g., genes, cells) through population and community levels (interactions between species) to global processes (Table 1). Specific biological traits or processes can be considered over multiple levels of organization (e.g., Blair 2004 studied bird nesting success at different levels). There is increasing interest in understanding the connections between different levels of organization or scales in disease systems (e.g., Matthews and Haydon 2007, Real and Biek 2007) and their effects on pathogen epidemiology and population regulation (Tompkins et al. 2011). Heterogeneity between individuals, and the interaction of individuals with spatio-temporal heterogeneity in environmental factors, can be critical in control of mosquito-borne disease (e.g., Lambrechts et al. 2009). This is a major contributing factor to the effect of scaling and interaction between scales or levels on pathogen epidemiology. While terminology has varied in these studies, we will use scale to refer to the dimension being considered (spatial, temporal, different dimensions within biological organization) and level to refer to points along those scales, as given above.
Levins (1992), using the term scale to denote points along the spatial scale, noted that there is no single or correct scale, and this is true for other scales as well. Organisms experience multiple spatial, temporal, and biological scales and levels within scales. It is critical that researchers be aware of the scales and levels at which observations are made and consider this in interpretation. Levels within scales are also not discrete but continuous. It is convenient to think of specific levels (individual, community, etc.) but there is overlap. However, the difference between the individual-level processes (occurring within an individual organism) and broader scale processes (interactions between individuals) is more discrete, and we will focus on that difference here.
There is a critical gap in our understanding of how processes on different scales of biological organization or at different levels influence transmission of vector-borne disease. Making connections between different scales is critical to our understanding of pathogen systems, as shown by Tompkins et al. (2011), who illustrated the links between within-organism dynamics and broader ecological scales for a parasite or pathogen using transmission between hosts as a theme (see their Fig. 2). For example, if the mechanisms controlling individual-level vector competence are critical components of transmission dynamics, then information at this level is needed for risk prediction in each population. Conversely, if an approximation using a constant transmission rate is adequate, then the individual-level detail becomes less important. Similar assessments will be necessary for many individual-level mechanisms and their impact on population and community levels. Modeling the dynamics of processes such as transmission and mortality will be valuable in assessing their importance to the epidemiology of vector-borne disease. Such studies should also assess how large-scale patterns in environment and community structure affect pathogen transmission at the individual level.
A key issue in vector-borne pathogen research is translation of field or laboratory studies to understanding the larger epidemiological system. For example, recent studies have shown that an individual mosquito’s susceptibility to infection with a pathogen can be affected by small changes in environmental factors (e.g., Kilpatrick et al. 2008, Richards et al. 2009) and within-individual factors (e.g., binding of viruses to midgut proteins, Mercado-Curiel et al. 2008), likely influencing population- and species-level variation in vector competence. However, how this individual variation affects transmission dynamics beyond the individual level is not known. Models have shown that the average transmission rate can influence transmission dynamics (e.g., Anderson and May 1991 and references therein; Lord and Day 2001a,b; Wonham et al. 2004, 2006), but the role of individual-level variation has not been explored extensively. Similarly, individual-level mechanisms affecting survival and movement patterns of mosquitoes lead to observable spatio-temporal mosquito abundance patterns, influencing transmission risk over space and time. Although some of these mechanisms have been studied (e.g., Day and Curtis 1993, Le Menach et al. 2005, Bustamante 2009), greater understanding of their effects on transmission dynamics is needed. We do not know when individual-level variation affects the risk of mosquito-borne disease or the ability of models to predict transmission.
This article examines two factors (transmission and mosquito mortality) involved in the epidemiology of mosquito-borne pathogens across the scale of biological organization, using selected examples to illustrate key points. We also consider how modeling can be used to investigate these factors, and the information needed to determine the level of detail needed in such models. Although our discussion focuses on a few arbovirus systems, the same issues apply to other vector-borne pathogens.
Transmission Between Vertebrate Hosts and Mosquitoes
Transmission between vertebrate hosts and mosquitoes is critical in determining the number of infectious mosquitoes, a major component in the risk of transmission at a population or community level. At the population level, this is often represented as a constant, as the proportion of mosquitoes becoming infectious after biting an infected vertebrate host. However, transmission occurs at an individual level and is more accurately considered a probabilistic process (Lord et al. 2006). The processes determining infection, dissemination, and transmission of an arbovirus in an individual mosquito are influenced by a variety of biotic and abiotic factors (Hardy et al. 1983, Tabachnick 1994, Beerntsen et al. 2000). Whether differences in these individual-level processes affect dynamics at coarser (e.g., ecosystem) scales, or how critical heterogeneity among individuals may be is unknown. Do we need to include details at the individual level to predict the risk of infection? It is critical that we improve our understanding of the connections between scales and levels if we hope to translate information from small scales to larger scale predictions. Vector competence can be considered an individual trait, whether or not an individual mosquito can be infected and transmit a pathogen. However, it can be considered at a broader scale, as the proportion of a population or species able to be infected and transmit. Following common usage in the literature, we use the term in both contexts, but with the different definitions as given above.
Individual Level
While acknowledging the importance of vertebrate hosts, we focus our discussion on the mosquito.
Vertebrate Host Viremia and Mosquito Infection
Ingestion of virions by a vector depends on the presence and concentration of virus in the vertebrate host blood. Viremias may be short lived (hours to days; e.g., St. Louis encephalitis virus [SLEV], McLean and Scott 1979; eastern equine encephalitis virus, Weaver et al. 1991), but can vary over time within one individual vertebrate host, between individuals of one species, and between species. After ingestion, infection and dissemination of arboviruses are often positively related to the dose in the infectious bloodmeal (Dohm et al. 2002; Tiawsirisup et al. 2004; Mahmood et al. 2006; Reisen et al. 2008; Pesko et al. 2009; Richards et al. 2009, 2010; Anderson et al. 2010). However, information about mechanisms governing the relationship between vertebrate host viremia and mosquito infection is limited.
Mosquito infection and infectiousness (ability to transmit), as a function of vertebrate host viremia are often treated as a threshold (e.g., Reisen et al. 2005, Turell et al. 2005), where only viremias above some fixed level result in infection. However, a probabilistic model, where the probability of becoming infected or infectious is a continuous function of dose, may be more realistic. In a probabilistic model, vertebrate hosts with different levels of viremia could be important in arbovirus transmission (Lord et al. 2006).
The number of infectious mosquitoes resulting from one viremic vertebrate host will depend on a complex interplay of mosquito and vertebrate host factors and the relationship between viremia and probability of vector infection (Lord et al. 2006). Correlations between infection or viremia in the vertebrate host and the probability of being bitten, as has been shown in malaria (e.g., Rossignol and Shieh 1993), may also be important and impact viral epidemiology. The relationship between vertebrate host viremia and mosquito infection for many arbovirus transmission cycles is unknown. Differences in vertebrate community composition (community level) affect the temporal and spatial availability of infectious hosts, influencing the viremias available for vector infection. Although diversity in the vertebrate host community at the species level has been considered (e.g., the dilution effect, Schmidt and Ostfeld 2001, LoGiudice et al. 2003, Keesing et al. 2006), the effects of variable levels of viremia at the individual level on pathogen epidemiology has hardly been explored.
Vector Competence
Biological transmission includes acquisition of pathogens from a bloodmeal, infection of midgut cells, dissemination to salivary glands and other tissues, and transmission during probing and feeding on a subsequent vertebrate host (horizontal) or to progeny (vertical; Hardy et al. 1983, Higgs and Beaty 2005). The vector also must survive the extrinsic incubation period (EIP), the period between ingestion of the pathogen and the ability to transmit it to a vertebrate host.
Mosquito barriers which prevent arbovirus transmission to subsequent vertebrate hosts may be mosquito species and virus strain-specific (e.g., Lorenz et al. 1984; Turell et al. 2001a,b; 2005; Davis et al. 2005; Moudy et al. 2007; Richards et al. 2007, 2009, 2010; Kilpatrick et al. 2008; Brault 2009; Kenney et al. 2012) and can be modified by environmental factors, such as temperature and viral dose (Dohm et al. 2002; Tiawsirisup et al. 2004; Mahmood et al. 2006; Richards et al. 2007, 2009, 2010; Reisen et al. 2008; Pesko et al. 2009; Anderson et al. 2010) or biological factors such as age (Richards et al. 2009, 2010). Environmental conditions encountered during immature stages may also affect adult mosquito barriers to susceptibility (e.g., Takahashi 1976; Grimstad and Haramis 1984; Alto et al. 2008a,b; Westbrook et al. 2010; Muturi and Alto 2011; Muturi et al. 2011). Interactions between environmental and biological factors may generate a complex landscape of infectious vectors that determines transmission dynamics and risk.
Mosquito Immunology
Another group of individual-level processes influencing mosquito–virus interactions and individual susceptibility is the mosquito innate immune response to arboviruses (Christophides et al. 2002; Sanders et al. 2003, 2005; Keene et al. 2004; Xi et al. 2008; Costa et al. 2009; Fragkoudis et al. 2009; Smartt et al. 2009; Souza-Neto et al. 2009; Bartholomay et al. 2010). Symbiotes and commensal microbes interact with the immune system and affect susceptibility and transmission of pathogens in a wide range of vector species (reviewed by Weiss and Aksoy 2011), providing a mechanism for community-level feedback into individual processes. The influence of immune responses at the individual level on the arbovirus transmission dynamics at population and community levels has not been explored.
Models of vertebrate immune systems and pathogen responses have provided valuable insights into within-host dynamics of pathogens (e.g., directly transmitted viruses, Iwasa et al. 2004, Alizon and van Baalen 2008, Fryer et al. 2010, Alizon et al. 2011; malaria, Gravenor et al. 1995; pathogen interactions, Xiao and Bossert 2010). There are few similar studies of the insect immune system (White et al. 2012). Arboviruses must cope with both invertebrate and vertebrate immunology that can impose strong selection on infectivity for both hosts (e.g., Venezuelan equine encephalitis virus, Coffey et al. 2008), but it is unknown how this affects arbovirus epidemiology. Models exploring these issues would be valuable.
Mosquito Abundance
The abundance of the adult vector population is a well known driver in models of transmission dynamics (e.g., Ross 1911, Macdonald 1957, Plaisier et al. 1990, Anderson and May 1991 and references therein), including West Nile virus (WNV) and SLEV (e.g., Lord and Day 2001a,b; Wonham et al. 2004). Many individual-level processes affect mosquito abundance. Individual mosquitoes use different microhabitats that affect developmental processes, resulting in variation between individuals in survival, growth, and reproduction. Temperature affects many factors influencing population abundance (e.g., immature development time, Day et al. 1990a, Reisen 1995, Mahmood and Crans 1998; growth, Day et al. 1990b; flight activity, Bidlingmayer 1974; adult survival, Alto and Juliano 2001, Delatte et al. 2009, Paaijmans et al. 2010, Lambrechts et al. 2011, Alto and Bettinardi 2013). The interaction of climate and individual-level development processes results in variable population dynamics across spatial scales. Climate also influences aspects of community structure, such as larval resources or vertebrate populations, which can affect mosquito survival and reproduction. Individual mosquitoes vary in sugar or blood feeding, and may exhibit repeatable preferences for specific types of bloodmeal hosts (Edman 1989) that affect fecundity and thus population abundance. Such factors have been included in models of mosquito populations or mosquito-borne disease at aggregate levels (e.g., Sota and Mogi 1989; Focks et al. 1995; Lord and Day 2001a,b) but rarely at an individual level, as is needed to assess how individual variation in microclimate choice, norms of reactions, and food choices interact to affect population characteristics. Individual-based models would be useful in understanding how different functional forms for individual-level relationships with environmental variables affect population characteristics. Using an individual-based model, Magori et al. (2009) showed that adult dispersal at an individual level interacted with environmental heterogeneity to affect population density in a relatively small population. Exploratory models would be valuable to identify individual-level factors that can be simplified and where details are needed to predict mosquito abundance and risk of transmission at the population or community levels.
Individual-Level Summary
Relationships among vertebrate host viremia, mosquito susceptibility, and virus diversity have direct implications for understanding larger scale issues. For example, the community composition of vertebrate hosts determines the viremias available to mosquito populations and interacts with individual-level mechanisms resulting in populations of infectious vectors. Individual-level mechanisms govern relationships between vertebrate host viremia, virus diversity, and vector susceptibility, and are essential to understanding the importance of different vertebrate hosts in disease epidemiology. It is important to understand the effects of ecosystem level factors such as community structure and environmental variation on interactions and vector competence at the individual and population level.
Beyond-Individual Levels
Beyond within-individual processes, we consider two types of effects: factors that are by nature functions of aggregated individuals (age structure in a mosquito population), and community interactions, where individuals of different species are interacting (a mosquito feeding on a bird). Clearly, individual-level factors will modify transmission dynamics at coarser levels, as host choice, species abundance, and behavior affects contact between individuals and thus community-level characteristics.
Vertebrate Defensive Behavior
Success in blood feeding depends on host defensive behavior (e.g., avian defensive behavior, Edman and Kale 1971, Webber and Edman 1972, Edman and Scott 1987). Highly defensive vertebrate hosts are unlikely to provide many bloodmeals and thus are unlikely to serve as efficient amplification hosts for viruses. Host defense depends on the total biting pressure experienced and thus on entire mosquito and vertebrate host communities. Community structure can impact individual-level processes by altering the likelihood of success of a feeding attempt and changing the distribution of vertebrate hosts used, consequently affecting vector survival and fecundity.
Mosquito Abundance
The individual-level characteristics discussed above influence the abundance of vectors at population and community levels. Environmental variation differentially influences development times of individuals from different species resulting in the asynchronous emergence of mosquito populations, changes in population age structure, and temporal differences in activity patterns. Seasonality in mosquito vectors can affect the dynamics of vector-borne pathogens (e.g., Anderson and May 1991 and references therein; Lord et al. 1996a,b; Randolph et al. 2000; Lord and Day 2001a,b; Bartley et al. 2002; Wonham et al. 2004; Lord 2010) and interacts with other seasonal elements to affect transmission in modeled systems (e.g., Lord and Day 2001a,b; Lord 2010).
Age Structure
The age structure of mosquito populations change over time, varies between species, and can be influenced by environmental factors (e.g., Ferro et al. 1995, Walker 2001, Vitek et al. 2008, Bustamante 2009). Older mosquito populations present a higher risk of arbovirus transmission (e.g., Day and Curtis 1994) because a higher proportion of the mosquito population may have taken infectious blood-meals and completed the EIP. Models have shown that variations in weather, landscape, and resources (oviposition sites and vertebrate hosts) can influence the age structure of a mosquito population (Bustamante 2009). The mechanisms driving the age structure of a vector population are complex and include individual, species, and environmental factors. Variations in age structure between vector populations may affect community dynamics and vertebrate host behavioral responses, modifying transmission risk at the community or ecosystem level. Conversely, environmental factors affect individual survival, oviposition, and host-seeking behavior, modifying population age structure.
Transmission Cycles
Vector-borne pathogen epidemiology requires the interaction of vectors, vertebrate hosts, and pathogens for transmission. A common approach to modeling these interactions is a population-level compartmental transmission model, focused on selected areas of interest and including a subset of species in a community. Such models require simplifications of the complex individual-level mechanisms and community structure, and the choice of these simplifications will affect the model outcomes and conclusions. In one example, Lord and Day (2001a,b) developed models for SLEV and WNV with the transmission rate defined as a fixed proportion of mosquitoes becoming infectious after biting infectious birds. Transmission rates varied between vertebrate host types (bird species or age-groups), and these rates affected likelihood and severity of outbreaks in bird populations. Assumptions about the transmission term in WNV models affect the modeled transmission dynamics (Wonham et al. 2006). Currently the effect of variation in individual-level mechanisms on pathogen dynamics at the population level is not predictable. Further work is needed to understand whether individual-level variation affects transmission in ways that cannot be simplified into transmission rates averaged over groups such as mosquito species or vertebrate host types.
Community Structure
Interactions at the community level influence vector-borne disease epidemiology. For example, competition between mosquito species for resources, predation, and interactions between pathogens in both vertebrates and mosquitoes may affect the transmission of a particular pathogen. This has not been examined in detail, although there are a few examples of such interactions (Alto et al. 2005, 2008a,b; Juliano 2009).
Diversity in the vertebrate host community variably affects pathogen epidemiology depending on the characteristics of the hosts and their interaction with the pathogen. When all vertebrate hosts are competent to some extent, additional vertebrate host species generally increases pathogen transmission (e.g., Rogers 1988; Lord et al. 1996b, 1997; Randolph and Dobson 2012; Roche et al. 2013). If some vertebrate host species are sources of blood for the vector but are not competent for the pathogen, transmission is reduced (the dilution effect, e.g., Schmidt and Ostfeld 2001, LoGiudice et al. 2003, Keesing et al. 2006, or zooprophylaxis, e.g., Sota and Mogi 1989). Roche et al. (2013) found that a similar effect may occur with multiple vectors. Some pathogens may have multiple vectors that overlap in space or time. Models of WNV indicate that vectors present at different times of the year influence the likelihood of virus establishment and persistence following an introduction, but the variation between vector species in components of transmission should also be considered (Lord 2010). Developing models considering complex associations of vectors and vertebrate hosts will aid in assessing which combinations are epidemiologically important.
Environmental Impact on Spatial and Temporal Pathogen Transmission Patterns: Case Study of SLEV
The interaction of competent mosquito vectors with competent vertebrate hosts depends on their overlap. This is often linked with environmental factors, particularly rainfall and temperature. These affect the population structures of mosquito vectors and avian amplification hosts and, in turn, affect the transmission of mosquito-borne pathogens such as SLEV (Day 2001). Regional climatic conditions directly affect sympatric mosquito and bird populations. The cycling of drought and rainfall drives the regional reproductive behaviors of mosquito vectors such as Culex nigripalpus Theobold (Shaman et al. 2002) and Culex tarsalis Coquillet (Shaman et al. 2010), avian amplification hosts (Shaman et al. 2003), and SLEV and WNV transmission in the southern half of Florida (Shaman et al. 2002) and Colorado (Shaman et al. 2010). For example, Cx. nigripalpus will wait in the gravid state for suitable oviposition conditions. Favorable environmental conditions will continue to produce young adults, but conditions reducing oviposition sites will result in an older female population with gravid females waiting for oviposition sites (Day and Curtis 1994). In contrast, Culex pipiens quinquefasciatus Say oviposit in permanent freshwater habitats (Bentley and Day 1989), so oviposition sites are constantly available. The result is a shortened gonotrophic cycle (Elizondo-Quiroga et al. 2006) and less variation in age structure. These two dramatically different oviposition strategies lead to different interactions with vertebrate hosts.
Epidemic transmission of SLEV in Florida is restricted to the southern half of the state (Day 2001). Epidemic transmission of SLEV requires the coexistence of large numbers of susceptible mosquitoes and avian amplification hosts and environmental conditions that slow the Cx. nigripalpus reproductive cycle. The drought–rainfall cycles found in south Florida result in Cx. nigripalpus transmission of SLEV and WNV. Such rainfall patterns do not occur in north Florida. Modeling of the hydrology in south Florida found patterns of water dynamics associated with SLEV transmission consistent with these biological responses (Shaman et al. 2002). This illustrates how an understanding of environmental modification of vector-borne pathogen cycles at community levels is a prerequisite for determining the spatial and temporal patterns of human disease outbreaks. Inclusion of these connections in models is necessary to improve comprehension of how epidemiology varies in space and time.
Future Directions
Critical questions remain: How does individual variation in mechanisms affect the population-level density of infectious mosquitoes and thus community-level transmission? How does individual mosquito and vertebrate behavior affect contact between mosquitoes and vertebrate hosts, and thus transmission? Experimental work on variation in vector competence mechanisms and the impact on species-level vector competence is critical. Heterogeneity and issues of scale should be considered during experimental design. Models of transmission cycles including individual-level mechanisms and interactions between individuals will be useful in assessing impact.
One approach might be to divide the host population into multiple classes with different average viremias, each affecting a class-dependent transmission rate. This would be expected to behave similarly to the transmission model of Lord and Day (2001a,b), albeit with more vertebrate host classes. The importance of different transmission rates in the vertebrate host classes is likely to depend on the parameter ranges. There are likely regions of parameter space where differences in transmission rates will affect the transmission dynamics; however, to determine this will require data on the interaction of mosquitoes and vertebrate hosts in a variety of systems. Another method might use a probabilistic approach as was used to consider the effect of vertebrate host viremia on vector infection (Lord et al. 2006). Both vertebrate hosts and mosquitoes could be tracked in terms of a distribution across a dimension of “transmissibility.” In some ways, this would be an intermediate between compartmental and individual-based models, with more variation in the interaction between vertebrate hosts and vectors than the compartmental approach but still using groups of individuals. Exploration of nonlinear relationships between environmental (e.g., temperature, rainfall) and biological variables (e.g., vector competence) is critical. Because there are few data available to estimate these relationships, modeling would be largely exploratory, but would focus research on critical areas.
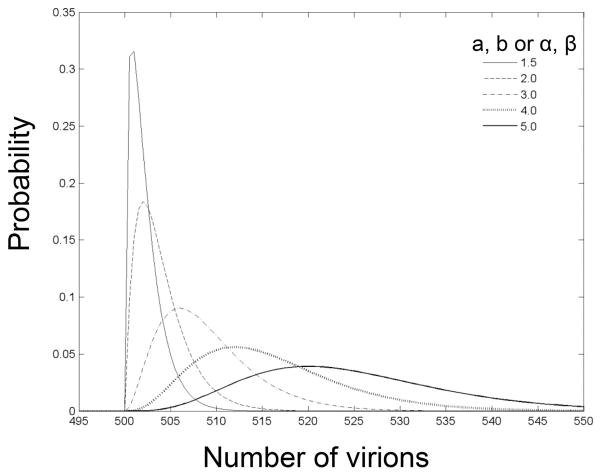
As an example of such exploratory work on the interactions between different processes, consider the probability distribution for the virions needed for a mosquito to become infected in addition to the distribution of virions in the bloodmeal as previously presented (Lord et al. 2006). Rather than assuming the distribution of virions in bloodmeals was normally distributed, and considering fixed numbers of virions needed for infection, we assume the distributions of virions in the bloodmeal and the number of virions needed for infection follow a gamma distribution, with different parameters. The number of virions needed for infection is assumed to be sufficient for the mosquito to become infectious, and losses during the latent period are ignored. The gamma distribution was chosen to illustrate the effect of variation in the shape of the distribution. The true distribution of these parameters is unknown, and is influenced by many of the mechanisms discussed here (e.g., vector competence, vertebrate host factors, virus genetics).
A population of 1,000 mosquitoes was generated, each with a number of virions x needed for infection randomly drawn from a gamma distribution (Devore 1982) with parameters a and b. Likewise, a population of 1,000 birds was generated, each of which would deliver y virions in a mosquito bloodmeal, randomly drawn from a gamma distribution with parameters α and β; 1
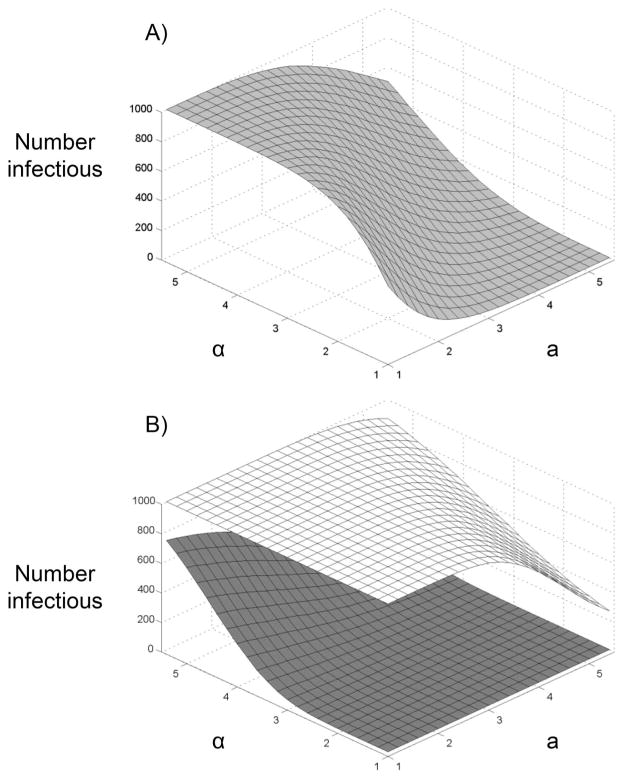
where φ and ψ are offsets that shift the starting point of the gamma distributions. This provides a lower bound to the distribution such that minimum value of x is φ and of y is ψ. Values of 500 or 520 virions were considered for both φ and φ. This placed the distributions in approximately the same range of virions as used previously (Lord et al. 2006). Γ(a) and Γ(α) are the standard gamma functions for a and α, respectively. For convenience, a = b and α = β; these parameters alter the shape of the distribution (Fig. 1). Each mosquito then randomly bit a bird. Birds could be bitten more than once but each mosquito only bit once. For each bite, if y ≥ x the mosquito became infectious; otherwise the mosquito remained uninfected. This was repeated 1,000 times for each value of a and α and for offsets of 500 and 520 (for both x and y), and a total infectious rate for the population (number infectious out of 1,000 mosquitoes) was determined. The highest number of infectious mosquitoes occurs when the distribution of the number of virions needed for infection (x) is relatively peaked (low a), but the virions in the bloodmeal (y) has a flatter distribution (high α; Fig. 2A). The offset also strongly affects the number of infectious mosquitoes in the population, changing the sensitivity to a and α (Fig. 2B). The most sensitivity to a and α is observed when both offsets were the same, indicating that the shapes of the distributions were important in determining the number of infectious mosquitoes. These distributions are likely to be affected by many factors, but currently data are not available to choose distributions or parameter values for specific situations. This example illustrates how models can provide insight into how factors interact and how individual-level mechanisms can influence.
Fig. 1.
Examples of gamma distributions with different parameter values. φ = 500; a = b (equivalently, α = β). Note the different shapes of the distribution with different parameter values. The offset (φ) moves the distributions right such that the curve starts at φ.
Fig. 2.
Number of infectious mosquitoes is affected by parameters of gamma distribution and offsets. (A) φ = ψ = 500; a is the value of both gamma distribution parameters (a = b) for the number of virions needed for infection; α is the value of both gamma distribution parameters (α = β) for the number of virions in the bloodmeal. The number of infectious mosquitoes is maximized when the number of virions in the bloodmeal has a relatively flat distribution (high α) and the number of virions needed for infection has a relatively peaked distribution (low a). Results were similar when both offsets were 520. (B) Sensitivity to the offset values used. Upper plane (white), ψ = 520 and φ = 500. Bottom plane (gray), ψ = 500 and φ = 520. The infection rate is higher and more sensitive to the shape of the distribution when ψ > φ, as this increases the probability that y > x.
In addition to theoretical exploration, empirical studies of how interactions among factors affect vector competence are needed. For example, Richards et al. (2007, 2009, 2010) showed that environmental variables influence mosquito vector competence in complex interactive nonlinear ways. Because of the complexity of the research required, use of modeling studies and appropriate statistical techniques in the planning stages will be essential.
Mosquito Mortality
Vector mortality influences vector-borne pathogen epidemiology, through effects on vector survival through the latent period (Dye 1992, Bellan 2010) and on vector abundance (Juliano 2007). Mortality rates depend on environmental influences, and vary spatially and temporally as well as between vector species or populations. Although vertebrate host mortality can also affect transmission cycles, typically vertebrate life spans are long relative to vector life spans and the duration of infection and so have less effect on the system. Here we will consider only mosquito mortality.
Individual Level
The probability of mortality of an individual mosquito is affected by many factors, including environmental factors such as temperature and humidity and biotic factors such as age, infection with pathogens, and behavior. Interactions with other individuals also affects mortality, either directly through predation or interference competition (Case and Gilpin 1974, Vance 1984, Amarasekare 2002), or indirectly through population-level effects such as vertebrate host defensive behavior and exploitative resource competition (Tilman 1982, Juliano 1998).
Adult Stages
Temperature and adult nutrient reserves (e.g., Nayar 1968; Nayar and Sauerman 1971a,b; 1975a,b,c; Alto and Juliano 2001; Delatte et al. 2009; Paaijmans et al. 2010; Vrzal et al. 2010; Xue et al. 2010; Lambrechts et al. 2011; Alto and Bettinardi 2013) have long been known to affect mortality, and recent work has shown effects of pathogens (Suchman et al. 2006, Dawes et al. 2009, Lambrechts and Scott 2009, McMeniman et al. 2009) and mosquito age (Styer et al. 2007a,b; Harrington et al. 2008; C.C.L., unpublished data). While temperature effects have been included in models (e.g., Focks et al. 1995; Lord and Day 2001a,b), few studies have examined the effects of age or behavior on mortality and consequent effects on community-level characteristics like transmission. Many studies underscore the importance of incorporating nonlinear rates of adult mortality in models of mosquito-borne pathogen transmission (e.g., Clements and Paterson 1981; Styer et al. 2007a,b; Bellan 2010) and heterogeneity between individuals or subgroups may also be important.
Models have shown that adult mosquito mortality affects mosquito-borne disease epidemiology (e.g., Ross 1911; Macdonald 1957; Garrett-Jones 1964; Dye 1986a,b, 1992; Lord and Day 2001b, Luz et al. 2003; Medlock et al. 2009, Bellan 2010), but this has largely been studied using population-level averages. The role of individual-level mechanisms driving mortality and heterogeneity in mortality is not well known.
Age-dependent mortality has been shown in several species, often increasing with age (e.g., Cx. nigripalpus, C.C.L., unpublished data; Aedes aegypti L., Styer et al. 2007a,b; Harrington et al. 2008), or with temperature and age, including interactions between these factors (Anopheles stephensi Liston, Dawes et al. 2009). However, age-dependent mortality has rarely been incorporated into theoretical studies of mosquito-borne pathogens (Bellan 2010). Age-specific hazard rates may change depending on reproductive status (number of gonotrophic cycles) and cumulative blood feeding (e.g., Aedes albopictus Skuse, Leisnham et al. 2008).
Both environmental factors and attributes of mosquito biology (e.g., senescence) contribute to nonlinear mortality rates and increased heterogeneity between individuals. Do we need to explore the individual-level mechanisms, or are population or species-level averages sufficient approximations to understand transmission?
Larval Stages
Larval mortality may have consequences for the phenotypes of survivors, affect other species in the community, and have direct impacts on mosquito population abundance. For example, predator-mediated release from larval competition allows accelerated development (Grill and Juliano 1996; Juliano et al. 2010; Alto et al. 2012a,b) and enhanced growth (Grill and Juliano 1996) among mosquito survivors. Other adult characteristics, such as size, may also be altered in the survivors and consequently affect adult survival and transmission. The presence of predators that induce mortality may lead to trophic cascades in food webs. For instance, predation by the backswimmer, Notonecta maculata F., on larval Culiseta mosquitoes results in increased abundance of diatoms (Blaustein et al. 1995). The mosquito Wyeomyia smithii Coquillet acts as a keystone predator in the pitcher plant Sarracenia purpurea L, and larval density can affect the architecture of the food web (Cochran-Stafira and von Ende 1998, Kneitel and Miller 2002). Such trophic cascades can affect larval resource availability, thus affecting the survival and adult characteristics of the emerging mosquitoes and potentially subsequent larval cohorts. More information is needed on the interactions of food webs involving mosquitoes and the potential effects on transmission.
Control practices inducing mortality in mosquito vectors are often assumed to act additively with other sources of mortality, resulting in lower adult population size. However, both compensatory mortality and overcompensation (Juliano 2007) can occur. Compensatory mortality occurs when the added mortality balances the release from competition, with no net change in the adult population (Washburn et al. 1991, Washburn 1995). Overcompensation occurs when the release from competition with added nondensity dependent mortality allows a greater proportion of the larval population to emerge than in the absence of the additional mortality factor (e.g., control; Service 1985, Washburn et al. 1991, Washburn 1995). For example, larval competition can be severe in container-inhabiting mosquitoes (e.g., Ae. albopictus, Ae. aegypti), such that many individuals die in the larval stage. If competition is reduced (fewer larvae in a container), more individuals may survive to contribute to the adult population. Sources of mortality may act in non-additive ways, particularly if some are density-dependent and some density-independent and the net effect on adult population size may be difficult to predict. Although there are multi-age-class models incorporating immature stages (e.g., Dye 1984, Gimnig et al. 2002, Ahumada et al. 2004, Hancock and Godfray 2007), understanding the relative impact of density-dependent mortality on recruitment to the adult stage and subsequent consequences for transmission is still in a rudimentary stage. Mortality of individuals via mosquito control, density-dependence, or predation could alter the distribution of phenotypes in the populations. For example, mortality could change the distribution of biting behavior and susceptibility for pathogens, affecting transmission.
Individual-level mechanisms such as predator avoidance can affect competitive interactions (Kesavaraju and Juliano 2004, Kesavaraju et al. 2007), with consequent effects on population size and transmission dynamics. Environmental conditions experienced by immature stages may have latent effects that affect adult mortality and other characteristics. Density-dependent effects from larval competition resulted in increased adult mortality in Aedes triseriatus Say and Aedes japonicus Theobold (Alto 2011). Effects on adult mortality from larval competition and nutrient limitation have also been observed for Cx.p. quinquefasciatus (Vrzal et al. 2010), Culex pipiens L. (Alto et al. 2012b), Wy. smithii (Bradshaw and Holzapfel 1992), and other Aedes species (Hawley 1985, Reiskind and Lounibos 2009). Larval nutrition can affect adult size (e.g., Juliano 1998, Reiskind and Zarrabi 2012), and larger mosquitoes have shown an increased life expectancy in some studies (Hawley 1985, Lounibos et al. 1990, Bradshaw and Holzapfel 1992, Takken et al. 1998), but not all (Moeur and Istock 1980, Leisnham et al. 2008). Heterogeneity in larval conditions likely increases heterogeneity in both larval survival and individual adult mosquito mortality, affecting community-level transmission dynamics. The inclusion of heterogeneity in mortality may improve the predictive power of models of vector-borne pathogens.
Beyond-Individual Levels
Individual-level mortality is often aggregated into a population level mortality rate, which influences both mosquito population dynamics and transmission. However, this assumes that mosquito mortality is constant over time (reviewed in Bellan 2010), age, and environment. These assumptions have not been verified for most vectors. Mortality rates in adult mosquitoes likely vary over time and influence transmission by effects on population age structure, survival during the EIP, and longevity after the mosquito becomes infectious (Styer et al. 2007a,b; Harrington et al. 2008; C.C.L., unpublished). Transmission dynamics are especially sensitive to population-level vector mortality rates when the EIP of the pathogen approaches life expectancy (Bellan 2010). Additional information is needed to determine how sensitive models of vector-borne diseases are to different mortality functions over age (e.g., Weibull, Gompertz, and logistic; Clements and Paterson 1981; Styer et al. 2007a,b; Delatte et al. 2009) and whether transmission is affected in nature.
Density-dependent processes that induce larval mosquito mortality may act to regulate adult populations (Juliano 2007; e.g., Ae. aegypti, Dye 1984; Cx. p. quinquefasciatus, Ahumada et al. 2004; Anopheles gambiae s.s Giles., Gimnig et al. 2002), modify characteristics of survivors, and influence community structure. Other sources of mortality may also influence adult populations and adult characteristics, and interact with density-dependent processes. Resulting compensatory mortality or overcompensation will affect community structure and interactions between the mosquitoes and hosts. The processes determining how these effects are related to transmission are not well understood.
Pathogens may be transmitted by multiple vectors with different life history traits. These variations can then affect their effectiveness as vectors. Interactions determined by community structure such as predation and interspecific competition may affect mortality, influencing the relative contributions of multiple vector species to transmission. Differential mortality among mosquito species may play a role in determining distribution patterns of mosquito vectors. The following two cases exemplify how community-level processes which partially determine vector distribution can influence disease transmission.
Case 1: Dengue Virus (DENV)
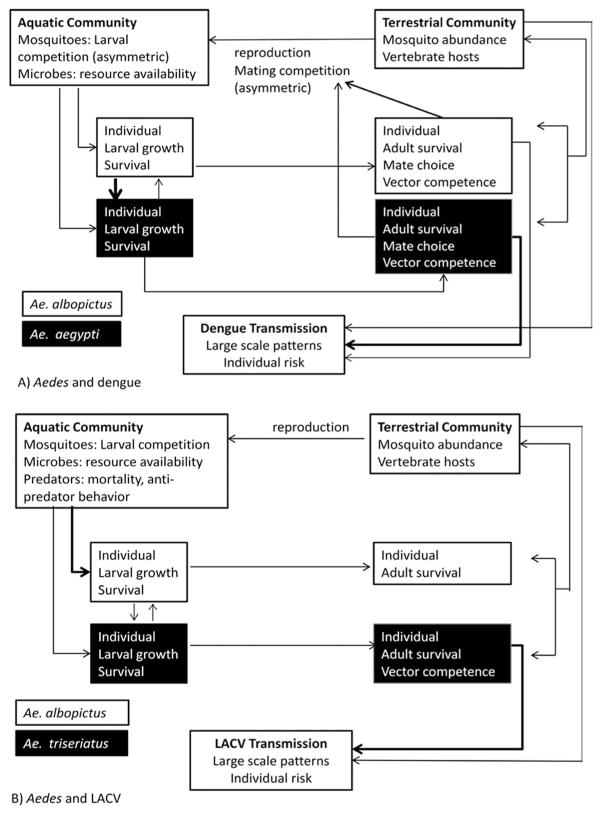
The range of the invasive mosquito Ae. albopictus has expanded in recent decades (O’Meara et al. 1995, Moore and Mitchell 1997, Moore 1999, Lounibos 2002, Santos and La 2003). In the continental United States, the spread of Ae. albopictus has been associated with declines in the abundance and distribution of Ae. aegypti. Field and laboratory investigations have demonstrated that larval resource competition (Barrera 1996, Juliano 1998, Braks et al. 2004, Juliano et al. 2004, Murrell and Juliano 2008) and adult interference competition (asymmetric mating interference, Bargielowski et al. 2013) are, in part, responsible for these observed changes in Ae. aegypti distribution. These species vary in their vector competence for DENV (Whitehead et al. 1971, Chen et al. 1993, Vazeille et al. 2003); changing abundance will affect DENV epidemiology. Differential mortality and reproduction of these species, attributable to competition favoring Ae. albopictus, has community-level consequences, influencing the distribution of both vectors and transmission of DENV (Fig. 3A).
Fig. 3.
Links and interactions between levels of biological organization. Only those interactions discussed in the text are shown; many more interactions are likely. Darker arrows indicate stronger effects when asymmetric. (A) Aedes and DENV, with asymmetric larval and mating competition. (B) Aedes and LACV, with predation on larvae differentially affecting growth and survival.
Case 2: La Crosse Encephalitis Virus (LACV)
Alterations in community processes that determine the geographic distribution of the primary vector, Ae. triseriatus, will influence the risk of LACV transmission. This species exists in a community with competing mosquito species (e.g., Ae. albopictus) and larval predators (e.g., Toxorhynchites rutilus Coquillet and Corethrella appendiculata Graham). Empirical studies and theoretical models have demonstrated that differential mortality attributable to predation on the immature stages of Ae. triseriatus and Ae. albopictus facilitates their coexistence (Juliano and Lounibos 2005, Alto et al. 2009, Juliano et al. 2010). In the presence of predators, Ae. triseriatus adopts antipredatory behaviors to a greater degree than Ae. albopictus, resulting in lower predation rates (Kesavaraju and Juliano 2004, Kesavaraju et al. 2007). This allows an increased likelihood of coexistence of the two species, likely altering the mosquito community dynamics and thus LACV epidemiology (Fig. 3B). Modeling studies would be valuable in exploring how the complex interactions between these mosquito species affects LACV epidemiology. These examples illustrate how community-level processes attributable to differential mortality among immature stages, operating at the small spatial scale of single water-filled containers, have large scale consequences on the regional and global distribution patterns of mosquito vectors.
Future Directions
Further studies are needed to determine how environmental conditions (e.g., temperature, water dynamics, resource availability), biotic interactions (e.g., competition, predation, parasitism), and attributes of mosquito biology (e.g., age, nutrient reserves, infection with pathogens) influence the shapes of mortality functions of adult mosquitoes. Investigations of the effects of population age-structure on transmission are critically needed. How sensitive is disease transmission to mortality rates when there is a stable age distribution compared with a variable age-structure, especially when multiple vectors may be responsible for transmission of a particular pathogen? There is a need for a better understanding of the relationship between larval and adult density, the influence of different sources of mortality, and the effect on transmission. Models which consider processes at different levels of biological organization will contribute to understanding disease transmission and developing effective mosquito management strategies. Interpretation of model results will be strengthened by validation with independent comparisons to field data, requiring carefully designed field experiments. Can we identify the key elements so models will be able to predict outbreaks or appropriately test control programs? Incorporation of different scales into models and validation studies will present challenges which will require a balance between the inclusion of adequate details and application of appropriate sensitivity analyses (Lord 2007).
Summary
Ultimately, our goal is to understand the dynamics of vector-borne disease to mitigate the effects on vertebrate hosts of interest, whether those are humans, domestic animals, or wild animals. The transmission dynamics of vector-borne pathogens are the result of an intricate interplay of individual characteristics which combine to influence population and community-level processes. Community structure influences individual processes in ways we are only beginning to understand for mosquitoes. Improving our understanding of these links between scales and levels will facilitate extrapolating information at one level or location to predictions of transmission dynamics across time, space, and communities.
There are many potential interactions between various scales and levels of biological organization. We have illustrated this with selected examples, and a few stand out as critical. Variation between individual vectors in mechanisms, determining individual vector competence or survival, will affect population level characteristics such as transmission or vector abundance. Variation in individual vector characteristics affects interactions with vertebrate hosts at the community level and will generate heterogeneity within and between communities that influence transmission. These community-level changes feed back into influence individual-level changes in ecological interactions such as blood feeding, predation, and competition. These ecological interactions then affect individuals in aspects such as movement patterns, survival, and fecundity, further influencing transmission between individuals and population-level dynamics. Heterogeneity and scale in environmental factors in both space and time will interact with these biological characteristics, resulting in dynamics that vary in space, time, and with community structure.
Issues of scale, particularly scales of biological organization, affect our understanding of vector-borne pathogen cycles. We must be aware of how this affects our research design and interpretations. Studies are needed at local levels with comparisons among and between habitats and mosquito and vertebrate communities. Laboratory studies on mechanisms at the individual level will be needed to assess variation between populations or species. Models incorporating different levels of biological organization and different biological scales will be needed to assess potential effects of variation at one scale on transmission dynamics at other scales. Specific questions and methods will depend on the system. Our challenge to the vector biology community is to be aware of the scales and levels of specific studies and how this influences our interpretation of results. The challenge to vector biology is enormous and will require studies by multiple researchers spanning multiple disciplines. This is illustrated by the wide range of studies and investigators cited in this selective discussion. It is essential that the research community encourages a longer view and the careful design of smaller scale studies so results can be integrated to provide information about how these complex vector–pathogen systems operate at larger scales.
Acknowledgments
J. Pohedra assisted in developing the model of infection. C. Thomas and H. Lynn assisted in manuscript preparation. We thank L. P. Lounibos, J. R. Rey, three anonymous reviewers, and the editor, S. A. Juliano, for reviewing the manuscript and providing many helpful comments. The research was partially supported by National Institutes of Health (NIH; R01-AI042164).
References Cited
- Ahumada JA, Lapointe D, Samuel FMD. Modeling the population dynamics of Culex quinquefasciatus (Diptera: Culicidae), along an elevational gradient in Hawaii. J Med Entomol. 2004;41:1157–1170. doi: 10.1603/0022-2585-41.6.1157. [DOI] [PubMed] [Google Scholar]
- Alizon S, vanBaalen M. Acute or chronic? Within-host models with immune dynamics, infection outcome, and parasite evolution. Am Nat. 2008;172:244–256. doi: 10.1086/592404. [DOI] [PubMed] [Google Scholar]
- Alizon S, Luciani F, Regoes RR. Epidemiological and clinical consequences of within-host evolution. Trends Microbiol. 2011;19:24–32. doi: 10.1016/j.tim.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Alto BW. Interspecific larval competition between invasive Aedes japonicus and native A. triseriatus (Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Alto BW, Bettinardi D. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am J Trop Med Hyg. 2013;88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J Med Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc B. 2008a;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008b;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Kesavaraju B, Juliano SA, Lounibos LP. Stage-dependent predation on competitors: consequences for the outcome of a mosquito invasion. J Anim Ecol. 2009;78:928–936. doi: 10.1111/j.1365-2656.2009.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Malicoate J, Elliott SM, Taylor J. Demographic consequences of predators on prey: trait and density mediated effects on mosquito larvae in containers. PLoS ONE. 2012a;7:e45785. doi: 10.1371/journal.pone.0045785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Muturi EJ, Lampman RL. Effects of nutrition and density in Culex pipiens. Med Vet Entomol. 2012b;26:396–406. doi: 10.1111/j.1365-2915.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- Amarasekare P. Interference competition and species coexistence. Proc R Soc Lond B. 2002;269:2541–2550. doi: 10.1098/rspb.2002.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford University Press; Oxford, United Kingdom: 1991. [Google Scholar]
- Anderson SL, Richards SL, Tabachnick WJ, Smartt CT. Effects of West Nile virus dose and extrinsic incubation temperature on temporal progression of vector competence in Culex pipiens quinquefasciatus. J Am Mosq Control Assoc. 2010;26:103–107. doi: 10.2987/09-5926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski IE, Lounibos LP, Carrasquilla MC. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci USA. 2013;110:2888–2892. doi: 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley LM, Donnelly CA, Garnett GP. The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Trans R Soc Trop Med Hyg. 2002;96:387–397. doi: 10.1016/s0035-9203(02)90371-8. [DOI] [PubMed] [Google Scholar]
- Beerntsen BT, James AA, Christensen BM. Genetics of mosquito vector competence. Microbiol Mol Biol Rev. 2000;64:115–137. doi: 10.1128/mmbr.64.1.115-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan SE. The importance of age dependent mortality and the extrinsic incubation period in models of mosquito-borne disease transmission and control. PLoS ONE. 2010;5:e10165. doi: 10.1371/journal.pone.0010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley MD, Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- Bidlingmayer WL. Influence of environmental factors and physiological stage on flight patterns of mosquitoes taken in vehicle aspirator and truck, suction, bait and New Jersey light traps. J Med Entomol. 1974;11:119–146. doi: 10.1093/jmedent/11.2.119. [DOI] [PubMed] [Google Scholar]
- Blair R. The effects of urban sprawl on birds at multiple levels of biological organization. Ecol Soc. 2004;9:2. http://www.ecologyandsociety.org/vol9/iss5/art2. [Google Scholar]
- Blaustein L, Kotler BP, Ward D. Direct and indirect effects of a predatory backswimmer (Notonecta maculata) on community structure of desert temporary pools. Ecol Entomol. 1995;20:311–318. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Reproductive consequences of density-dependent size variation in the pitcherplant mosquito, Wyeomyia smithii (Diptera: Culicidae) Ann Entomol Soc Am. 1992;85:274–281. [Google Scholar]
- Braks MAH, Honorio NA, Lounibos LP, Lourenco-De-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet Res. 2009;40:43. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante DM. PhD thesis. University of Florida; Gainesville: 2009. Culex nigripalpus (Diptera: Culicidae) population age structure under heterogeneous environments and sources of error on the estimation of mosquito infection rates. [Google Scholar]
- Case TJ, Gilpin ME. Interference competition and niche theory. Proc Natl Acad Sci USA. 1974;71:3073–3077. doi: 10.1073/pnas.71.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Wei HL, Hsu EL, Chen ER. Vector competence of Aedes albopictus and Ae. aegypti (Diptera: Culicidae) to dengue 1 virus on Taiwan: development of the virus in orally and parenterally infected mosquitoes. J Med Entomol. 1993;30:524–530. doi: 10.1093/jmedent/30.3.524. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov EM, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Clements AN, Paterson GD. The analysis of mortality and survival rates in wild populations of mosquitoes. J Appl Ecol. 1981;18:373–399. [Google Scholar]
- Cochran-Stafira DL, von Ende CN. Integrating bacteria into food webs: studies with Sarracenia purpurea inquilines. Ecology. 1998;79:880–898. [Google Scholar]
- Coffey LL, Vasilakis N, Brault AC, Powers AM, Tripet F, Weaver SC. Arbovirus evolution in vivo is constrained by host alternation. Proc Natl Acad Sci USA. 2008;105:6970–6975. doi: 10.1073/pnas.0712130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CA, Santos IG, da Barbosa MG. Detection and typing of dengue viruses in Aedes aegypti (Diptera: Culicidae) in the City of Manaus, State of Amazonas. Rev Soc Bras Med Trop. 2009;42:677–681. doi: 10.1590/s0037-86822009000600013. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species, a facsimile of the 1st ed, 1981. Harvard University Press; Cambridge, MA: 1859. [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DWC, Novak RJ, et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basanez MG. Anopheles mortality is both age-and Plasmodium-density dependent: implications for malaria transmission. Malaria J. 2009;8:228. doi: 10.1186/1475-2875-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- Day JF, Curtis GA. Annual emergence patterns of Culex nigripalpus females before, during, and after a widespread St. Louis encephalitis epidemic in South Florida. J Am Mosq Control Assoc. 1993;9:249–255. [PubMed] [Google Scholar]
- Day JF, Curtis GA. When it rains, they soar -and that makes Culex nigripalpus a dangerous mosquito. Am Entomol. 1994;40:162–167. [Google Scholar]
- Day JF, Curtis GA, Edman JD. Rainfall-directed oviposition behavior of Culex nigripalpus (Diptera: Culicidae) and its influence on St. Louis encephalitis virus transmission in Indian River County, Florida. J Med Entomol. 1990a;27:43–50. doi: 10.1093/jmedent/27.1.43. [DOI] [PubMed] [Google Scholar]
- Day JF, Ramsey AM, Zhang JT. Environmentally mediated seasonal variation in mosquito body size. Environ Entomol. 1990b;19:469–473. [Google Scholar]
- Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- Devore JL. Probability and statistics for engineering and the sciences. Brooks/Cole Publishing Company; Monterey, CA: 1982. [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Dye C. Models for the population dynamics of the yellow fever mosquito, Aedes aegypti. J Anim Ecol. 1984;53:247–268. [Google Scholar]
- Dye C. A comparative approach to the measurement of disease transmission by mosquitoes. Trans R Soc Trop Med Hyg. 1986a;80:842–842. [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitol Today. 1986b;2:203–208. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Dye C. The analysis of parasite transmission by bloodsucking insects. Annu Rev Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. [DOI] [PubMed] [Google Scholar]
- Edman JD. Are mosquitoes gourmet or gourmand? J Am Mosq Control Assoc. 1989;5:487–497. [PubMed] [Google Scholar]
- Edman JD, Kale HW. Host behavior: its influence on the feeding success of mosquitoes. Ann Entomol Soc Am. 1971;64:513–516. [Google Scholar]
- Edman JD, Scott TW. Host defensive behaviour and the feeding success of mosquitoes. Insect Sci Applic. 1987;8:617–622. [Google Scholar]
- Elizondo-Quiroga A, Flores-Suarez A, Elizondo-Quiroga D, Ponce-Garcia G, Blitvich BJ, Contreras-Cordero JF, Gonzalez-Rojas JI, Mercado-Hernandez R, Beaty BJ, Fernandez-Salas I. Gonotrophic cycle and survivorship of Culex quinquefasciatus (Diptera: Culicidae) using sticky ovitraps in Monterrey, northeastern Mexico. J Am Mosq Control Assoc. 2006;22:10–14. doi: 10.2987/8756-971X(2006)22[10:GCASOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ferro C, Morrison AC, Torres M, Pardo R, Wilson ML, Tesh RB. Age structure, blood-feeding behavior, and Leishmania chagasi infection in Lutzomyia longipalpus (Diptera: Psychodidae) at an endemic focus of visceral leishmaniasis in Columbia. J Med Entomol. 1995;32:618–629. doi: 10.1093/jmedent/32.5.618. [DOI] [PubMed] [Google Scholar]
- Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- Fragkoudis R, Attarzadeh-Yazdi G, Nash AA, Fazakerley JK, Kohl A. Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol. 2009;90:2061–2072. doi: 10.1099/vir.0.013201-0. [DOI] [PubMed] [Google Scholar]
- Fryer HR, Frater J, Duda A, Roberts M, Phillips R, Mclean AR. Modelling the evolution and spread of HIV immune escape mutants. PLoS Pathog. 2010;6:e1001196. doi: 10.1371/journal.ppat.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol. 2002;39:162–172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Gravenor MB, Mclean AR, Kwiatowski D. The regulation of malaria parasitemia -parameter estimates for a population-model. Parasitology. 1995;110:115–122. doi: 10.1017/s0031182000063861. [DOI] [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behavior: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–76. [Google Scholar]
- Grimstad P, Haramis L. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition-deprived mosquitoes. J Med Entomol. 1984;21:249–256. doi: 10.1093/jmedent/21.3.249. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Godfray HCJ. Application of the lumped age-class technique to studying the dynamics of malaria-mosquito-human interactions. Malaria J. 2007;6:98. doi: 10.1186/1475-2875-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Vermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, Scott TW. Age-dependent survival of the dengue vector Aedes aegypti (Diptera: Culicidae) demonstrated by simultaneous release-recapture of different age cohorts. J Med Entomol. 2008;45:307–313. doi: 10.1603/0022-2585(2008)45[307:asotdv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hastings A, Petrovskii S, Morozov A. Spatial ecology across scales. Biol Lett. 2011;7:163–165. doi: 10.1098/rsbl.2010.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. Anim Ecol. 1985;54:955–964. [Google Scholar]
- Higgs S, Beaty BJ. Natural cycles of vector-borne pathogens. In: Marquardt WC, Kondratieff BC, Moore CG, Freier JE, Hagedorn HH, Black WC IV, James AA, Hemingway J, Higgs S, editors. Biology of disease vectors. 2. Elsevier; San Diego, CA: 2005. pp. 167–185. [Google Scholar]
- Iwasa Y, Michor F, Nowak M. Some basic properties of immune selection. J Theor Biol. 2004;229:179–188. doi: 10.1016/j.jtbi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Population dynamics. J Am Mosq Control Assoc. 2007;23:265–275. doi: 10.2987/8756-971x(2007)23[265:pd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, Nishimura N, Greene K. Your worst enemy could be your best friend: predator contributions to invasion resistance and persistence of natives. Oecologia. 2010;162:709–718. doi: 10.1007/s00442-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus: Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Kenney JL, Adams AP, Gorchakov R, Leal G, Weaver SC. Genetic and anatomic determinants of enzootic Venezuelan equine encephalitis virus infection of Culex (Melanoconion) taeniopus. PLoS Negl Trop Dis. 2012;6:e1606. doi: 10.1371/journal.pntd.0001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitel JM, Miller TE. Resource and top-predator regulation in the pitcher plant (Sarracenia purpurea) inquiline community. Ecology. 2002;83:680–688. [Google Scholar]
- Lambrechts L, Scott TW. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc R Soc B. 2009;276:1369–1378. doi: 10.1098/rspb.2008.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Knox TB, Wong J, Liebman KA, Albright RG, Stoddard ST. Shifting priorities in vector biology to improve control of vector-borne disease. Trop Med Int Health. 2009;14:1505–1514. doi: 10.1111/j.1365-3156.2009.02401.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Sala LM, Juliano SA. Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J Med Entomol. 2008;45:210–221. doi: 10.1603/0022-2585(2008)45[210:gviasa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Menach A, Mckenzie FE, Flahault A, Smith DL. The unexpected importance of mosquito oviposition behaviour for malaria: non-productive larval habitats can be sources for malaria transmission. Malaria J. 2005;4:23. doi: 10.1186/1475-2875-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC. Modeling and biological control of mosquitoes. J Am Mosq Control Assoc. 2007;23:252–264. doi: 10.2987/8756-971x(2007)23[252:mabcom]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC. The effect of multiple vectors on arbovirus transmission. Israel J Ecol Evol. 2010;55:371–392. doi: 10.1560/IJEE.55.3-4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Day JF. Simulation studies of St. Louis encephalitis in South Florida. Vector Borne Zoonotic Dis. 2001a;1:299–316. doi: 10.1089/15303660160025921. [DOI] [PubMed] [Google Scholar]
- Lord CC, Day JF. Simulation studies of St. Louis encephalitis and West Nile viruses: the impact of bird mortality. Vector Borne Zoonotic Dis. 2001b;1:317–330. doi: 10.1089/15303660160025930. [DOI] [PubMed] [Google Scholar]
- Lord CC, Woolhouse MEJ, Heesterbeek JAP, Mellor PS. Vector-borne diseases and the basic reproduction number: a case-study of African horse sickness. Med Vet Entomol. 1996a;10:19–28. doi: 10.1111/j.1365-2915.1996.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Lord CC, Woolhouse MEJ, Rawlings P, Mellor PS. Simulation studies of African horse sickness and Culicoides imicola (Diptera: Ceratopogonidae) J Med Entomol. 1996b;33:328–338. doi: 10.1093/jmedent/33.3.328. [DOI] [PubMed] [Google Scholar]
- Lord CC, Woolhouse MEJ, Mellor PS. Simulation studies of vaccination strategies in African horse sickness. Vaccine. 1997;15:519–524. doi: 10.1016/s0264-410x(97)00220-x. [DOI] [PubMed] [Google Scholar]
- Lord CC, Rutledge CR, Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz L, Beaty BJ, Aitken TH, Wallis GP, Tabachnick WJ. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, V, Larson L, Morris CD. Parity, fecundity and body size of Mansonia dyari in Florida. J Am Mosq Control Assoc. 1990;6:121–126. [PubMed] [Google Scholar]
- Luz PM, Codeco CT, Massad E, Struchiner CJ. Uncertainties regarding dengue modeling in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2003;98:871–878. [PubMed] [Google Scholar]
- Macdonald G. The epidemiology and control of malaria. Oxford University Press; Oxford, United Kingdom: 1957. [Google Scholar]
- Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, Gould F. Skeeter Buster: A stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PloS Neglect Trop Dis. 2009;3:e508. doi: 10.1371/journal.pntd.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood F, Crans WJ. Effect of temperature on the development of Culiseta melanura (Diptera: Culicidae) and its impact on the amplification of eastern equine encephalomyelitis. J Med Entomol. 1998;35:1007–1012. doi: 10.1093/jmedent/35.6.1007. [DOI] [PubMed] [Google Scholar]
- Mahmood F, Chiles RE, Fang Y, Green EN, Reisen WK. Effects of time after infection, mosquito genotype, and infectious viral dose on the dynamics of Culex tarsalis vector competence for western equine encephalomyelitis virus. J Am Mosq Control Assoc. 2006;22:272–281. doi: 10.2987/8756-971X(2006)22[272:EOTAIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Matthews L, Haydon D. Introduction. Cross-scale influences on epidemiological dynamics: from genes to ecosystems. J R Soc Interface. 2007;4:763–765. [Google Scholar]
- McLean RG, Scott TW. Avian hosts of St. Louis encephalitis virus. Proceedings of the Bird Control Seminar. 1979 ( http://digitalcommons.unl.edu/icwdmbirdcontrol/20)
- McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O’Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- Medlock J, Luz PM, Struchiner CJ, Galvani AP. The impact of transgenic mosquitoes on dengue virulence to humans and mosquitoes. Am Nat. 2009;174:565–577. doi: 10.1086/605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Curiel RF, Black WC, Iv, Munoz MD. A dengue receptor as possible genetic marker of vector competence in Aedes aegypti. BMC Microbiol. 2008;8:118. doi: 10.1186/1471-2180-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeur JE, Istock CA. Ecology and evolution of the pitcher-plant mosquito. 4. Larval influence over adult reproductive-performance and longevity. J Anim Ecol. 1980;49:775–792. [Google Scholar]
- Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. J Am Mosq Control Assoc. 1999;15:221–227. [PubMed] [Google Scholar]
- Moore CG, Mitchell CJ. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg Infect Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy RM, Meola MA, Morin LLL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- Murrell E, Juliano S. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Alto BW. Larval environmental temperature and insecticide exposure alters Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 2011;11:1157–1163. doi: 10.1089/vbz.2010.0209. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Constanzo K, Kesavaraju B, Alto BW. Can insecticides and larval competition alter susceptibility of Aedes mosquitoes to arbovirus infection? J Med Entomol. 2011;48:429–436. doi: 10.1603/me10213. [DOI] [PubMed] [Google Scholar]
- Nayar JK. The biology of Culex nigripalpus Theobald (Diptera: Culicidae) Part 2. Adult characteristics at emergence and adult survival without nourishment. J Med Entomol. 1968;5:203–210. doi: 10.1093/jmedent/5.2.203. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. The effects of diet on life-span, fecundity and flight potential of Aedes taeniorhynchus adults. J Med Entomol. 1971a;8:506–513. doi: 10.1093/jmedent/8.5.506. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. Physiological effects of carbohydrates on survival, metabolism, and flight potential of female Aedes taeniorhynchus. J Insect Physiol. 1971b;17:2221–2233. doi: 10.1016/0022-1910(71)90180-6. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 1. Utilization of sugar for survival. J Med Entomol. 1975a;12:92–98. doi: 10.1093/jmedent/12.1.92. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 2. Utilization of a blood meal for survival. J Med Entomol. 1975b;12:99–103. doi: 10.1093/jmedent/12.1.99. [DOI] [PubMed] [Google Scholar]
- Nayar JK, Sauerman DM. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 3. Utilization of blood and sugar for fecundity. J Med Entomol. 1975c;12:220–225. doi: 10.1093/jmedent/12.2.220. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol. 2005;20:328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull SH, Song S, Mcclure KM, Sackett LC, Kilpatrick AM, Johnson PTJ. From superspreaders to disease hotspots: linking transmission across hosts and space. Front Ecol Environ. 2012;10:75–82. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko K, Westbrook CJ, Mores CN, Lounibos LP, Reiskind MH. Effects of infectious virus dose and bloodmeal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J Med Entomol. 2009;46:395–399. doi: 10.1603/033.046.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisier AP, Van Oortmarssen GJ, Habbema JDF, Remme J, Alley ES. ONCHOSIM: a model and computer simulation program for the transmission and control of onchocerciasis. Compt Meth Prog Biomed. 1990;31:43–56. doi: 10.1016/0169-2607(90)90030-d. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Dobson ADM. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Green RM, Peacey MF, Rogers DJ. Seasonal synchrony: the key to tick-borne encephalitis foci identified by satellite data. Parasitology. 2000;121:15–23. doi: 10.1017/s0031182099006083. [DOI] [PubMed] [Google Scholar]
- Real LA, Biek R. Spatial dynamics and genetics of infectious diseases on heterogeneous landscapes. J R Soc Interface. 2007;4:935–948. doi: 10.1098/rsif.2007.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK. Effect of temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J Med Entomol. 1995;32:636–645. doi: 10.1093/jmedent/32.5.636. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–483. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- Reisen W, Barker C, Fang Y, Martinez V. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med Vet Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind MH, Zarrabi AA. Is bigger really bigger? Differential responses to temperature in measures of body size of the mosquito, Aedes albopictus. J Insect Physiol. 2012;58:911–917. doi: 10.1016/j.jinsphys.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko K, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for St. Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko K, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for West Nile virus. Am J Trop Med Hyg. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S. Large-scale spatial-transmission models of infectious disease. Science. 2007;316:1298–1301. doi: 10.1126/science.1134695. [DOI] [PubMed] [Google Scholar]
- Roche B, Rohani P, Dobson AP, Guegan JF. The impact of community organization on vector-borne pathogens. Am Nat. 2013;181:1–11. doi: 10.1086/668591. [DOI] [PubMed] [Google Scholar]
- Rogers DJ. A general model for the African trypanosomiases. Parasitology. 1988;97:193–212. doi: 10.1017/s0031182000066853. [DOI] [PubMed] [Google Scholar]
- Ross R. The prevention of malaria. 2. Murray; London, United Kingdom: 1911. [Google Scholar]
- Rossignol PA, Shieh JN. Feeding success of vectors on infected hosts. Parasitol Today. 1993;9:442–443. doi: 10.1016/0169-4758(93)90095-w. [DOI] [PubMed] [Google Scholar]
- Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, Nimmannitya S, Kalayanarooj S, Jarman RG, Thomas SJ, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA. 2012;109:9535–9538. doi: 10.1073/pnas.1120621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Sanders HR, Foy BD, Evans AM, Ross LS, Beaty BJ, Olson KE, Gill SS. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:1293–1307. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Santos R, La C. Updating the distribution of Aedes albopictus in Brazil (1997–2002) Revista de Saude Publica Sao Paulo. 2003;37:671–673. [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RS. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Service MW. Population dynamics and mortalities of mosquito preadults. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 185–201. [Google Scholar]
- Seuss D. Horton Hears a Who. Random House; New York: 1954. [Google Scholar]
- Shaman J, Day JF, Komar N. Hydrologic conditions describe West Nile virus risk in Colorado. Int J Environ Res Public Health. 2010;7:494–508. doi: 10.3390/ijerph7020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg Infect Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. St. Louis encephalitis virus in wild birds during the 1990 South Florida epidemic: the importance of drought, wetting conditions, and the emergence of Culex nigripalpus (Diptera: Culicidae) to arboviral amplification and transmission. J Med Entomol. 2003;40:547–554. doi: 10.1603/0022-2585-40.4.547. [DOI] [PubMed] [Google Scholar]
- Smartt C, Richards S, Anderson S, Erickson J. West Nile virus infection alters midgut gene expression in Culex pipiens quinquefasciatus Say (Diptera: Culicidae) Am J Trop Med Hyg. 2009;81:258–263. [PMC free article] [PubMed] [Google Scholar]
- Sota T, Mogi M. Effectiveness of zooprophylaxis in malaria control: a theoretical inquiry, with a model for mosquito populations with two bloodmeal hosts. Med Vet Entomol. 1989;3:337–345. doi: 10.1111/j.1365-2915.1989.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Souza-Neto J, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Carey JR, Wang JL, Scott TW. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007a;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Minnick SL, Sun AK, Scott TW. Mortality and reproduction dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector Borne Zoonotic Dis. 2007b;7:86–98. doi: 10.1089/vbz.2007.0216. [DOI] [PubMed] [Google Scholar]
- Suchman EL, Kononko A, Plake E, Doehling M, Kleker B, Black WC, Buchatsky L, Carlson J. Effects of AeDNV infection on Aedes aegypti lifespan and reproduction. Biol Control. 2006;39:465–473. [Google Scholar]
- Tabachnick WJ. Genetics of insect vector competence for arboviruses. Adv Dis Vector Res. 1994;10:93–108. [Google Scholar]
- Takahashi M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on pattern of transmission of Japanese encephalitis virus. J Med Entomol. 1976;13:275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S, Platt KB, Evens RB, Rowley WA. Susceptibility of Ochlerotatus trivittatus (Coq.), Aedes albopictus (Skuse), and Culex pipiens (L.) to West Nile virus infection. Vector Borne Zoonotic Dis. 2004;4:190–197. doi: 10.1089/vbz.2004.4.190. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton University Press; Princeton, NJ: 1982. [PubMed] [Google Scholar]
- Tilman D, Kareiva P. Spatial ecology: the role of space in population dynamics and interspecific interactions. Princeton University Press; Princeton, NJ: 1997. [Google Scholar]
- Tompkins DM, Dunn AM, Smith MJ, Telfer S. Wildlife diseases: from individuals to ecosystems. J Anim Ecol. 2011;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001a;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, Dohm DJ, O’Guinn ML. Potential North American vectors of West Nile virus. Ann N Y Acad Sci. 2001b;951:317–324. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Vance RR. Interference competition and the coexistence of two competitors on a single limiting resource. Ecology. 1984;65:1349–1357. [Google Scholar]
- Vazeille M, Rosen L, Mousson L, Failloux AB. Low receptivity for dengue type 2 viruses of Aedes albopictus from southeast Asia compared with that of Aedes aegypti. Am J Trop Med Hyg. 2003;68:203–208. [PubMed] [Google Scholar]
- Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culex nigripalpus in Florida, 2005. J Med Entomol. 2008;45:483–493. doi: 10.1603/0022-2585(2008)45[483:atbcni]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrzal EM, Allan SA, Hahn DA. Amino acids in nectar enhance longevity of female Culex quinquefasciatus mosquitoes. J Insect Physiol. 2010;56:1659–1664. doi: 10.1016/j.jinsphys.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Walker AR. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull Entomol Res. 2001;91:69–78. [PubMed] [Google Scholar]
- Washburn JO. Regulatory factors affecting larval mosquito populations in container and pool habitats - Implications for biological control. J Am Mosq Control Assoc. 1995;11:279–283. [PubMed] [Google Scholar]
- Washburn JO, Mercer DR, Anderson JR. Regulatory role of parasites: impact on host population shifts with resource availability. Science. 1991;253:185–188. doi: 10.1126/science.1906637. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Scott TW, Ricohesse R. Molecular evolution of eastern equine encephalomyelitis virus in North America. Virology. 1991;182:774–784. doi: 10.1016/0042-6822(91)90618-l. [DOI] [PubMed] [Google Scholar]
- Webber LA, Edman JD. Anti-mosquito behavior of ciconiiform birds. Anim Behav. 1972;20:228–232. [Google Scholar]
- Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook CJ, Reiskind MH, Pesko KN, Greene KE, Lounibos LP. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 2010;10:241–247. doi: 10.1089/vbz.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SM, Burden JP, Maini PK, Hails RS. Modelling the within-host growth of viral infections in insects. J Theor Biol. 2012;312:34–43. doi: 10.1016/j.jtbi.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, Yuill TM, Gould DJ, Simasathien P. Experimental infection of Aedes aegypti and Aedes albopictus with dengue viruses. Trans R Soc Trop Med Hyg. 1971;65:661–667. doi: 10.1016/0035-9203(71)90051-4. [DOI] [PubMed] [Google Scholar]
- Wiens JA. Spatial scaling in ecology. Funct Ecol. 1989;3:385–397. [Google Scholar]
- Wonham MJ, De-Camino-Beck T, Lewis MA. An epidemiological model for West Nile virus: invasion analysis and control applications. Proc R Soc B. 2004;271:501–507. doi: 10.1098/rspb.2003.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonham MJ, Lewis MA, Renclawowicz J, Van Den Driessche P. Transmission assumptions generate conflicting predictions in host-vector disease models: a case study in West Nile virus. Ecol Lett. 2006;9:706–725. doi: 10.1111/j.1461-0248.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- Xi ZY, Ramirez JL, Dimopoulos G. The Aedes aegypti Toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Bossert W. An intra-host mathematical model on interaction between HIV and malaria. Bull Math Biol. 2010;72:1892–1911. doi: 10.1007/s11538-010-9515-6. [DOI] [PubMed] [Google Scholar]
- Xue RD, Barnard DR, Muller GC. Effects of body size and nutritional regimen on survival in adult Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2010;47:778–782. doi: 10.1603/me09222. [DOI] [PubMed] [Google Scholar]