Abstract
S-(−)7-hydroxy-3-(4′-hydroxyphenyl)-chroman, or S-(−)equol, a biologically active intestinally derived bacterial metabolite of the soy isoflavones daidzin/daidzein, is not produced in neonatal life. Because its synthesis is dependent on equol-producing bacteria, we hypothesized that early nutrition may influence equol production. This prospective 2.5-year study determined the frequency of S-(−)equol production in healthy infants (n = 90) fed breast milk, soy infant formula, or cow’s milk formula in their first year. Urinary S-(−)equol and daidzein were quantified by mass spectrometry after a standardized 3.5-day soy isoflavone challenge. Infants were tested at 6, 9, 12, 18, 24, and 36 months of age, and 3-day diet records were obtained at each visit to explore the effect of early and postweaning (>12 months) macronutrient and micronutrient dietary composition and S-(−)equol production. Use of antibiotics was also recorded. At age 6 months, none of the breast-fed infants produced S-(−)equol, whereas 3.8% and 6.0%, respectively, of soy and cow’s milk formula–fed infants were equol producers. By age 3 years, 50% of the formula-fed infants were equol producers, compared with 25% of breast-fed infants. Use of antibiotics was prevalent among infants and may have impacted the stability of S-(−)equol production. No significant differences among the groups were observed in postweaning dietary intakes of total energy, carbohydrate, fiber, protein, fat, saturated fatty acids, or polyunsaturated fatty acids and the propensity to make S-(−)equol. In conclusion, S-(−)equol production is developmentally regulated and initially related to diet composition with the proportion of equol producers increasing over the first 3 years of life, with a trend for formula feeding favoring S-(−)equol production.
Keywords: Equol, Isoflavones, Soy, Infant, Diet, Antibiotics
1. Introduction
S-(−)7-hydroxy-3-(4′-hydroxyphenyl)-chroman, or S-(−)equol, is a metabolite of the soy isoflavones daidzin/daidzein formed by the action of bacteria in the distal region of the intestine and colon [1–4]. It is not produced in all adults who consume soy foods [4]. The frequency of “equol producers” in Western adults, when consuming soy foods, has been consistently shown to be 25% to 35% [5–8], much lower than the 50% to 60% frequency reported for adult equol producers in Japan, China, and Korea, where traditional soy foods are regularly consumed [9–12]. It is presently unclear what factors determine an individual’s ability to produce S-(−)equol when consuming soy foods, but understanding why equol production varies among individuals is important because the health benefits of a soy-based diet appear to be greater in equol producers than in non–equol producers [1,8,13–15]. This relevance is further underscored by the fact that S-(−)equol is now being commercialized as a supplement [16,17] and developed as a pharmaceutical agent [18,19] targeted to adults who are unable to produce S-(−)equol. Interest in this soy isoflavone metabolite is because of its potent biological properties [20], including its selective affinity toward estrogen receptor β [2,21,22], and its androgen-modulating effects in vivo [23], which promise potential therapeutic actions in numerous hormone-dependent diseases.
What is known is that the conversion of daidzin/daidzein to S-(−)equol requires the presence of specific equol-producing bacteria, of which numerous species have been recognized [8]. In addition, optimal conditions are required within the gastrointestinal tract for the reduction and deoxygenation reactions to transform these dietary isoflavones into S-(−)equol [8]. The gastrointestinal tract of newborn infants is sterile at birth but rapidly develops a bacterial flora, and presumably, at some point, the equol-producing bacteria colonize the intestinal tract of a proportion of these infants; ultimately, S-(−)equol is produced when soy foods are consumed. Little is known about the developmental aspects of equol production beyond our earlier work that demonstrated that S-(−)equol is first detectable in the plasma of 4-month-old infants fed soy infant formula exclusively and consequently exposed to high intakes of daidzin/daidzein from this nutritional regimen [24,25]. Several other more recent studies have shown that S-(−)equol is barely detectable in these early months of life [26,27]. One fundamental question not addressed in previously reported studies of isoflavone metabolism in infants and children was whether differences in the type of early nutrition have an impact on the ability, ultimately, to produce S-(−)equol.
Because diet can affect the intestinal microbiome, the intraluminal pH, and redox potential [28], we hypothesized that the frequency of equol producers might be anticipated to differ among breast-fed, cow’s milk formula–fed, and soy infant formula–fed infants and that the form of early nutrition could be a key determinant of whether equol is ultimately produced. We describe, for the first time, findings from a longitudinal prospective study of 90 infants who were breast-fed, cow’s milk formula fed, or soy infant formula fed in the first year of life and followed up from 6 months to 3 years of age, during which time they were repeatedly tested for their ability to produce S-(−)equol in response to a standard soy challenge [7]. In addition, a detailed dietary analysis was performed to determine whether specific components of the diet, particularly the postweaning diet (>12 months), could be identified as being associated with a greater propensity to produce S-(−)equol.
2. Methods and materials
This study was conducted at Cincinnati Children’s Hospital Medical Center (CCHMC) in the Center for Clinical and Translational Science and Training and supported by funding from the National Institutes of Health (NIH) Grant (R01 AT002190). The Center for Clinical and Translational Science and Training is supported by an Institutional Clinical and Translational Science Award (NIH/National Center for Advancement of Translational Science Grant No. 8UL1 TR000077-05). The study protocol and informed consent form were reviewed and approved by the institutional review board at CCHMC (protocol CCHMC no. 05-06-12). This study was a free-living dietary study of healthy infants; no drugs were being administered, and the study was initiated prior to requirement for registration of clinical studies.
2.1. Subjects
Healthy infants (n = 93) were recruited through advertisements placed in a parenting magazine distributed throughout the Greater Cincinnati metropolitan area and on bulletin boards in the Cincinnati Children’s Hospital clinical areas. The initial screening of infants was performed by a telephone or e-mail interview with the parent(s) to whom the study protocol was explained, including all inclusion and exclusion criteria for participation. Exclusion criteria included a history of liver or gastrointestinal disease, more than 2 courses of antibiotic prior to 6 months of age, a family history of allergy to soy or peanuts, and a height or weight below the 5th or above the 95th percentile for age. An infant was enrolled in the study only if her/his nutritional regimen had been relatively constant from 6 weeks to 6 months of age with regard to the choice of infant feeding. In general, once the milk source (breast milk, cow’s milk, or soy infant formula) was well established, it was continued to 1 year of life. Based on this early nutrition regimen, the infants were stratified to 1 of the 3 dietary groups, that is, breast-fed, cow’s milk formula–fed, or soy formula–fed. Infants deemed suitable for participation attended CCHMC for a screen visit that included recording height and weight and a “well-baby” check-up by a pediatrician, and parents were instructed by a dietitian on how to keep a 3-day diet record. Written informed consent was obtained from the parent(s) of each child. The parents were compensated for time and travel expenses, and a gift card was given for the baby at each study visit.
2.2. Experimental design
The primary objective of the study was to determine whether the timing and frequency of equol production was influenced by the choice of early feeding regimen in infants in the first 3 years of life. A secondary objective was to determine whether differences in specific components of the postweaning diet influenced the production of S-(−)equol. To do this, infants in all 3 groups were tested for S-(−)equol production in response to a standard soy isoflavone challenge [7], substituting Similac Isomil Advanced Soy Formula (Abbott Nutrition Columbus, OH) for adult soy milk, and this challenge was performed at 6 months of age and then again at 9, 12, 18, 24, 30, and 36 months of age. Before each visit, the parents were asked if any antibiotics had been prescribed to the child since the last visit. Visits were not scheduled within 30 days of antibiotic treatment to allow recolonization of normal gut bacteria. A parent kept a 3-day diet record of all foods consumed by the infant in the week prior to each clinic visit and immediately before challenging them with soy isoflavones to test for S-(−)equol production. Testing involved giving the equivalent of 7 mg/d of soy isoflavone (as aglycone equivalents) contained in 237 mL of Similac Isomil Advanced Soy Formula. The soy formula was divided between morning and evening feedings for 3 consecutive days, and on the morning of the fourth day, a further 118 mL of soy infant formula was consumed. After 1 year of age, the mothers were given the option of continuing to use soy infant formula as the challenge or to substitute a soy isoflavone supplement (Nature’s Bounty, Bohemia, NY 21.5 mg isoflavones/capsule) for the soy isoflavone challenge. When the soy isoflavone supplement was chosen, the capsule was opened and the contents were mixed in yogurt, apple sauce, the icing of an Oreo, Nabisco, East Hanover, NJ cookie or some other food that the child preferred. At the end of day 3, or during the morning of day 4, a spot urine sample was collected and stored at −20°C until analyzed.
2.3. Dietary analysis
The 3-day diet records were analyzed using the Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA), as described previously [29,30].
2.4. Urinary isoflavone analysis
S-(−)equol and its precursor isoflavone daidzein were quantified in urine by stable-isotope dilution with liquid chromatography–mass spectrometry (MS) using electrospray ionization tandem MS to determine equol producer status [7]. The stable-labeled internal standards [2,3,4-13C3]daidzein and [2,3,4-13C3]equol were added to 0.1 mL of urine, and the samples were subjected to overnight enzymatic hydrolysis at 37°C. The isoflavones were then extracted by solid-phase extraction, converted to the dansylated derivatives, and quantified by electrospray ionization using tandem MS. The derivatized daidzein and equol were monitored by the positively charged molecular ion transition mass/charge ratios of m/z 721/>170 for daidzein and 709/>170 for equol, which resulted from cleavage of the dansyl groups for both compounds. This assay is linear over the dynamic range of 0 to 400 µg/L, with an interassay precision (coefficient of variation) determined from replicate analysis of quality control samples of less than 5%. These methods have been described in detail previously [17]. Equol producer status was defined by a urinary log10 S-(−)equol/daidzein ratio greater than −1.75 [7].
2.5. Statistical analyses
We anticipated the highest incidence of equol production to be in the breast-fed infants, and sample size calculations were based on their having an equol production frequency similar to Western adults (35%) [6–8]. We hypothesized a lower equol production rate for cow’s milk formula–fed (10%) and soy infant formula–fed (10%) groups. An assumption was made that the correlation of individual data across time would be 0.5. In order to detect a statistically significant difference in the proportion of equol producers among the 3 groups, 27 infants/group (n = 81) were needed. The test would have 80% power at α = .05 and be a 2-tailed test. The number of infants enrolled was ultimately increased to 93 to compensate for dropouts.
In the presentation of results, demographic data were summarized using mean ± SD for continuous measures and frequency, and with percentages for categorical data. To evaluate the effect of early nutrition on equol production during the first 12 months, the percentage of babies who were equol producers was compared among the 3 groups (breast-fed, cow’s milk formula–fed, soy infant formula–fed) using a χ2 test. Infants were classified as equol producers if there was evidence of equol production based on the S-(−)equol/daidzein ratio, as defined previously [7]. P values less than .05 were considered significant.
For evaluation of the relationship between postweaning diet and equol production from 18 to 36 months, principal component analysis was conducted including the specified standardized dietary components for each macromolecular category to identify the factors that explained a large percent of the variance of that category. An individual principal component (PC) was included for further analysis if the PC explained at least 10% of the total variance of the data. The PCs were described based on the loadings. The changes over time in macronutrient composition of the diet were evaluated by analyzing each PC separately within each category to determine if there were any statistically significant differences between equol producers and non–equol producers using repeated-measures analysis of variance based on the infant diet data at 18, 24, 30, and 36 months. This analysis was performed for the major macronutrients and micronutrients including, carbohydrates, lipids, proteins, vitamins (A, B, C, D, E, and K), and minerals. P values less than .05 were considered significant.
3. Results
3.1. Screening and retention
More than 300 parents responded to the study advertisements, and 98 healthy infants met the entry criteria for enrollment and were consented to the study. Of these infants, 5 withdrew prior to the intervention phase of the study and were considered “in screen” but not “enrolled.” Of the 93 infants enrolled, 3 withdrew after the first day of soy isoflavone challenge (6-month time point) because of intolerance to soy formula. Complete or partial data were obtained from 90 infants over the 7 clinic visits. The numbers of infants who successfully completed all 7 visits and provided urine samples for analysis were 24 (86%) of 28 in the breast-fed group, 28 (82%) of 34 in the cow’s milk formula–fed group, and 18 (64%) of 28 in the soy infant formula–fed group.
3.2. Demographics
The demographics of the infants enrolled in the study are detailed in the Table. Comparison of the groups by analysis of variance for continuous variables and χ2 test for categorical data found no significant differences among the groups with regard to screening age, height, weight, or sex (see Table). The racial composition of the cohort was 80.6% white, 12.9% black, 2.2% Asian, and 4.3%mixed race. Ethnically, 99% of the subjects were non-Hispanic; there was 1 Hispanic baby enrolled.
Table.
Demographics for the infant cohort enrolled in the studya
| Breast-fed (n = 28) |
Cow’s milk formula–fed (n = 34) |
Soy milk formula–fed (n = 28) |
|
|---|---|---|---|
| Ageb | 6.0 ± 0.7 | 5.8 ± 1.1 | 6.0 ± 1.2 |
| Heightb | 66.2 ± 2.5 | 66.3 ± 3.6 | 66.4 ± 3.4 |
| Weightb | 7.5 ± 0.8 | 7.7 ± 1.3 | 7.5 ± 1.1 |
| Male | 14 | 15 | 14 |
| Female | 14 | 19 | 14 |
Values for age, height, and weight are arithmetic means (±SD).
Groups were compared by analysis of variance for continuous variables and χ2 test for categorical data, and no significant differences were found among the groups in any category.
3.3. Equol producer status
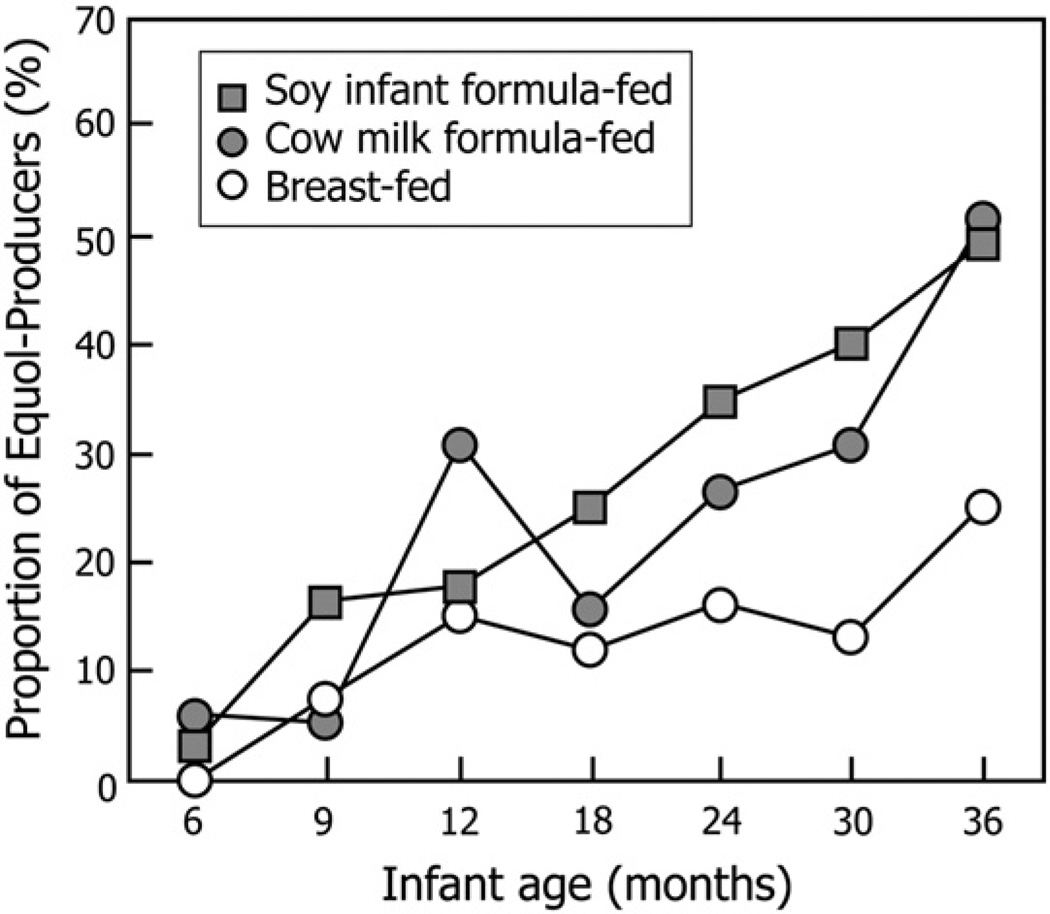
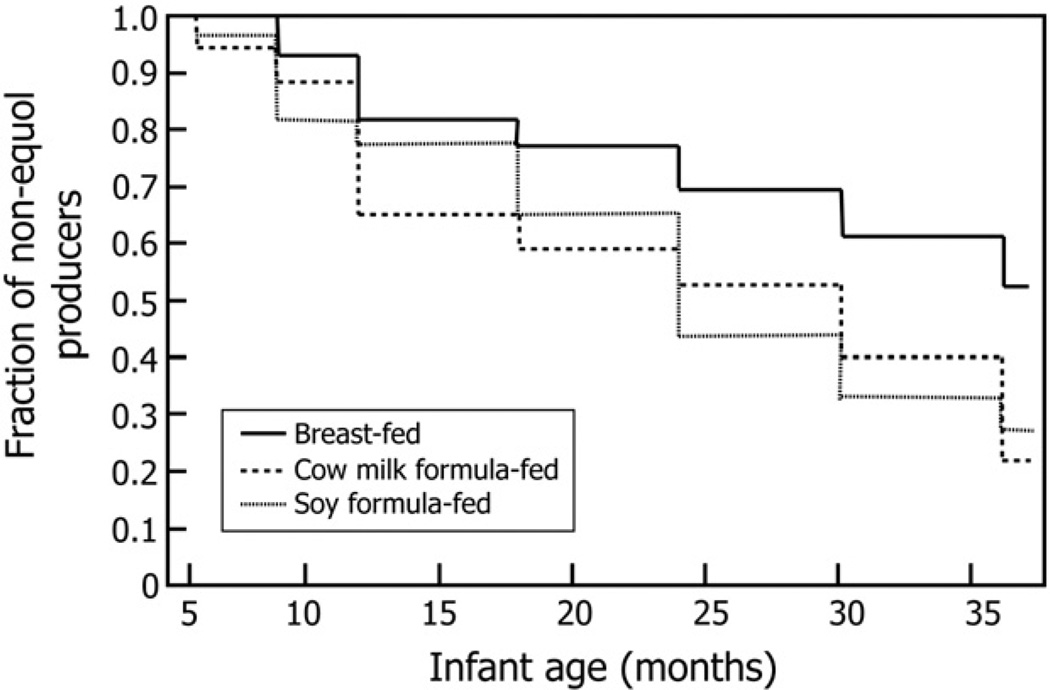
The proportion of infants who were equol producers, defined by the log10 transformed urinary S-(−)equol/daidzein ratio, at each of the 7 study visits for the different groups is shown in Fig. 1. At the start of the study, none of the 6-month-old breast-fed infants produced S-(−)equol when challenged with soy isoflavones, whereas 6.1% and 3.8% of the of the cow’s milk formula–fed and soy infant formula–fed infants proved to be equol producers by age 6 months. At 9 months of age, equol producers were observed in all 3 groups, but the proportion of equol producers in the soy infant formula–fed and cow’s milk formula–fed groups was higher than in breast-fed infants. Kaplan-Meier plots revealed that in breast milk–fed infants, it took a longer time to the first appearance of S-(−)equol when compared with either formula-fed infants (Fig. 2). By 3 years of age, 50% of the infants in both the formula-fed groups produced S-(−)equol when tested compared with only 25% of the infants who were breast-fed (Fig. 1).
Fig. 1.
Age-related changes in the proportion of breast-fed, cow’smilk formula–fed, or soy infant formula–fed infants who tested positive for equol production when challenged with soy isoflavones prospectively from 6 months to 3 years of age1,2,3. 1Values are the percentage of infants per breast-fed, cow’s milk formula–fed, or soy formula–fed infants producing equol from the total of those attending each visit. 2Data represent all measurements for each variable at each time point for all infants attending the visits. 3No significant differences were found between any of the groups at any of the time points.
Fig. 2.
Kaplan-Meier plots showing the time to first appearance of positive S-(−)equol production for breast-fed infants, cow’s milk formula–fed, or soy infant formula–fed infants in the first year of life1,2,3. 1Values represent the fraction of breast-fed, cow’s milk formula–fed, or soy formula–fed infants who did not produce equol at any one visit over the time course of the study. 2Data represent all measurements for each variable at each time point for all infants attending the visit. 3Statistics: data were analyzed by Kaplan-Meier time-to-event analysis. A P value of .07 was the result of the Kaplan-Meier time-to-event analysis that was an overall comparison of the 3 individual groups. The time to event in this analysis was the time to first occurrence of equol production.
A Kaplan-Meier time-to-event analysis, which was an overall comparison of the 3 individual groups, resulted in a P value of .07. The “time to event” in this analysis was the time to first occurrence of equol production. Because the overall P values did not reach the P = .05 level of significance, pairwise comparisons were not performed. When the 3 food groups are compared at each individual time point, no significant differences among the groups were found at any time point at the P = .05 level (minimum P value = 0.14). Although not significant, these findings indicate a trend (P = .07) to higher S-(−)equol production by formula feeding from 18 months of age onward.
3.4. Impact of antibiotics on equol production
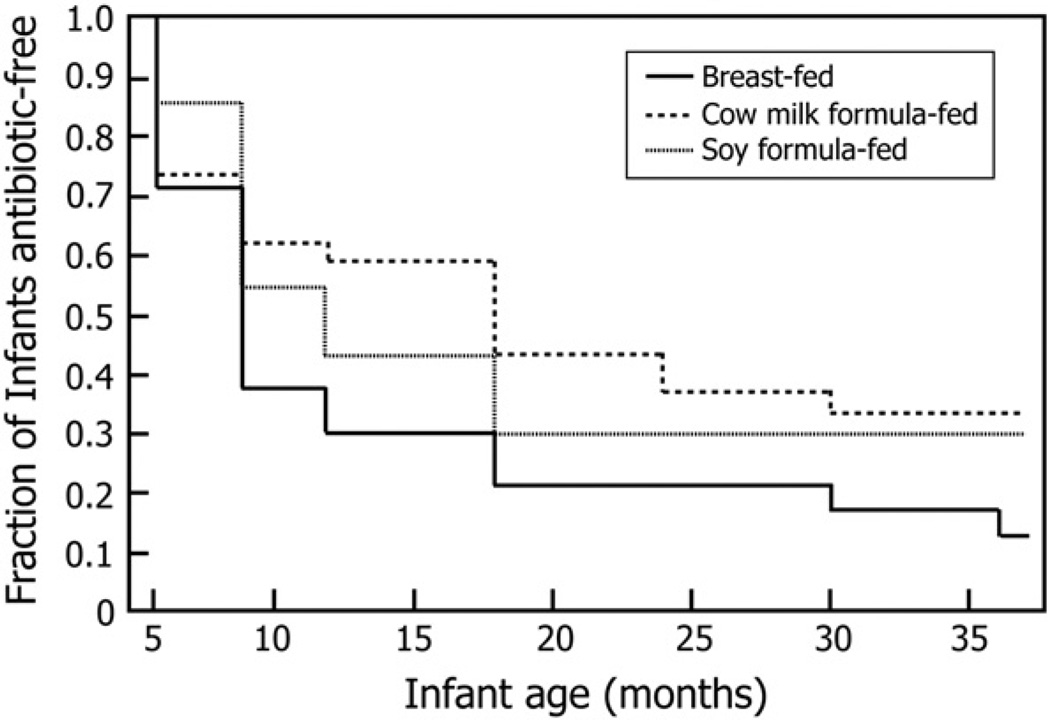
From a total of 564 study visits, the parents reported that infants had been administered antibiotics on 152 occasions prior to a visit (27% of the visits). For 139 of the infant visits, the parents were able to provide the name(s) of the antibiotics given, and in only 13 cases, the antibiotic information could not be obtained. Of the known cases, 71.7% of the time (n = 109), only 1 course of antibiotic was administered, and 28.3% of the time (n = 43), 2 or more courses of different antibiotics were administered to treat a variety of infections. The antibiotics were classified into 3 main groups. The first group (1) was the β-lactam’s, which included (1a) the penicillin (amoxicillin) subclass, (1b) the cephalosporin subclass, and (1c) a β-Lactam plus clavulanate (augmentin). The second group (2) was the trimemthroprim dihydrofolate inhibitors (Bactin, Bactrim, Trimethoprim, Sulfamethoxazole, and Septra), and the third group (3) was the azalide’s (Azithromycin, Zithromax, Azithrocin, Zmax, and Azin). The proportion of infants who had never been given a course of antibiotics in each of the groups was 5 of 28, 12 of 34, and 10 of 28 for the breast-fed, cow’s milk formula–fed, and soy formula–fed groups. Thus, in our study, breast-fed infants were observed to be more likely to received antibiotic treatment in the first 3 years of life than infants fed formulas. A Kaplan-Meier plot showing the time to first use of antibiotics revealed that breast-fed infants were also more likely to have been administered antibiotics at an earlier age than either formula-fed infants (Fig. 3; P = .079).
Fig. 3.
Kaplan-Meier plots depicting the proportion of infants who remained antibiotic-free over the first 3 years of life stratified according to whether they were breast-fed, cow’s milk formula fed, or soy infant formula fed during the first year1,2,3. 1Values represent the fraction of breast-fed, cow’s milk formula–fed, or soy formula–fed infants not treated by antibiotics at any one visit over the time course of the study. 2Data represent all measurements for each variable at each time point for all infants attending the visit. 3Statistics: χ2 test was performed at each age to evaluate the association between antibiotic use and equol production. No significant association was found when all food groups were combined for each age, except for age 36 months, where there was a lower percentage of equol producers in infants who received antibiotics. When the association was evaluated for each age group within each nutrition group, no significant associations were observed.
When the relationship between antibiotic use and equol production was analyzed by χ2 test, no significant association was found when the 3 nutrition groups were combined for each age, except for age 36 months, where there was a lower percentage of equol producers in infants who received antibiotics. Because antibiotic use can influence S-(−)equol production, this fairly common use of antibiotics could explain why in some infants S-(−)equol production was not consistently maintained throughout the entire study period.
3.5. Early nutrition and S-(−)equol production
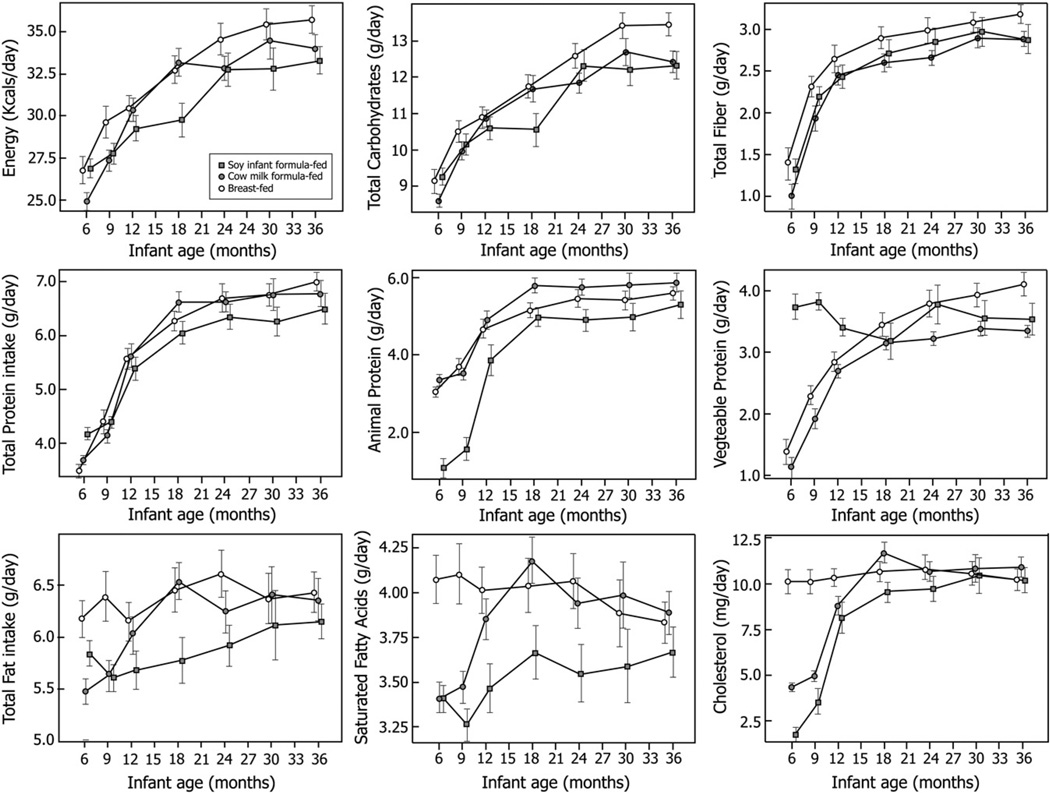
Changes in the mean (±SD) total energy intake and the intake of total carbohydrate, fiber, protein, animal protein, vegetable protein fat, saturated fatty acids, and polyunsaturated fatty acids over the 2.5-year follow-up of the infants fed breast milk, soy formula, and cow’s milk formula are plotted in Fig. 4. There were no major differences among the groups with respect to the macronutrient or micronutrient intakes over the time course of the study, save an expected higher intake of vegetable protein and concomitant lower intake of animal protein in those infants fed the plant-based soy formula during the first year of life. Dietary analysis after 1 year and hence postweaning was found to be similar among the groups. Furthermore, no significant associations could be found between infants who were equol producers when compared with non–equol producers for individual components of the diet.
Fig. 4.
Changes in the arithmetic mean (±SD) for total energy intake and intakes of total carbohydrate, fiber, protein, animal protein, vegetable protein fat, saturated fatty acids, and polyunsaturated fatty acids over the 2.5-year follow-up of the infants who were breast-fed, cow’s milk formula fed, or soy infant formula fed1,2,3. 1Values represent the number of kilocalories per day, grams per day, or milligrams per day as indicated for each food category. 2Data represent all measurements for each variable at each age time point for all infants attending the visit from the breast-fed, cow’s milk formula–fed, or soy formula–fed infants. 3No statistical differences were seen between the breast-fed, cow’s milk formula–fed, or soy formula–fed infants for any of the food categories at any of the study visit time points.
4. Discussion
In 1997, it was first reported that the plasma concentrations of genistein and daidzein in 4-month-old soy formula–fed infants were extremely high (mean ± SD, 684 ± 443 and 295 ± 60 ng/mL, respectively), whereas only traces of these isoflavones were present in the plasma of age-matched cow’s milk formula–fed and breast-fed infants [24]. The isoflavone metabolite S-(−)equol [8] was barely detectable in most Western infants fed soy formula, presumably because the equol-producing bacteria are absent at birth and colonization of the intestinal microbiome is a time-dependent and adaptive process. By adult life, typically 25% to 35% of Western adults consistently produce S-(−)equol when consuming soy foods [5–8], and this proportion is far higher in Asian adults who consume traditional soy foods (50%–60%) [9–12]. There is evidence for greater clinical responses to soy-based diets in equol producers compared with non–equol producers [1,8,13–15]. Also, given the low incidence of hormone-dependent conditions in Asians, which has been suggested to be due to exposure to dietary isoflavones, particularly S-(−)equol [4], understanding the determinants of equol production has important relevance to disease prevention and treatment. The production of S-(−)equol is developmentally regulated. A limited number of studies have reported on isoflavone excretion in infants and children [26,27,31–36], but only 2 previous studies attempted to investigate the developmental aspects of S-(−)equol production [26,27]. In a prospective “free-living” study of 166 full-term infants followed up from birth to 1 year of age in which plasma, saliva, and urine samples were collected, S-(−)equol was not detectable in the urine of 98%, 95%, and 78%, respectively, of breast-fed, soy infant formula–fed and cow’s milk formula–fed infants [27]. However, in this study, a soy challenge was not used to assess the frequency of equol producers in the breast-fed or cow’s milk formula–fed infants. Without exposure to daidzin/daidzein, the precursor to S-(−)equol, it is not possible to knowwhether or not an infant is an equol producer. The finding of low levels of S-(−)equol in the urine from cow’s milk formula–fed infants can be explained by low levels of S-(−)equol naturally present in cow’s milk [37]. The frequency of equol producers was also examined in a nonprospective manner in groups of infants and children aged 4 months to 7 years [26] and in children of 6 to 18 years of age [36]. The latter, a study of 90 German children, found equol in the urine of 31% of the children, but concentrations were extremely low (<9 ng/mL) and indicative of negligible soy food intakes. However, again, in the absence of a significant soy isoflavone challenge, it is not possible to determine with accuracy the frequency of equol producers [7]. The study from Hoey et al [26] only compared soy infant formula–fed and cow’smilk formula–fed infants and reported equol producers to account for 19% and only 5%, respectively, in these groups. The difference in frequency was also associated with differences in fecal bacterial composition, with an increased number of Bifidobacteria, Bacteroides, and Clostridia spp in the cultured fecal samples from the cow’s milk formula–fed children [26]. In none of the previous studies of infants and children was there an attempt to examine differences in diet composition. Although our study did not attempt to monitor changes in intestinal bacteria through fecal analysis, it does represent, to our knowledge, the first attempt to examine the impact of the choice of early nutritional regimens and the post weaning diet, on the developmental production of S-(−)equol in the first 3 years of life in a prospective manner, while using the conventional soy isoflavone challenge to determine the ability of the infant to produce S-(−)equol. Equol producer status was determined from the ratio of S-(−)equol (the product) to its precursor daidzein in urine and is independent of S-(−)equol concentration [7]. Total S-(−)equol production is not necessary to determine equol producer status and would be difficult to determine in infants because of practical limitations of collecting accurate 24-hour urines. The study commenced at 6 months of age because previous data had shown that very few infants before this age are able to produce S-(−)equol because of an immature intestinal microbiome [24–27,32]. This study was particularly challenging because of social and practical demands of recruitment and of following up the same infant prospectively for 2.5 years in a study requiring 7 separate clinic visits. Some parents were unable to bring their infant to every study visit. Nevertheless, once enrolled, the retention rate was deemed to be good, and data reported here represent 564 (89%) clinic visits of a possible 630 for the 90 infants who completed the first study visit.
The findings from our study indicate that independent of the form of early nutrition, only a small proportion of infants of 6 months of age have the ability to metabolize daidzin/daidzein to S-(−)equol when challenged with soy formula, and thus, the bacteria that produce S-(−)equol are presumably absent, or the conditions are not optimal in the intestinal lumen for catalyzing the multiple reactions leading to S-(−)equol formation. This observation is consistent with data from other studies [24–27,32]. During development, there was a trend to increasing proportions of infants becoming equol producers, so that by 3 years of age, 25% to 50% of infants were found to be equol producers. [8]. Interestingly, the frequency of equol producers in the formula-fed infants (ie, soy and cow’s milk formulas) was consistently higher than that for breast-fed infants throughout the 3 years of age, although statistical treatment of these data found the differences among the groups to not quite reach significance (P = .07) presumably because of sample size limitations. Why the prevalence of equol-producing adults is generally higher in Asians (50%–60%) compared with Westerners (25%–35%) remains unclear, but it is possible that exposure to traditional soy foods in early life and throughout childhood could explain the different frequencies. It has been reported that repeated soy food consumption by 20 Chinese adults who were equol nonproducers led to 40% converting to equol producers after 16 weeks [35], but similar chronic exposure to soy foods in Western adults was reported to have no effect on stimulating S-(−)equol production [38]. It is conceivable that dietary factors play a role in the ability to make S-(−)equol [8,29], and in this regard, the intake of a number of dietary components has been found to differ between adult equol producers and nonproducers, including notably polyunsaturated fatty acid intake [29,39].
In this infant study, 3-day diet records were obtained by the parents for each infant at 6 months of age and at each test point up to 3 years of age, and a detailed dietary analysis of the intake of 143 macronutrients and micronutrients was calculated [29,30], providing comparison of similarities or differences in the postweaning diet. In this regard, our study appears to be unique in providing the first detailed information comparing the diets of infants over the first 3 years of life stratified according to early feeding regimen. Significant differences were observed in the diet composition of soy formula–fed infants in the first year of life, when compared with breast-fed and cow’s milk formula–fed infants, most notably with regard to intakes of animal protein, which was markedly and expectedly lower in the soy formula–fed infants, as was total cholesterol intake. The inverse relationship was evident for vegetable protein intake, as expected. Significant effects on cholesterol synthesis rates have been documented for infants fed soy formula [31], which were associated with high isoflavone intakes, but contributing to this effect is the fact that this plant protein is devoid of cholesterol. Postweaning (>12 months of age) total energy intake increased with age consistent with increasing demands for growth, and we found no significant differences in the dietary intake of any of the macronutrients among the 3 groups of infants. Thus, there appears to be no obvious qualitative or quantitative associations between the postweaning diet and the ability of the infant to ultimately be able to make S-(−)equol. These findings indicate that the most critical determinant of S-(−)equol production is colonization of the intestinal tract with specific equol-producing bacteria, of which numerous species have been identified. Unlike the study by Hoey et al [26] that showed significant differences in fecal bacteria between soy infant formula–fed and cow’s milk formula–fed infants, we are unable to corroborate possible differences in the microflora among the 3 feeding groups because we did not determine fecal microflora. Our study did, however, provide insight into the stability of S-(−)equol production over this 2.5-year period. Of those infants where 2 sequential visits are available (excluding those who produced equol for the first time at age 36 months) once S-(−)equol was produced, 34% continued to produce S-(−)equol throughout the study, whereas 66% tested negative for S-(−)equol on at least one occasion during follow-up. In adults, we have reported that equol production is generally a stable phenomenon [1,7,8,29], although antibiotics may or may not abolish S-(−)equol production [29,34,40–43]. It is impossible to perform a prospective study over the first 3 years of life and expect infants to be completely free of antibiotics. Antibiotics were frequently administered over the course of the study, and for 28% of the infants, this occurred on multiple occasions. Interestingly, it was observed that antibiotic use was greatest in the breast-fed infant group, where 82% of these infants were treated with antibiotics at some point in the first 3 years of life, compared with 62.5% and 64%, respectively, of the infants in the cow’s milk formula–fed and soy formula–fed groups. We attribute some of the variability in the consistency of testing positive in equol producers to the high frequency of antibiotic use. Whether the overall lower frequency of equol producers in the breast-fed infants may be the result of a greater frequency of antibiotic use is difficult to determine. Two previous studies describing the effects of oral antibiotics on urinary isoflavone excretion in older children reported reduced urinary isoflavone excretion [33,34] while taking broad spectrum antibiotics, but the small sample size precluded conclusions regarding the impact of antibiotic use on S-(−)equol formation. Given the wide range of antibiotics prescribed to our infants, it was not possible to make associations between the type of antibiotic administered and its effect on S-(−)equol formation, and whether the higher frequency of antibiotic use in the breast-fed group could explain the lower frequency of equol producers is unclear.
In conclusion, this study confirms our hypothesis that the formation of S-(−)equol in early life is a developmentally diet-related and time-dependent process that appears to be unrelated to the postweaning intake of macronutrient or micronutrients and did not support a higher incidence of equol producers occurring in breast-fed infants. Very few infants produce S-(−)equol in the first year of life when challenged with soy isoflavones, but by 3 years of age, the proportion of equol producers reached 25% to 50% which is similar to that observed in adults from Western and Asian populations. There is, however, a trend toward a greater propensity to be an equol producer when the infants were bottle-fed with either cow’s milk or soy infant formula, rather than breast-fed in the first year of life.
Abbreviations
- CCHMC
Cincinnati Children’s Hospital Medical Center
- HPLC
high-pressure liquid chromatography
- MRM
multiple-reaction ion monitoring
- MS
mass spectrometry
- PC
principal component
- S-(−)equol
S-(−)7-hydroxy-3-(4′-hydroxyphenyl)-chroman
- SFA
saturated fatty acids
Footnotes
Author’s contribution
K.D.R.S. (PI) and N.M.B. (Co-investigator) wrote the NIH grant, designed the research, and prepared the manuscript; N.M.B. and S.L.G. conducted the clinical component of the study; S.S. S. provided food databases and analyzed diet data; X.Z. performed the MS analysis of the urine samples; J.E.H. was the supervising physician. All authors read and approved the manuscript and accept full responsibility for its contents. K.D.R.S. accepts primary responsibility for the final manuscript content.
Conflict of interest
None of the authors have a conflict of interest related to this study.
REFERENCES
- 1.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 2.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 3.Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KDR. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem J. 1982;201:353–357. doi: 10.1042/bj2010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- 5.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 6.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–2193. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KDR, Clerici C. Equol. history, chemistry, and formation. J Nutr. 2010;140:1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128:1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 10.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 11.Akaza H, Miyanaga N, Takahima N, Naito S, Hirao Y, Tsukamoto T, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 12.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wahala K, Thomas WK, et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136:1347–1351. doi: 10.1093/jn/136.5.1347. [DOI] [PubMed] [Google Scholar]
- 13.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem. 2007;18:380–390. doi: 10.1016/j.jnutbio.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KW, Ko KP, Ahn Y, Kim CS, Park SJ, Park JK, et al. Epidemiological profiles between equol producers and non producers: a genome wide association study of the equol-producing phenotype. Genes Nutr. 2012;7:567–574. doi: 10.1007/s12263-012-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, et al. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol. 2008;46:2713–2720. doi: 10.1016/j.fct.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(−)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–2043. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RL, Greiwe JS, Desai PB, Schwen RJ. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor beta-agonist being developed for the treatment of menopausal symptoms. Menopause. 2011;18:185–193. [PubMed] [Google Scholar]
- 19.Schwen RJ, Nguyen L, Plomley JB, Jackson RL. Toxicokinetics and lack of uterotropic effect of orally administered S-equol. Food Chem Toxicol. 2012;50:1741–1748. doi: 10.1016/j.fct.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 22.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Lund TD, Munson DJ, Haldy ME, Setchell KDR, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 26.Hoey L, Rowland IR, Lloyd AS, Clarke DB, Wiseman H. Influence of soya-based infant formula consumption on isoflavone and gut microflora metabolite concentrations in urine and on faecal microflora composition and metabolic activity in infants and children. Br J Nutr. 2004;91:607–616. doi: 10.1079/BJN20031083. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, et al. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19:223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Setchell KDR, Brown NM, Summer S, King EC, Heubi JE, Cole S, et al. Dietary factors influence production of the soy isoflavone metabolite S-(−)equol in healthy adults. J Nutr. 2013;143(12):1950–1958. doi: 10.3945/jn.113.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summer SS, Ollberding NJ, Guy T, Setchell KD, Brown N, Kalkwarf HJ. Cross-border use of food databases: equivalence of US and Australian databases for macronutrients. J Acad Nutr Diet. 2013;113:1340–1345. doi: 10.1016/j.jand.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz ML, Wong WW, Mimouni F, Hachey DL, Setchell KDR, Klein PD, et al. Effects of infant nutrition on cholesterol synthesis rates. Pediatr Res. 1994;35:135–140. doi: 10.1203/00006450-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am J Clin Nutr. 2006;84:406–413. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 33.Halm BM, Ashburn LA, Franke AA. Isoflavones from soya foods are more bioavailable in children than adults. Br J Nutr. 2007;98:998–1005. doi: 10.1017/S0007114507771866. [DOI] [PubMed] [Google Scholar]
- 34.Franke AA, Halm BM, Ashburn LA. Urinary isoflavones are increased in adults, but decreased in children, consuming soy when on oral antibiotic therapy. Nutr Cancer. 2008;60:627–635. doi: 10.1080/01635580802065310. [DOI] [PubMed] [Google Scholar]
- 35.Ko TF, Tsai HS, Lin SM, Liu CD, Learn SP, Chiou RY. GC-MS determined distribution of urinary equol producers as affected by age, gender, and repeated ingestions of soymilk. J Food Sci. 2010;75:H306–H310. doi: 10.1111/j.1750-3841.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- 36.Degen GH, Blaszkewicz M, Shi L, Buyken AE, Remer T. Urinary isoflavone phytoestrogens in German children and adolescents—a longitudinal examination in the DONALD cohort. Mol Nutr Food Res. 2011;55:359–367. doi: 10.1002/mnfr.201000325. [DOI] [PubMed] [Google Scholar]
- 37.Hoikkala A, Mustonen E, Saastamoinen I, Jokela T, Taponen J, Saloniemi H, et al. High levels of equol in organic skimmed Finnish cow milk. Mol Nutr Food Res. 2007;51:782–786. doi: 10.1002/mnfr.200600222. [DOI] [PubMed] [Google Scholar]
- 38.Vedrine N, Mathey J, Morand C, Brandolini M, Davicco MJ, Guy L, et al. One-month exposure to soy isoflavones did not induce the ability to produce equol in postmenopausal women. Eur J Clin Nutr. 2006;60:1039–1045. doi: 10.1038/sj.ejcn.1602415. [DOI] [PubMed] [Google Scholar]
- 39.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–2246. doi: 10.1093/jn/137.10.2242. [DOI] [PubMed] [Google Scholar]
- 40.Blair RM, Appt SE, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis) J Nutr. 2003;133:2262–2267. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson C, Berman S, Humbert O, Lampe JW. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J Nutr. 2004;134:596–599. doi: 10.1093/jn/134.3.596. [DOI] [PubMed] [Google Scholar]
- 42.Franke AA, Lai JF, Halm BM, Pagano I, Kono N, Mack WJ, et al. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2012;23:573–579. doi: 10.1016/j.jnutbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Br J Nutr. 2012;107:1201–1206. doi: 10.1017/S0007114511004223. [DOI] [PMC free article] [PubMed] [Google Scholar]