Abstract
Huntington's disease (HD) is a progressive neurodegenerative condition. At-risk individuals have accessed predictive testing via direct mutation testing since 1993. The UK Huntington's Prediction Consortium has collected anonymised data on UK predictive tests, annually, from 1993 to 2014: 9407 predictive tests were performed across 23 UK centres. Where gender was recorded, 4077 participants were male (44.3%) and 5122 were female (55.7%). The median age of participants was 37 years. The most common reason for predictive testing was to reduce uncertainty (70.5%). Of the 8441 predictive tests on individuals at 50% prior risk, 4629 (54.8%) were reported as mutation negative and 3790 (44.9%) were mutation positive, with 22 (0.3%) in the database being uninterpretable. Using a prevalence figure of 12.3 × 10−5, the cumulative uptake of predictive testing in the 50% at-risk UK population from 1994 to 2014 was estimated at 17.4% (95% CI: 16.9–18.0%). We present the largest study conducted on predictive testing in HD. Our findings indicate that the vast majority of individuals at risk of HD (>80%) have not undergone predictive testing. Future therapies in HD will likely target presymptomatic individuals; therefore, identifying the at-risk population whose gene status is unknown is of significant public health value.
Introduction
Huntington's disease (HD) is a slowly progressive autosomal dominant neurodegenerative disorder characterised by the development of abnormalities in movement, cognitive decline and behavioural disturbances. It is caused by an expanded CAG repeat in the first exon of the HTT gene that encodes an abnormal polyglutamine expansion in the Huntingtin protein, resulting in selective neuronal degeneration.1
Predictive testing for HD first became available in 1986 using linkage analysis;2 this was superseded by direct mutation analysis in 1993. Guidelines for testing have been well established and updated periodically3 with strong recommendations to avoid testing those under the age of 18 years. In our earlier report the cumulative uptake of predictive testing was expressed as the number of predictive tests performed as a proportion of those estimated to be at 50% risk in the population.4 Previous studies have overestimated the cumulative uptake of predictive tests because, as new cases of HD are diagnosed, the number of individuals known to be at risk of HD in a given population (the denominator in the equation) increases over time. To overcome this problem, Tassicker et al5 proposed a method to determine the cumulative at-risk population over time that accounts for the changing at-risk population and disease duration.5 This method has been applied to small populations in Victoria, Australia5 and Northern Ireland.6 Here, using the UK Huntington's Prediction Consortium (UK HPC) data from 1993 to 2014, we update our previous study4 and report on the largest study ever conducted on the experience of predictive testing for HD.
Materials and methods
The UK HPC was launched in 1989 to collect anonymised data systematically on all completed presymptomatic HD tests in the United Kingdom and to provide a forum for professional discussion.4 Forms were completed by a nominated participant from each centre and entered into a central database by a consortium coordinator. All 23 centres in the United Kingdom offering predictive testing have participated contemporaneously from the outset giving near complete coverage of data.
Data recorded included the testing centre, gender, age of testee, prior genetic risk, details of the type of genetic test and final result. From 1993 to 2006, the test results were characterised as being normal/unaffected (<36 CAG repeat length on largest allele) or abnormal/affected (≥36 repeat length on the largest allele). From 2007, centres also began to report on intermediate alleles (28–35 CAG repeat length on the largest allele) and reduced penetrance alleles (36–39 CAG repeat length on the largest allele). In order to maximise engagement from the centres, data collection was kept to a minimum; consequently, information on the smaller allele was not collected. From 2010, data on the ethnicity of testees started to be collected. From 2012, maternal/paternal origin of mutation and reasons for predictive testing, using a standardised list after reviewing the genetic file, started to be collected.
Calculations for cumulative uptake of predictive testing (1994–2014)
Data on predictive tests were available for 23 testing centres for the period 1993 to 2014. Because predictive testing only became available towards the end of 1993, this year was excluded from the analysis. Within each year, tests were included for those who were ≥18 years old, and who were identified as being at 50% risk. There were 483 centre-level data collection periods in total (ie, 21 years multiplied by 23 centres). Ten centres did not report data for one or more years, and this resulted in 26 of the 483 (5.4%) centre-level data collection periods having missing data. We assumed missing data were missing at random (‘MAR') and used multiple imputation based on predictive mean matching to impute plausible values based on year and centre.7
The cumulative uptake of predictive testing over the study period was determined using the formula described by Tassicker et al.5 The numerator is the cumulative number of predictive tests performed. The denominator is number of eligible participants, given by:
Where pop (y) denotes the UK population aged ≥18 years in year y.
Classically, the ratio of the number of symptomatic individuals (prevalence) to individuals at 50% risk of developing HD in a population has been described as being, on theoretical grounds, 1:5.8 Tassicker et al5 revised this to a ratio of 1:4.2 based on their own empirical data from multiple source ascertainment of at-risk individuals in Victoria, Australia; this revised ratio is similar to the empirical data from Northern Ireland.6 A ratio of 1:4.2 was used to calculate the cumulative uptake as it is based on empirical evidence.
In order to calculate reliably the population at 50% risk, accurate estimates of the prevalence of HD in the general population are required. This is itself a contentious issue (see Discussion). In the present study, the prevalence figure of 12.3 per 100 000 in the adult population was used to calculate the cumulative uptake of predictive testing over the study period.
Discrepancies in the estimated prevalence of HD and the ratio of symptomatic individuals to individuals at 50% risk in the research literature were accounted for by performing a sensitivity analysis to calculate multiple uptake figures based on several different parameter estimates.
Disease duration, from onset of symptoms to death, was taken as being 18.8 years based on the average of the reported median disease duration in two large cohort studies.9, 10 Mid-year population estimates for those aged ≥18 years were obtained for the United Kingdom for each year in the period 1994 to 2014.11
Statistical analysis
Descriptive analysis was carried out using SPSS Statistics software (version 22; Armonk, NY, USA). For the main results, point estimates and 95% confidence intervals are reported. In order to calculate confidence intervals, observed counts were assumed to follow a Poisson distribution. To detect the effect of any change in the age distribution of participants between the early years and the more recent years, a comparison was made between the age distribution in the first 5 years (1994–1998) and the last 5 years (2010–2014). Comparisons were made using the Kolmogorov–Smirnov test for difference in distribution, and the Mann–Whitney test for difference in median. For the main analysis, missing data were imputed via predictive mean matching based on centre and year. Imputation was carried out using the mice package in R (Version 3.2.2).
Results
Demographic information and prior risk
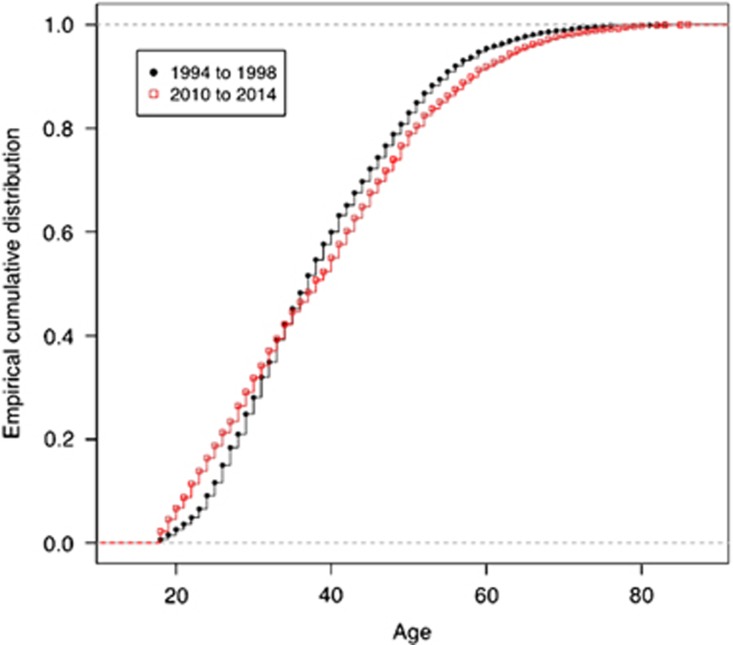
Between 1993 and 2014, 9407 predictive tests for HD were performed in 23 centres in the United Kingdom. Of these, 8441 (89.9%) were at 50% prior risk; 642 (6.8%) were at 25% prior risk; 13 (0.1%) were at 12.5% prior risk; and in 311 cases (3.3%) the information was missing or uninterpretable on the database. The median age at testing was 37 years with an interquartile range of 29–47 years. There was no significant difference in the median age of participants at 50% risk of HD in the first 5 years of predictive testing (1994–1998) compared with the last 5 years (2010–2014) (P=0.60). However, a comparison of the age distributions of participants between these two periods showed a statistically significant difference (P<0.0001), with proportionally more older individuals undertaking the test in the earlier years (1994–1998), and proportionally more younger individuals undertaking the test in the later years (2010–2014) (Figure 1). In the first 5-year period, 42% of results were positive and 58% were negative but this changed significantly in the last 5-year period with 51% positive and 49% negative (χ2=20.6, P<0.0001).
Figure 1.
Age distribution of those tested in 1994–1998 (black) compared with 2010–2014 (red).
Predictive testing by year
In the initial years of predictive testing (1994–1998), the mean number of tests performed annually on those ≥18 years old with a 50% prior risk, after imputation of missing data, was 535 (SD 2.9). From 1999 to 2014, the corresponding figure had fallen to 362 (SD 1.9). Overall, there have been 123 retests that were mostly from previous linkage analyses.
Reasons for testing
Patient-reported reasons for undergoing testing were not recorded uniformly for all centres or consistently throughout the study. Reasons for predictive testing were recorded for 4743 (50.4%) participants and we report on the number of responses recorded for the five most common reasons for predictive testing. Participants were allowed to cite multiple reasons for predictive testing and this is reflected in the results. The most common patient-reported reasons for undertaking predictive testing were to reduce uncertainty (70.5% of responders) and for future planning (57.7%). Other reasons included to provide information to relatives (38.3%), reproductive decision making (23.0%) and the hope for future treatments (9.6%). Rarer reasons for predictive testing included: testing as part of the assisted reproduction protocols, insurance/mortgage purposes, ‘curiosity', patient-reported symptoms, the absence of prior genetic confirmation in families with a clinical diagnosis of HD and to plan social care in individuals with pre-existing learning or physical disabilities.
Test outcomes
In all, 9372 tests (99.6%) were performed by direct mutation analysis characterising the CAG repeat length. A total of 27 tests (0.3%) were performed by linkage analysis and 8 participants had both tests (0.1%). The majority of linkage tests (93%) were carried out before 1994.
Table 1 summarises the outcome of the predictive tests. There were slightly more females requesting testing (55.7% of cases where the gender was known) and a small excess of negative results (54.8%). From 2010, data on intermediate and reduced penetrance alleles became available: 77 results were reported as intermediate alleles (4.2%) and 82 results were reported as reduced penetrance alleles (4.5%).
Table 1. Summary of UK HPC data 1993–2014.
| Variable | |
|---|---|
| Gender | |
| Male | 4077 (43.3%) |
| Female | 5122 (54.5%) |
| Missing information | 208 (2.2%) |
| Total | 9407 |
| A priori risk of Huntington's disease | |
| 12.5% | 13 (0.1%) |
| 25% | 642 (6.8%) |
| 50% | 8441 (89.7%) |
| Other/missing information | 311 (3.3%) |
| Total | 9407 |
| Results of predictive testing for individuals with a priori risk of 50% | |
| Outcome | |
| 1993–2014 | |
| Negative (<36 repeats) | 4629 (54.8%) |
| Positive (≥36 repeats) | 3790 (44.9%) |
| Result missing/uninterpretable | 22 (0.3%) |
| Total | 8441 |
| 2010–2014 | |
| Normal (0–27 repeats) | 857 (46.9%) |
| Intermediate alleles (28–35 repeats) | 77 (4.2%) |
| Reduced penetrance alleles (36–39 repeats) | 82 (4.5%) |
| Affected (40+ repeats) | 812 (44.4%) |
| Total | 1828 |
Cumulative uptake of predictive testing
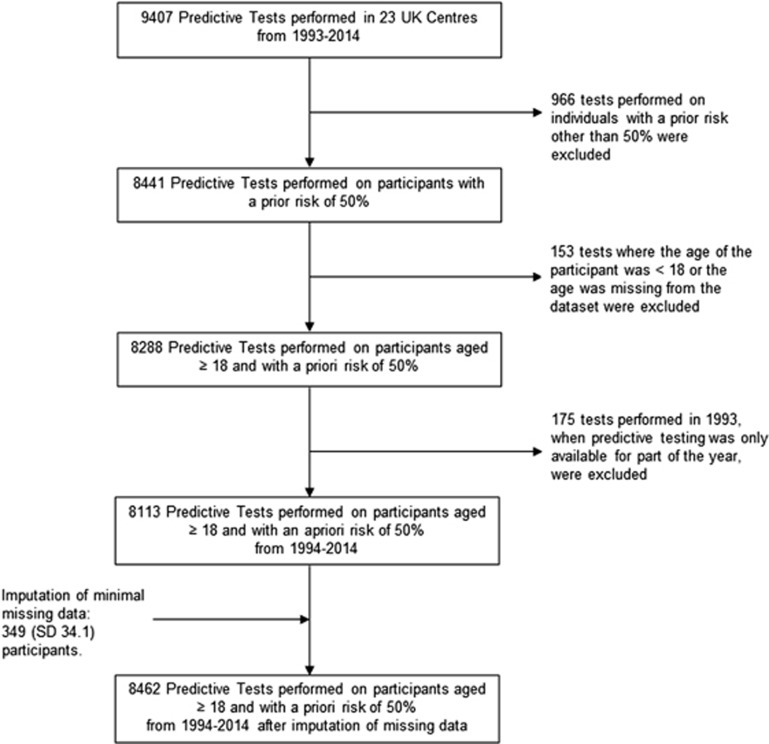
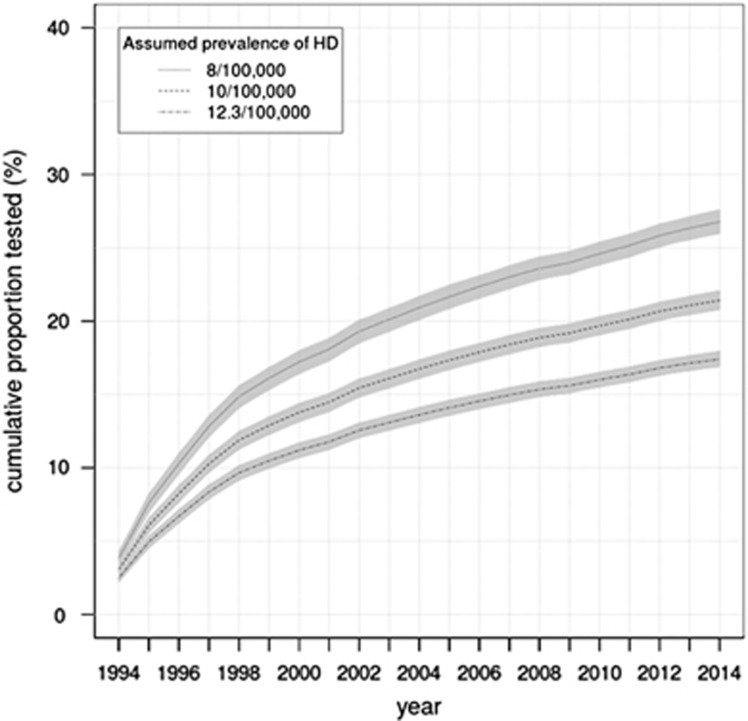
From 1994 to 2014, 8113 predictive tests performed on participants aged ≥18 years and with a prior risk of 50% were recorded in the database. After imputation of minimal missing data, an estimated 8462 predictive tests were performed in the same group. Figure 2 illustrates the selection criteria for cases for the analysis of the cumulative uptake of predictive testing. The estimated cumulative number of individuals aged ≥18 years and at 50% risk from 1994 to 2014 was 48 591, giving a cumulative uptake of 17.4% (95% CI: 16.9–18.0%). Figure 3 illustrates the cumulative uptake of predictive testing in the 50% at-risk population from 1994 to 2014 over time for 3 different estimates of the UK prevalence in the adult population. A sensitivity analysis giving results for different estimates of the prevalence of HD in the UK population over the age of 18 years and ratios of prevalence to those at 50% risk of 4.2 and 5 is given in Table 2.
Figure 2.
Selection of cases for analysis of cumulative uptake.
Figure 3.
Summary of sensitivity testing for cumulative uptake of predictive tests in the United Kingdom. For the sake of clarity, not all scenarios are shown. The ratio of symptomatic individuals to those at risk in this figure is 1:4.2. Thick lines represent the confidence interval.
Table 2. Calculated figures for cumulative uptake of predictive testing based on alternative parameter estimates.
| UK prevalence of HD (per 100 000 adult population) | Ratio of symptomatic individuals to individuals at 50% risk of developing HD | Calculated cumulative uptake (95% CI) (%) |
|---|---|---|
| 8 | 1:4.2 | 26.8 (25.9–27.6) |
| 8 | 1:5 | 22.3 (21.6–23.0) |
| 10 | 1:4.2 | 21.4 (20.7–22.1) |
| 10 | 1:5 | 17.8 (17.3–18.4) |
| 12 | 1:4.2 | 17.8 (17.3–18.4) |
| 12 | 1:5 | 14.9 (14.4–15.3) |
| 12.3 | 1:4.2 | 17.4 (16.9–18.0) |
| 12.3 | 1:5 | 14.5 (14.0–15.0) |
| 13 | 1:4.2 | 16.5 (16.0–17.0) |
| 13 | 1:5 | 13.7 (13.3–14.2) |
The effect of three of these scenarios on cumulative uptake of predictive testing is illustrated in Figure 3.
Discussion
The data presented here represent the largest study ever conducted on the uptake of predictive testing for HD in a single population. Over a 22-year period from 1993 to 2014, 9407 predictive tests were recorded and the estimated cumulative uptake of predictive testing for the UK population at 50% risk of developing HD from 1994 to 2014 was 17.4% (95% CI: 16.9–18.0%).
Our group previously reported a similar cumulative uptake figure of 18% after only four complete years of predictive testing (1993–1997).4 Interestingly, even though this is a similar figure to that which we now report, this study used a lower prevalence of 7.5 per 100 000 and did not correct for the increasing at-risk population over time, and hence represents a significant overestimation of the uptake of predictive testing in 1997. Table 3 summarises the key features of other studies of the uptake of predictive testing in HD. Our estimated uptake of 17.4% is comparable to figures of 12.3–14.6% in Northern Ireland6 and 15.4% in Victoria, Australia5 where the formula of Tassicker et al5 was applied. In the remaining studies, the reported uptake of predictive testing varies from 5 to 44.7% however, it is imperative to note that there are major methodological differences in the approaches to these calculations of uptake that invalidates a direct comparison with our findings. For instance, in Slovenia, where an uptake figure of 44.7% was reported, the authors included tests performed on those at prior risks <50% and expressed uptake as the fraction of tests performed on those identified at risk from their registries and medical records rather than the estimated at-risk population.12 Using this method, any incomplete ascertainment of at-risk individuals will lead to an overestimate of cumulative uptake. The sources of variation in uptake measurements are summarised in Table 4.
Table 3. Studies of uptake of predictive testing in Huntington's disease.
| Population | Study period | Reported HD prevalence (per 100 000) | Number of completed tests | Use of the Tassicker et al5 formula | Reported uptake (%) | Authors |
|---|---|---|---|---|---|---|
| Europe | ||||||
| France | 1993–1999 | 5 | 409 | No | 5 | Goizet et al19 |
| Greece | 1995–2008 | 5.4 | 256 | No | 8.6 | Panas et al20 |
| The Netherlands | 1987–1997 | 6.5 | 752 | No | 24 | Maat-Kievit et al21 |
| North Rhine-Westphalia, Germany | 1993–2004 | 10 | 248 | No | _ | Bernhardt et al22 |
| Northern Ireland | 1990–2009 | 10.6 | 212 | Yes | 14.4–14.6 | Morrison et al6 |
| Slovenia | 1997–2007 | 5.16 | 68 | No | 44.7 | Peterlin et al12 |
| United Kingdom | 1987–1997 | 7.5 | 2722 | No | 18 | Harper et al4 |
| America | ||||||
| Canada | 1987–2000 | 8.4 | 1061 | No | 18 | Creighton et al23 |
| Montreal, Canada | 1994–2008 | _ | 135 | No | 9.2 | Dufrasne et al24 |
| Australia | ||||||
| Victoria, Australia | 1996–2003 | 8 | 333 | Yes | 15.4 | Tassicker et al5 |
Table 4. Sources of variation in estimated cumulative uptake of predictive testing in different studies.
| Sources of variation | |
|---|---|
| Potential overestimation | Inclusion of tests performed on those with a priori risk of 25% in the numerator but not the denominator (Maat-Kievit et al21). |
| No adjustment for the increasing number of at-risk individuals over the study period (Goizet et al,19 Panas et al,20 Bernhardt et al,22 Harper et al,4 Creighton et al,23 Dufrasne et al24). | |
| Expressing uptake as the fraction of tests performed in individuals who engaged in the counselling process rather than the estimated at-risk population (Bernhardt et al22). | |
| Expressing uptake as a fraction of tests done in individuals registered being at risk on HD registries rather than the estimated, total at-risk population (Maat-Kieivit et al21). | |
| Use of the Conneally ratio of symptomatic individuals: 50% at risk individuals as being 1:5 on theoretical grounds that may overestimate the at-risk population when compared with the ratio of 1:4.2 given found empirically (Creighton et al,23 Panas et al20). | |
| Underascertainment of HD cases may underestimate disease prevalence and thereby underestimate the population at 50% risk. | |
| Potential underestimation | Inclusion of individuals under 18 years of age in the at-risk population who are generally unable to access predictive testing. Those under 18 years may represent 5.6–9% of the 50% at-risk population (Harper et al,4 Creighton et al,23 Panas et al20). |
A low uptake of predictive testing in HD has been reported consistently in several different populations. Broadly, this may be explained by factors related to autonomous decision making by individuals or by issues related to accessing services. The individual choice to undertake predictive testing may be affected by the absence of disease-modifying treatments, anxiety about an abnormal result, the financial implications of an abnormal result, personal experiences of caring for relatives with HD or perceived stigma associated with the condition. Barriers to accessing predictive testing may include the travel requirements required to access specialist counselling and testing services, the stress of travelling, opportunity costs of missed work or time with family members, the inflexibility of the testing process and difficulty in accessing support.13 In the United Kingdom, health care is funded from general taxation and is free at the point of access; in addition, the 23 centres have a wide geographical spread, and hence the financial and geographic barriers to testing may be less influential in determining the uptake rate. A moratorium between the government and the insurance industry is in place; restricting access of insurers to predictive test results for policies below specified financial limits thereby removing another potential barrier to predictive testing.14 We do not know the extent to which those at 50% risk who do not come forward do not want/need to know whether they have inherited the mutation or are unaware of its availability. There are methodological problems associated with studies on this topic that include: biased samples, low response rates, measuring different stages of the decision-making process and differences between questionnaires and face-to-face interviews.15
Uptake of testing is of course rather higher for other, different conditions, where not only predictive testing but also therapeutic interventions are available; for example, in a systematic review of 14 studies of BRCA1/2 testing, mean uptake was 59% with a range 25–96%.16 In a recent UK study, uptake of predictive testing for Lynch syndrome was 55.7%.17
Of the 9199 predictive tests where the gender was recorded, 55.7% of the participants were female. This is consistent with the majority of studies of uptake in predictive testing where there is a slight female preponderance among those tested.12, 18, 19, 20, 21, 22, 23, 24 Several theories have been proposed to explain this finding; it may be that females are more likely to address questions relevant to reproductive planning and may feel better equipped to cope with an abnormal result.25
The median age for participants who underwent predictive testing was 37 years. This is slightly higher than Greece,20 Slovenia12 and France,19 but slightly younger than the figures of 39.3 and 41 reported in Canada23 and Victoria,18 respectively. An interesting finding of our study was that the age distribution of individuals undertaking predictive testing changed from the beginning to the end of the study with proportionally more older testees in the early years, and proportionally more younger testees in the later years. As life expectancies increase, there may be a trend towards the presentation of HD later in life. Interestingly, the frequency of reduced penetrance alleles in the 50% at-risk group >60 years old was 6.4% compared with 4.5% for all age groups at 50% risk.
In the initial years of predictive testing the number of tests performed annually was significantly higher than in subsequent years. This may be explained, in part, by a backlog of participants who were unable to access predictive testing via linkage analysis before 1993 with only a small contribution from those requesting retesting.
Of the predictive tests performed on participants with a prior risk of 50%, 54.8% were gene negative whereas 44.6% were gene positive. The tendency to acquire slightly more normal results when the expected frequencies would be 50:50 is a phenomenon that is consistent with other studies.12, 19, 21, 23, 24 This may be explained by the fact that the 50% risk of developing HD is the risk at birth and those who are asymptomatic in adult life when they present for testing have a slightly reduced risk by virtue of the fact that they have not developed symptoms thus far. In keeping with this, we observed a lower frequency of abnormal results in the higher age groups (data not shown). The change in the ratio of positive to negative results between the first and last 5-year period was highly significant. This may be explained by change in the age distribution with more young individuals requesting the test and by the fact that some people in the early stage of the disease request a predictive test.
Genotypes with intermediate alleles (IAs) and reduced penetrance (RP) alleles were recorded consistently from 2010 onwards with their observed frequencies in the 50% at-risk population being a minimum of 4.2 and 4.5%, respectively. Sequeiros et al26 assessed CAG repeat length in the general population in Portugal and determined the frequency of genotypes with IAs and RP alleles to be 6 and 0.1%, respectively. In Canada, the corresponding figures were 5.8 and 0.4%, respectively.27 The current study demonstrates a higher proportion of reduced penetrance alleles in the predictive testing population and is in keeping with the figure of Sequeiros et al26 of 4.8% in the Portuguese predictive testing population. Furthermore, the current study only reports on the largest allele size; therefore, it may underestimate the presence of an additional IA/RP allele in genotypes where a larger CAG expansion was present.
The participant-reported reasons for undergoing predictive testing were recorded for 4743 (50.4%) individuals. Decreasing uncertainty was the most commonly cited reason for having the predictive test, with planning for the future and reproductive options also being important factors. These reasons are similar to those given by participants when predictive testing was first introduced.28 It is important to note that although an abnormal predictive test informs the testee they will develop the disease, it does not predict the age of onset, thereby introducing a different uncertainty. Furthermore, a result demonstrating a reduced penetrance allele maintains a level of uncertainty for both the individuals and their offspring. One can anticipate that the development of effective disease-modifying therapies targeted at those in the presymptomatic phase of the disease would provide a major reason for at-risk individuals to undergo predictive testing.
Limitations of the current study
In the current study, basic information on participants undergoing predictive testing was collected annually from 23 centres over a 22-year period. Over the course of the study, some centres did not provide data for every year of predictive testing (overall, a maximum of 5.4% of yearly centre-level reports were missing), and therefore imputation of missing data was required to calculate more reliably an estimate of cumulative uptake of predictive testing. In some instances, there was incomplete or uninterpretable information on prior risk, gender and test results recorded on the database. A further issue is that the codes of practice were not standardised across the 23 centres and multiple laboratories; for example, in some centres, individuals who presented as being at risk may have had neurological signs of HD but still went through a predictive testing protocol rather than diagnostic testing, and this may have contributed to the difference in the proportion of positive to negative test results between the first and last 5 years of the study period.
The method of Tassicker et al5 for calculation of the cumulative uptake of testing relies on an accurate measure of prevalence for HD in the adult population and the ratio of symptomatic cases/population at 50% risk. Based on highest quality and most current evidence we estimated these to be 12.3 per 100 000 and 1:4.2, respectively. The UK prevalence of HD is a contentious issue: two recent studies calculating prevalence figures based on two different GP research databases in the United Kingdom gave vastly different estimates of 5.96 (from The Health Network Improvement database)29 and 12.3 (from the General Practice Research Database)30 per 100 000 of the population. This significant discrepancy may, in part, be explained by the fact that the former study looked at the prevalence in the whole population, whereas the latter exclusively looked at the population over 20 years old. The significance of this is that the prevalence of cases under the age of 20 years is very much lower than in the older population.31 In the present study, the figure of 12.3 per 100 000 in the >20-year-old population was used to calculate the cumulative uptake of predictive testing over the study period. The rationale for this is that, firstly, it is based on recent evidence and, secondly, the present study is principally interested in the prevalence of those who can reliably give informed consent for predictive testing, which is ≥18 years of age, and close enough to the age given for that prevalence study.
If our parameter estimates are inaccurate, then the estimated cumulative uptake figure will vary. However, as the sensitivity analysis shows, regardless of the probable parameter estimates used, <27% of individuals at 50% risk of HD have undergone predictive testing and our best estimate is that the cumulative uptake of predictive testing is ∼17.4% (95% CI: 16.9–18.0%).
Another limitation of the method of Tassicker et al5 is the assumption of a constant disease duration throughout the course of the study. We used the average disease duration from symptom onset to death as being 18.8 years, based on the average of two large cohort studies. However, it may be argued that the disease duration has increased over the past 22 years because of direct mutation testing leading to earlier diagnoses. The current study demonstrates that the vast majority of those at risk of HD in the United Kingdom (>80%) have not participated in the predictive testing programme.
Implications for practice
This study is important as an awareness of the size of the at-risk population who have not yet had their gene status confirmed is necessary in order to plan the best services for this population. There are a number of active clinical trials;32 if even one of these identifies an agent that provides a neuroprotective effect in HD, then services will need to change. It is likely that the number of individuals requesting testing will increase. Services will therefore need to be able to manage an increased demand for testing, as well as being able to offer appropriate interventions and follow-up in the presymptomatic phase of the illness. Individuals who have decided that they wish to be tested, or who have just learned that they are at risk, may question genetic counsellors about the need to follow the current predictive guidelines; it is useful to say that <20% of people who are at risk have chosen to have a predictive test, hence there is still a need to proceed cautiously.
The authors declare no conflict of interest.
References
- Bates GP, Dorsey R, Gusella JF et al: Huntington disease. Nat Rev Dis Prim 2015; 1: article number 15005. [DOI] [PubMed] [Google Scholar]
- Meissen GJ, Myers RH, Mastromauro CA et al: Predictive testing for Huntingtons disease with use of a linked DNA marker. N Engl J Med 1988; 318: 535–542. [DOI] [PubMed] [Google Scholar]
- MacLeod R, Tibben A, Frontali M et al: Recommendations for the predictive genetic test in Huntington's disease. Clin Genet 2013; 83: 221–231. [DOI] [PubMed] [Google Scholar]
- Harper PS, Lim C, Craufurd D: Ten years of presymptomatic testing for Huntington's disease : the experience of the UK Huntington's Disease Prediction Consortium. J Med Genet 2000; 37: 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassicker RJ, Teltscher B, Trembath MK et al: Problems assessing uptake of Huntington disease predictive testing and a proposed solution. Eur J Hum Genet 2009; 17: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P, Harding-Lester S, Bradley A: Uptake of Huntington disease predictive testing in a complete population. Clin Genet 2011; 80: 281–286. [DOI] [PubMed] [Google Scholar]
- van Buuren S: Flexible Imputation of Missing Data. Chapman and Hall/CRC, 2012. [Google Scholar]
- Conneally PM: Huntington disease: genetics and epidemiology. Am J Hum Genet 1984; 36: 506–526. [PMC free article] [PubMed] [Google Scholar]
- Roos RA, Hermans J, Vegter-van der Vlis M, van Ommen GJ, Bruyn GW: Duration of illness in Huntington's disease is not related to age at onset. J Neurol Neurosurg Psychiatry 1993; 56: 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Gray J, Ivashina J, Conneally PM: Differences in duration of Huntington's disease based on age at onset. J Neurol Neurosurg Psychiatry 1999; 66: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. Available from https://ons.gov.uk.
- Peterlin B, Kobal J, Teran N, Flisar D, Lovrecic L: Epidemiology of Huntington's disease in Slovenia. Acta Neurol Scand 2009; 119: 371–375. [DOI] [PubMed] [Google Scholar]
- Hawkins AK, Creighton S, Hayden MR: When access is an issue: exploring barriers to predictive testing for Huntington disease in British Columbia, Canada. Eur J Hum Genet 2013; 21: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HM Government and Association of British Insurers. Concordat and moratorium on genetics and insurance, 2014. Available from https://www.gov.uk/government/publications/agreement-extended-on-predictive-genetic-tests-and-insurance (accessed 25 February 2016).
- Binedell J, Soldan JR: Noparticipation in Huntington's disease predictive testing: reasons for caution in interpreting findings. J Genet Couns 1997; 6: 419–432. [DOI] [PubMed] [Google Scholar]
- Ropka ME, Wenzel J, Phillips EK, Siadaty M, Philbrick JT: Uptake rates for breast cancer genetic testing: asystematic review. Cancer Epidemiol Biomarker Prev 2006; 15: 840–855. [DOI] [PubMed] [Google Scholar]
- Barow P, Green K, Clancy T, Lalloo F, Hill J, Evans DG: Improving the uptake of predictive testing and colorectal screening in Lynch syndrome: a regional primary care survey. Clin Genet 2015; 87: 517–524. [DOI] [PubMed] [Google Scholar]
- Trembath MK, Tassicker RJ, Collins VR, Mansie S, Sheffield LJ, Delatycki MB: Fifteen years of experience in predictive testing for Huntington disease at a single testing center in Victoria, Australia. Genet Med 2006; 8: 673–680. [DOI] [PubMed] [Google Scholar]
- Goizet C, Lesca G, Dürr A, French Group for Presymptomatic Testing in Neurogenetic Disorders: Presymptomatic testing in Huntington's disease and autosomal dominant cerebellar ataxias. Neurology 2002; 59: 1330–1336. [DOI] [PubMed] [Google Scholar]
- Panas M, Karadima G, Vassos E et al: Huntington's disease in Greece: the experience of 14 years. Clin Genet 2011; 80: 586–590. [DOI] [PubMed] [Google Scholar]
- Maat-Kievit A, Vegter-van der Vlis M, Zoeteweij M, Losekoot M, van Haeringen A, Roos R: Paradox of a better test for Huntington's disease. J Neurol Neurosurg Psychiatry 2000; 69: 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt C, Schwan A-M, Kraus P, Epplen JT, Kuntsmann E: Decreasing uptake of predictive testing for Huntington's disease in a German centre: 12 years' experience (1993–2004). Eur J Hum Genet 2009; 17: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton S, Almqvist EW, MacGregor D et al: Predictive, pre-natal and diagnostic genetic testing for Huntington's disease: the experience in Canada from 1987 to 2000. Clin Genet 2003; 63: 462–475. [DOI] [PubMed] [Google Scholar]
- Dufrasne S, Roy M, Galvez M: Experience over fifteen years with a protocol for predictive testing for Huntington disease. Mol Genet Metab 2011; 102: 494–504. [DOI] [PubMed] [Google Scholar]
- Taylor S: Gender differences in attitudes among those at risk for Huntington's disease. Genet Test 2005; 9: 152–157. [DOI] [PubMed] [Google Scholar]
- Sequeiros J, Ramos EM, Cerqueira J et al: Large normal and reduced penetrance alleles in Huntington disease: instability in families and frequency at the laboratory, at the clinic and in the population. Clin Genet 2010; 78: 381–387. [DOI] [PubMed] [Google Scholar]
- Semaka A, Kay C, Doty CN, Collins JA, Tam N, Hayden MR: High frequency of intermediate alleles on Huntington disease-associated haplotypes in British Columbia's general population. Am J Med Genet B Neuropsychiatr Genet 2013; 162: 864–871. [DOI] [PubMed] [Google Scholar]
- Evers-Kiebooms G, Cassiman JJ, van den Berghe H: Attitudes towards predictive testing in Huntington's disease: a recent survey in Belgium. J Med Genet 1987; 24: 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackley C, Hoppitt TJ, Calvert M et al: Huntington's disease: current epidemiology and pharmacological management in UK primary care. Neuroepidemiology 2011; 37: 216–221. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Douglas I, Rawlins MD, Wexler NS, Tabrizi SJ, Smeeth L: Prevalence of adult Huntington's disease in the UK based on diagnoses recorded in general practice records. J Neurol Neurosurg Psychiatry 2013; 84: 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas I, Evans S, Rawlins MD et al: Juvenile Huntington's disease: a population-based study using the General Practice Research Database. BMJ Open 2013; 3e002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild EJ, Tabrizi SJ: Targets for future clinical trials in Huntington's disease: what's in the pipeline? Mov Disord 2014; 29: 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]