ABSTRACT
We hypothesized that treatment of methamphetamine (METH) effects with a mixture of 2 high affinity anti-METH monoclonal antibodies (mAb) with differing molecular recognition for METH-like structures could increase efficacy compared to treatment with a single mAb. The antibodies studied were mAb7F9 (METH and amphetamine [AMP] KD = 7.7 and 270 nM) and mAb4G9 (16 nM and 110 nM, respectively) in a 50:50 mixture.
Adult male Sprague Dawley Rats were treated with iv saline or a loading dose of mAb7F9-mAb4G9 (141 mg/kg of each mAb) followed by 2 weekly doses (70.5 mg/kg total) on days 7 and 14. METH challenge doses (0.56 mg/kg) were administered 4 hrs and 3 days after each mAb7F9-mAb4G9 treatment, and 7 days after the final treatment (day 21). Locomotor activity (0–4 hrs) and serum METH and AMP concentrations (at 5 hrs) were measured after each METH challenge. MAb7F9-mAb4G9 treatment significantly reduced the duration of locomotor activity after 6 of the 7 METH doses (P < 0.05) and significantly increased serum METH and AMP concentrations. Administering three-fold higher METH doses (1.68 mg/kg) on days 24 and 28 showed mAb7F9-mAb4G9 treatment had negligible effects on the duration of METH-induced locomotor activity.
These data were then compared to previous monotherapy data. While mAb7F9-mAb4G9 therapy inhibited the effects of multiple METH challenge doses, the inhibition was not as profound or as long lasting as the effects of mAb7F9 treatment alone. These data demonstrate the importance of both mAb affinity and specificity in the production of effective, long-lasting anti-METH mAb therapies.
KEYWORDS: combination therapy, locomotor activity, Methamphetamine, monoclonal antibody, rat, substance abuse
Introduction
The use of multiple monoclonal antibodies (mAbs) against a protein or viral disease target is often used to increase mAb efficacy. For example, in primates, the ZMApp anti-Ebola virus mAb cocktail which consists of 3 chimeric mAbs increases survival from Ebola infection relative to monotherapy (i.e., one mAb).1 In a humanized murine HIV model, treatment with more than one mAb against multiple viral epitopes leads to a substantial reduction in viral load for 60 days while the use of monotherapy is less efficacious, less consistent, and demonstrates a shorter duration of effect.2 In mice, mAbs against multiple ghrelin (a protein associated with obesity) epitopes result in the antagonism of endogenous ghrelin-mediated effects (i.e., decreased feeding), while monotherapy is ineffective.3
Use of mAb therapy has been proposed to treat (+)-methamphetamine (METH) addiction and decrease METH toxicity. High affinity anti-METH mAb bind METH in the serum and extracellular fluid4,5 and are thought to both slow the entry and decrease the accumulation of METH in the central nervous system which decreases METH-induced behavioral effects. Compared to large protein or multi-protein disease targets, the METH molecule (149 g/mol) lacks the size and chemical complexity to permit simultaneous mAb binding to more than one unique epitope. Indeed, X-ray crystallography studies show METH is completely engulfed in the METH mAb binding site, without external exposure of different parts (or epitopes) of the molecule.6 However, METH in solution exists in different rotational conformations.7-9 For a given mAb with unique affinity and specificity for METH, these potential differences in METH configuration could lead to increases or decreases in in vivo anti-METH mAb binding over time.
We hypothesized that a 50:50 mixture of 2 different high affinity anti-METH mAbs with different specificities for METH-like molecules could increase efficacy over previously reported single mAb therapy.10 To test this hypothesis, we administered a mixture of equal parts mAb7F9 and mAb4G9 to male Sprague-Dawley rats (METH KD = 7.7 and 16 nM, respectively; amphetamine [AMP] KD = 270 nM and 110 nM).11,12 Over a one-month period, we used METH-induced behavioral and METH concentration measures to assess the efficacy of the anti-METH mAb mixture in clinically relevant scenarios of chronic mAb treatment for METH addiction. As part of this assessment, we also determined the potential consequences of missing or stopping mAb treatments, a likely scenario for patients undergoing treatment for METH addiction. Finally, we compared the mAb7F9-mAb4G9 treatment to previously reported results of mAb7F9 monotherapy10 by assessing how continuously each treatment affected the duration of action of METH-induced locomotor activity during and after the mAb dosing regimens, as well as comparing each treatments' effect on METH and AMP concentrations. This comparison showed the combination mAb7F9-mAb4G9 therapy was much less effective than treatment with mAb7F9 alone. Additionally, the results demonstrated the importance of affinity and specificity as critical mAb properties for effective, long-lasting anti-METH mAb function.
Results
MAb7F9-mAb4G9 dissociation constants (KD) for METH and AMP
We characterized the combined KD of the 50:50 mixture of mAb7F9 and mAb4G9. The KD value for METH determined with the 50:50 mixture was 7.6 nM. This was essentially the same value as previously reported for METH binding to mAb7F9 (KD = of 7.7 nM), but was different from the 16 nM KD value for mAb4G9.11,12 The Ki value for AMP binding with the 50:50 mixture was 145 nM. This was similar to the reported AMP binding with mAb4G9 (KD = 110 nM) rather than the 270 nM AMP KD value reported for mAb7F9.12 Because both antibodies had higher affinity for METH than AMP, Ki values of AMP binding determined using a [3H]-METH radioligand are higher than KD values determined using a [3H]-AMP radioligand.
METH-induced locomotor activity with increasing METH doses
The effects of METH dose on locomotor response were determined to establish a model for assessing mAb effects on METH-induced behavior. The distance traveled vs time after METH administration for 4 different METH doses is shown in Figure 1A. There were no significant differences (P < 0.05) in total distance traveled between any of the 4 doses (Fig. 1B). There were, however, significant dose-dependent increases in the duration of METH-induced locomotor activity as the METH dose increased (Fig. 1C).
Figure 1.
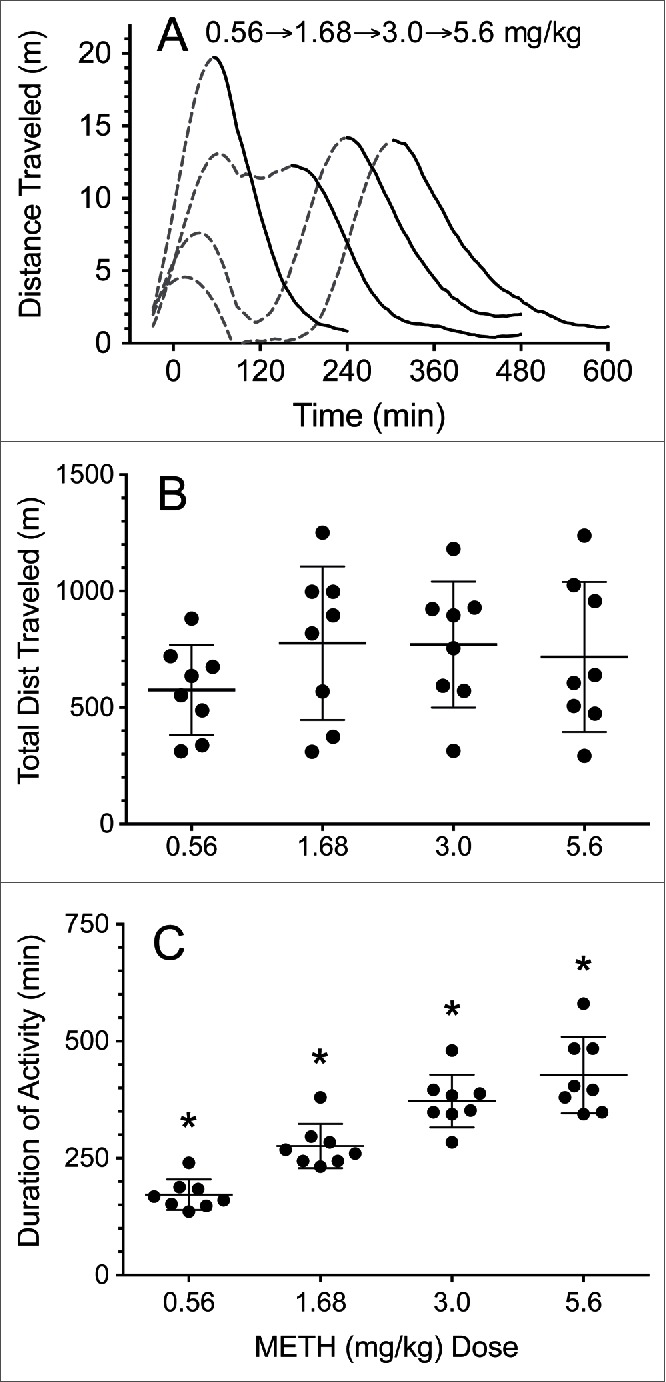
METH effects on distance traveled and duration of locomotor activity after 0.56–5.6 mg/kg increasing weekly METH doses in male rats (n = 8). (A) Distance traveled over time. Early data after METH dosing are depicted as a dashed line. The solid line denotes the terminal phase of the horizontal distance traveled data. These data were fit to a smoothing function for clarity. Curves for increasing doses are plotted from left to right. (B) Total average distance traveled in meters for each dose (±SD). (C) Average duration of action for each dose of METH induced locomotion (±SD). The * denotes a statistically significant difference (P < 0.05) from all other groups.
Phase 1. MAb7F9-mAb4G9 effects on repeated 0.56 mg/kg METH challenges on days 0–21
Over the first 21 days of the study, 7 METH challenge doses were administered to assess the efficacy of chronic mAb treatment. Efficacy was measured by analysis of locomotor activity and serum METH and AMP concentrations in control and treatment groups.
Representative plots of the average METH-induced distance traveled over time during the Phase 1 studies (control vs, mAb7F9-mAb4G9 treatments) are depicted in Figure 2. Similar representative plots of rearing behavior are not shown. The average rearing activity in the vehicle treated animals was lower in the current study than in the previous study with mAb7F9 alone,10 and mAb7F9-mAb4G9 treatment appeared to have less effects on METH-induced rearing than treatment with mAb7F9 alone. Table 1 summarizes the measurements of METH-induced locomotor activity in vehicle- and mAb-treated groups. Figure 3 shows the METH-induced distance traveled for vehicle and mAb7F9-mAb4G9 treatments. Rearing plots are not shown, but rearing behavior was not significantly decreased in the mAb7F9-mAb4G9 group compared to controls in any measurement interval.
Figure 2.
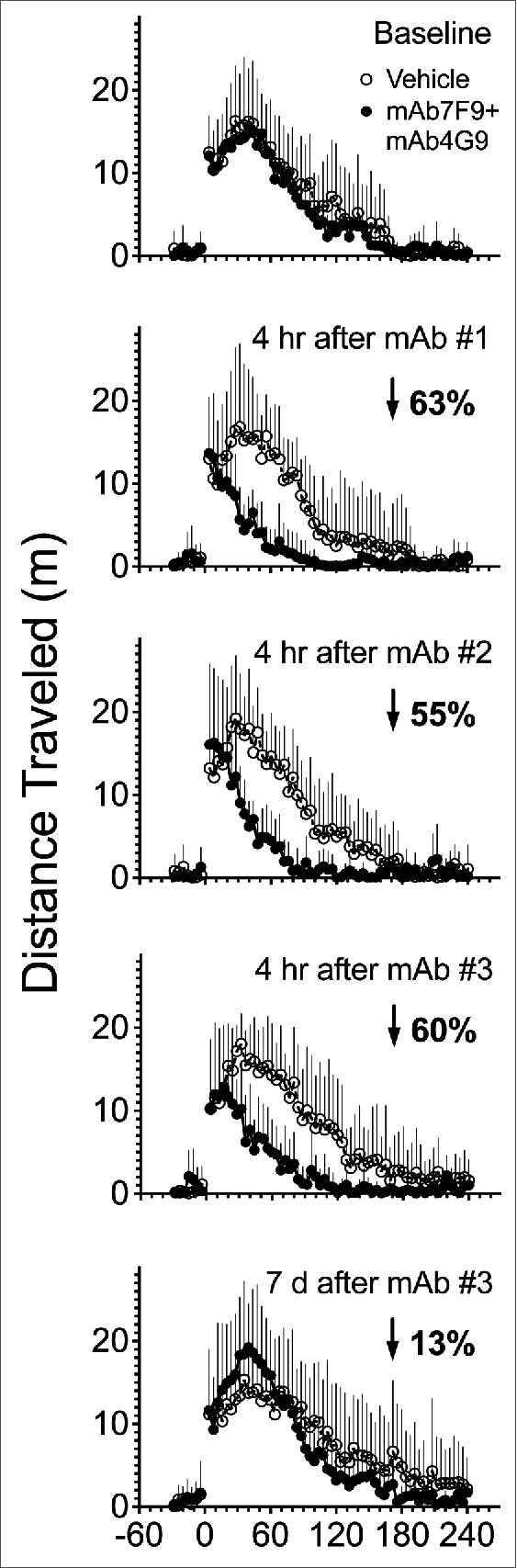
Profile of average distance traveled after 0.56 mg/kg METH challenge doses in control (open circle) and mAb7F9-mAb4G9 (closed circle) treated rats (+SD). Each measurement was collected in 4 min bin intervals, and the average value of these intervals for each group is reported. The METH-induced distance traveled before vehicle or mAb7F9-mAb4G9 administration (baseline) is shown in the top panel. The next 4 panels show the change in distance traveled over time in mAb7F9-mAb4G9 vs. vehicle-treated rats throughout the first 21 days of the study. The first mAb dose (mAb #1) was on day 0, the second mAb dose (mAb #2) was on day 7, and the final mAb dose was day 14 (mAb #3). Other time points are reported relative to mAb dosing. The average percentage decrease in total distance traveled in mAb7F9-mAb4G9 compared to vehicle-treated rats is indicated by the downward pointing arrow and the percentage decrease compared to vehicle-treated rats.
Table 1.
Analysis of distance traveled data to determine time of peak effect and duration of action after 0.56 mg/kg METH challenge doses in vehicle- and mAb7F9-mAb4G9 treated rats in Phase 1 studies.
| Time of Peak Effect (min ± SD) |
Duration of Action (min ± SD) |
|||||
|---|---|---|---|---|---|---|
| Day | Vehicle or mAbDose # | Time of METH Challenge after Vehicle or mAb | Vehicle | mAb | Vehicle | mAb |
| −4 | Pre-treatment Baseline | 35 ± 10 | 42 ± 20 | 134 ± 37 | 140 ± 40 | |
| 0 | 1 | 4 hr post | 44 ± 35 | 15 ± 16 | 124 ± 38 | 61 ± 19a |
| 3 | 3 day post | 48 ± 27 | 27 ± 16 | 129 ± 31 | 90 ± 9a | |
| 7 | 2 | 4 hr post | 37 ± 9 | 18 ± 21 | 132 ± 39 | 73 ± 12a |
| 10 | 3 day post | 60 ± 30 | 20 ± 13a | 164 ± 31 | 105 ± 26a | |
| 14 | 3 | 4 hr post | 44 ± 33 | 24 ± 11 | 146 ± 47 | 74 ± 27a |
| 17 | 3 day post | 54 ± 33 | 35 ± 18 | 165 ± 52 | 106 ± 31a | |
| 21 | 7 day post | 69 ± 51 | 37 ± 13 | 172 ± 67 | 141 ± 38 | |
aStatistical significance compared to time-matched vehicle controls (P < 0.05).
Figure 3.
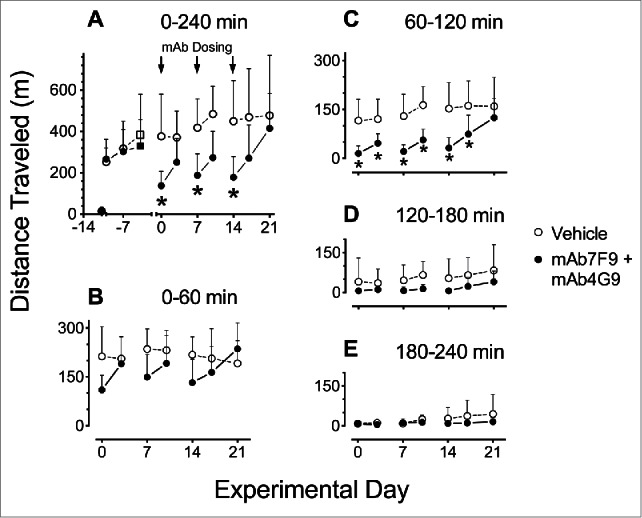
Average distance traveled after 0.56 mg/kg METH challenge doses in vehicle- (open circles, n = 8) vs. mAb7F9-mAb4G9 treated rats (closed circles, n = 7) throughout the Phase 1 studies (days 0–21) (+SD). Panel A shows total measurements over 4 hrs on each experimental day including the vehicle and mAb with saline or METH administrations. Measurements are shown in progressive 1 hr intervals in Panels B-E. The * denotes statistical significance compared to time-matched vehicle controls (P < 0.05). The differences between vehicle and mAb treated rats on days 3 and 10 in panel A were not significantly different, but showed P values of 0.06 and 0.07, respectively.
MAb7F9-mAb4G9 induced changes in METH and AMP serum concentrations compared to vehicle treatment are shown in Figure 4.
Figure 4.
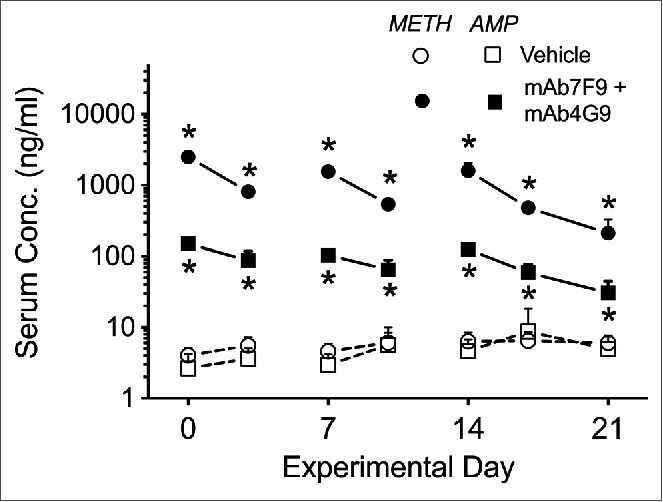
Average serum METH (circles) and metabolite AMP (squares) concentrations 5 hrs after the 0.56 mg/kg METH dose in vehicle- (open symbols) and mAb7F9-mAb4G9-treated (filled symbols) rats. The * denotes statistical significance compared to time-matched vehicle controls (P < 0.05).
Phase 2. MAb7F9-mAb4G9 effects on a 1.68 mg/kg METH challenge dose 10 and 14 days after discontinuation of mAb therapy (study days 24 and 28)
Ten and 14 days after the end of mAb treatment, the rats were administered a 3-fold higher METH dose (1.68 mg/kg) to test the remaining functional capacity of the mAb. We estimated this METH dose was about an 8- and 12-fold excess to the available METH binding sites on days 10 and 14 (respectively).
Figure 5 shows average distance traveled over time after METH administration. The best-fit line to the terminal distance traveled data in vehicle- and mAb7F9-mAb4G9-treated animals had an r2 = 0.86 and 0.92 (respectively) on day 24 and an r2 = 0.72 and 0.84 (respectively) on day 28. The best-fit line equations were y = −0.159x + 47 and y = −0.112x + 31 on day 24 and y = −0.127x + 40 and y = −0.106x+32 in vehicle- and mAb7F9-mAb4G9-treated animals respectively. Due to poor linearity of the rearing data points in the terminal phase of rearing activity, we did not attempt to fit a best-fit line to the terminal phase of the rearing data points.
Figure 5.
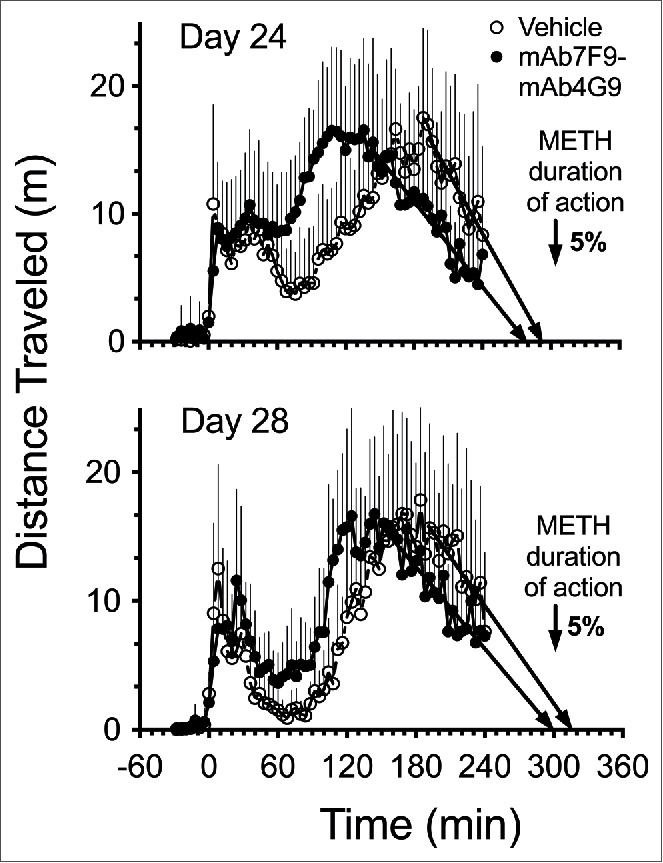
Average distance traveled over time after a 1.68 mg/kg METH dose (3-fold greater than Phase 1) in vehicle- (open circle, n = 8) and mAb7F9-mAb4G9-treated (closed circle, n = 7) rats on day 24 and day 28 in Phase 2 studies (+SD). A best-fit linear regression line was fit to to the terminal phase distance traveled-time data points. This line was used to show the direction of the accumulating data and exactly where it intersected the time axis to estimate the time of the end of METH-induced horizontal distance traveled.
Table 2 shows total (4-hrs) and 1-hr interval METH-induced distance traveled and rearing data from the Phase 2 experiments. In the mAb7F9-mAb4G9-treated rats, the individual METH-induced locomotion in 3 of 7 rats on day 24 and 2 of 7 rats on day 28 decreased to saline treatment induced baseline values during the measurement period. This return to baseline values was only achieved in one of 8 vehicle-treated animals on both day 24 and 28.
Table 2.
Analysis of distance traveled (from Figure 5) and number of rearing events (plots not shown) after a 1.68 mg/kg METH challenge dose in vehicle- and mAb7F9-mAb4G9-treated rats in the Phase 2 studies. This table shows the analysis of the total distance traveled data from 0–240 min after METH dosing as well as the analysis of the sequential 1 hr interval data over the 4 hrs of measurement.
| Distance traveled (in meters ± SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–240 min (Total) |
0–60 min |
60–120 min |
120–180 min |
180–240 min |
||||||
| Day | Vehicle | mAb | Vehicle | mAb | Vehicle | mAb | Vehicle | mAb | Vehicle | mAb |
| 24 | 586 ± 202 | 650 ± 235 | 117 ± 67 | 130 ± 46 | 88 ± 62 | 199 ± 68a | 190 ± 58 | 207 ± 84 | 191 ± 81 | 116 ± 73 |
| 28 | 526 ± 212 | 585 ± 183 | 77 ± 50 | 100 ± 33 | 48 ± 30 | 124 ± 30 a | 204 ± 88 | 220 ± 81 | 197 ± 82 | 140 ± 77 |
| Number of rearing events (± SD) | ||||||||||
| 24 | 552 ± 269 | 852 ± 301 | 158 ± 139 | 317 ± 84 | 97 ± 87 | 222 ± 86b | 143 ± 59 | 183 ± 90 | 154 ± 57 | 130 ± 77 |
| 28 | 509 ± 208 | 755 ± 288 | 98 ± 93 | 189 ±77 | 51 ± 42 | 160 ± 91b | 176 ± 78 | 236 ± 81 | 184 ± 71 | 170 ± 93 |
aStatistical significance compared to time-matched vehicle controls (P < 0.05)
bP = 0.06.
Two of 7 mAb treated rats achieved saline baseline rearing activity on both days 24 and 28. Three of 8 and one of 8 vehicle-treated rats reached baseline rearing activity on days 24 and 28, respectively.
Five hours after 1.68 mg/kg METH administration, serum METH concentrations were significantly elevated in the mAb7F9-mAb4G9-treated rats compared to the vehicle-treated rats on day 24 (162 ± 80 vs 21 ± 5 ng/ml) and day 28 (90 ± 54 vs 18 ± 4 ng/ml, P<0.05). Serum concentrations of AMP were significantly elevated in the mAb-treated rats relative to the vehicle-treated rats on day 24 (37 ± 15 vs 17 ± 8 ng/ml, P < 0.05) but not on day 28 (25 ± 12 vs 15 ± 6 ng/ml, P = 0.05). Whole brain METH and AMP concentrations (not corrected for METH in the serum in brain tissue) were not significantly different between groups (79 ± 22 vs 96 ± 44 and 103 ± 25 vs 145 ± 65 ng/g, respectively). These data are reported as the mean ± SD.
Because the combined treatment with mAb7F9-mAb4G9 was not as effective as the use of mAb7F9 alone, we also compared the current data set with the key findings from the report of monotherapy with mAb7F9 only.10 See Figure 6 for a comparison of day 28 serum and brain METH concentrations and duration of locomotor activity between mAb7F9-mAb4G9- and mAb7F9-treated animals.
Figure 6.
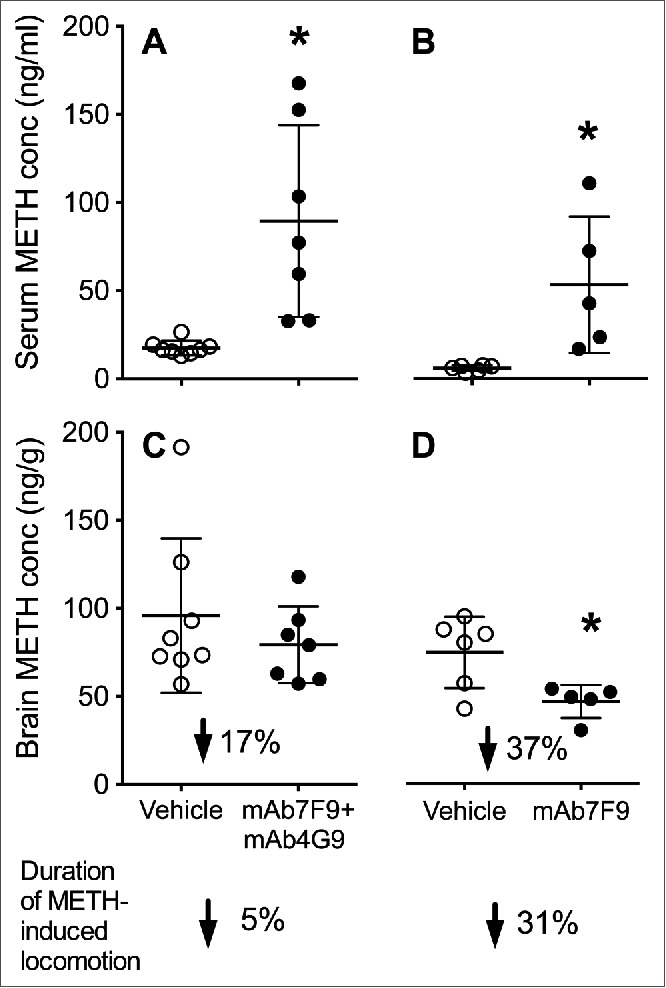
Average day 28 METH serum (Panels A and B) and brain (Panels C and D) concentrations in mAb7F9-mAb4G9- (Panels A and C) and mAb7F9-treated rats (Panels B and D) (±SD). All data for mAb7F9 alone are from a previously reported study from our group.10 The * denotes statistical significance compared to time-matched vehicle-treated controls (P < 0.05). The percent reduction in the brain concentration values denotes the mAb-induced reduction in brain concentration compared to vehicle treated controls in each study. The lowest arrows and values denote the percentage reduction in the duration of METH-induced locomotion in the vehicle- and mAb-treated groups.
Discussion
We hypothesized that treatment of METH pharmacological effects with a mixture of 2 high affinity anti-METH monoclonal antibodies (mAb) with differing molecular recognition of the METH-like structure could increase efficacy compared to treatment with a single mAb. Both anti-METH mAbs chosen for our studies were generated from vaccinations with the same METH-like hapten, but with differing carrier proteins (See Figure 1 in Hambuchen et al. 2015).13 While both mAb7F9 and mAb4G9 have relatively high affinity for METH (KD = 7.7 vs 16 nM, respectively), they differ in their absolute affinity for METH analogs like AMP and (+)-3,4-methylenedioxymethamphetamine (MDMA).11,12,14 Due to these differences in affinity and specificity for METH analogs, we reasoned that the use of these 2 unique mAb binding sites might provide the potential for enhanced in vivo METH binding, leading to benefits in therapeutic efficacy. Indeed, the affinity measurement of the 50:50 mAb combination was reflective of the of the relatively higher METH affinity of mAb7F9 and the relatively higher AMP affinity of mAb4G9.
METH-induced locomotor activity was a primary measure of chronic anti-METH mAb effects. Drugs that inhibit METH-induced locomotor activity have also been shown to inhibit METH effects in conditioned place preference15 and self-administration16 assays. During the development of this model of chronic treatment, we had to consider the effects of METH-induced stereotypy on horizontal motion. After high doses of stimulant, the effects are predominately repetitive stereotypic behaviors with limited horizontal motion. Over time this transitions into predominately horizontal motion, which is the predominate locomotor activity found with lower doses of METH.17 In addition, stereotypy-induced suppression of stimulant-induced locomotion occurs with repeated dosing (even at low doses).18 This can complicate the analysis of mAb-induced effects on each METH dose, especially in studies in which METH is repeatedly administered.
To better understand the implication of these changes for our experiments, we studied locomotor effects in a control group of rats following 4 escalating METH doses (Fig. 1). Surprisingly, despite the log difference between the 0.56 and 5.6 mg/kg METH doses, there was no significant difference in total distance traveled for any of the 4 METH doses (Fig. 1B). This apparently stable response at different METH doses was likely a product of the increasing suppression of early locomotion as the METH dose increased, which resulted in a METH dose-dependent delay in maximal locomotor activity (Fig. 1A). Importantly, the duration of the end of METH-induced locomotor activity was progressively longer as the METH dose increased and considerably less variable between rats than the total distance traveled measurements (Fig. 1C). Accordingly, there were significant differences (P < 0.05) in duration of locomotion between all doses. This phenomena is similar to that reported in the 1987 study by Segal et al.19 In their study, rats were administered a 1.75 mg/kg AMP dose and divided into groups based on whether or not stereotypy suppressed the measured locomotor activity. Regardless of the activity being suppressed or not, the duration of the resulting activity appears to be similar between the groups.
These behavioral control data from the METH dose escalation study support our use of METH-induced locomotor activity as a consistent, reliable measure of METH-induced duration of action and mAb inhibition of these effects. They also demonstrated the need to analyze discrete time intervals (e.g., 60–120 min) for determining METH duration of action, rather than comparing total locomotor activity (i.e., total distance traveled) as a primary endpoint.
The study showed by all measures that the combination therapy with mAb7F9-mAb4G9 was not better than mAb7F9 alone. Like mAb7F9 monotherapy,10 mAb7F9-mAb4G9 treatment significantly reduced the duration of METH-induced locomotion during Phase 1 of the studies on days 0–17 (Fig. 3C and Table 1, duration), but unlike mAb7F9 these beneficial effects ceased by day 21 which was one week after the final mAb administration.
The mAb7F9-mAb4G9 combination compared to mAb7F9 monotherapy also appeared to have a lesser effect on the METH-induced locomotion immediately after METH administration.10 In the chronic mAb7F9 monotherapy study, most time of peak locomotor activity values appeared significantly earlier than those in the vehicle treated group. The earlier peak activity with mAb7F9 treatment is likely due to the inhibition of the locomotor suppressing METH effects that occur just after a 0.56 mg/kg dose (Fig. 1A, Fig. 2 vehicle). This finding (except for day 10) did not occur in the animals treated with the combination therapy (Fig. 2, Table 1 left column).
There were not significant reductions in rearing behaviors in the mAb7F9-mAb4G9 treated group compared to control. This was likely due to both the large variation in the rearing behavior values in the vehicle control group and the apparent lower effects on rearing produced by the combination treatment. In contrast, MAb7F9 alone produced significant reductions in rearing behavior on days 0–17.10
During Phase 2 of the study (days 24 and 28), the important differences between mAb7F9-mAb4G9 combination therapy and mAb7F9 monotherapy were even more pronounced. The mAb7F9-mAb4G9 combination caused only a modest 5% reduction in the duration of action of the 1.68 mg/kg METH dose (Fig. 5). In the previous study with mAb7F9, the duration of locomotor activity resulting from the increased METH dose on days 24 and 28 was decreased by 27% and 31%, respectively.10 Compared to vehicle- and mAb7F9-mAb4G9-treated rats, more mAb7F9-treated rats returned to baseline rearing and distance traveled values during the 4 hr study period. Additionally, there was a significant reduction of rearing behavior compared to vehicle-treated rats in the last hour of the day 24 and 28 METH challenges in the mAb7F9-treated rats10 that was not found in the combination-treated rats (Table 2).
Differences were also found between the 2 studies in the METH and AMP metabolite concentrations 5 hrs after METH dosing. As suggested by the behavior measures, the combination therapy did not have enhanced effects on METH binding. Like mAb7F9 monotherapy during Phase 1,10 mAb7F9-mAb4G9 significantly elevated both METH and AMP metabolite concentrations (Fig. 4) compared to vehicle-treated animals after each METH challenge on days 0–21, and it did so in a similar pattern to simulated mAb concentrations resulting from weekly mAb administration (See Hambuchen et al. 2014 Fig. 1B). Due to mAb4G9's higher affinity for AMP, the combination therapy appeared to result in higher AMP concentrations than mAb7F9 treatment alone at all Phase 1 time points. This change is consistent with data from previous studies of pharmacokinetic effects of mAb4G9 monotherapy.12 It is also possible that mAb7F9, through high affinity binding of METH, is showing greater inhibition of metabolic conversion of METH to AMP.
As in Phase 2 of the mAb7F9 study,10 there was a significant mAb-induced elevation in serum METH concentrations on days 24 and 28 compared to vehicle, and the combination therapy additionally increased AMP concentrations on day 24. Despite the elevations of serum METH and AMP, mAb7F9-mAb4G9 treatment did not result in reductions in brain concentrations of either METH or AMP on day 28 (the final day). However, mAb7F9 treatment produces significant reductions in brain METH concentrations compared to vehicle treatment (Fig. 6). This significant change in brain concentrations is consistent with the substantial reduction in the duration of METH-induced locomotion. The modest, not significant reduction in brain METH concentrations produced by mAb7F9-mAb4G9 is consistent with the modest reductions in METH duration of action on day 28. This highlights the important relationship between reductions in brain METH concentrations and reductions in behavioral effects in anti-METH mAb studies. An elevation of serum METH concentrations is not a reliable measure of changes in anti-METH effects. Note that these total brain concentrations underestimate the true reductions in brain concentrations since total brain METH concentrations (brain plus blood) do not correct for the effects of high METH concentrations in the blood stream due to mAb binding of METH.
MAb7F9's higher affinity for METH and the presence of a 50% greater dose of mAb7F9 in the monotherapy studies is likely the major reasons for the greater efficacy of mAb7F9 alone. MAb4G9 pharmacokinetics are not likely a contributing factor, as the concentration time curve of mAb7F9 and mAb4G9 binding METH in rat serum is virtually identical over a 2 week period.5 Considering the greater AMP binding with mAb7F9-mAb4G9 and overall lesser effects of the combination therapy on METH-induced behaviors, it is likely that the increased AMP metabolite formation in male rats20,21 has negligible effects on overall METH actions. Furthermore, it is also possible, as suggested by Laurenzana et al.,12 that the AMP metabolite is partially inhibiting the binding of METH to mAb4G9. Nevertheless, the contribution of AMP (as a metabolite of METH) would be less of a concern in humans because only ∼14% of a METH dose is converted to AMP,22 compared to the 34–48% conversion to AMP found in male rats.20,21
While the replacement of half the mAb7F9 dose with mAb4G9 increased the AMP binding activity, this therapeutic strategy did not increase the overall METH binding or clinically relevant beneficial anti-METH effects as hypothesized. Differences in pharmacokinetic properties of the antibodies were unlikely a contributor since mAb7F9 and mAb4G9 have very similar pharmacokinetic properties.5 These data emphasize the importance of mAb affinity and specificity for the target ligand. These data do not rule out the possibility that 2 or more unique anti-METH mAbs with more favorable METH binding properties (i.e., ≤7.7 nM KD for METH and low affinity for metabolites) could potentially result in enhanced anti-METH effects. Except for cost, the use of anti-METH mAb has advantages over the use of an active vaccine for chronic treatment of METH addiction. Anti-METH mAb treatment can produce immediate beneficial effects, instead of waiting 2–3 months for the immune response to rise to maximum levels.13 With mAb treatment, substantially higher anti-METH antibody concentrations can be achieved. Finally, mAb treatment will be effective in immune compromised individuals, unlike vaccines.
Overall, these studies provide preclinical evidence in a chronic mAb administration model that mAb7F9 is a superior treatment option compared to mAb7F9-mAb4G9 or mAb4G9 alone. This is due to mAb7F9's longer functional duration, more continuous anti-METH effect during administration, and greater effect on METH disposition than mAb4G9. These data provide additional support for the choice of chimeric mAb7F9 for clinical trials as an addiction therapy (ClinicalTrials.gov identifier: NCT01603147).11,23
Materials and methods
Drugs, chemicals, and supplies
(+)-Methamphetamine hydrochloride was obtained from the National Institute on Drug Abuse. The LC-MS/MS standards (±)-METH, (±)-AMP, and (±)-1-phenyl-1,2-dideutero-2-[trideuteromethyl]aminopropane (METH-d5) internal standard in methanol were purchased from Cerilliant. All doses and standards were calculated as free base. All other reagents and supplies were purchased from Sigma Chemical Company or Thermo-Fisher Scientific, unless otherwise stated.
Anti-METH monoclonal antibodies
The production of endotoxin free mAb7F9 and mAb4G9 is previously described.14,24,25 MAb7F9 is a murine IgG1 isotype mAb (κ light chain) with a KD of 7.7 nM for METH and 270 nM for AMP.11,12 MAb4G9 is a murine IgG2b isotype (κ light chain) with a KD 16 nM for METH and 110 nM for AMP.12,14 The affinity of the combined mAb7F9 and mAb4G9 mixture for METH was determined by a previously reported bead-based radioimmunoassay.11 This radioimmunoassay was performed in the same manner as the assays used to generate the previously mentioned values. The t1/2 of mAb4G9 in Sprague-Dawley rat serum is 6.9 days, and the values for mAb7F9 were assumed to be similar.5,26
Animals
Non-catheterized male Sprague Dawley rats (n = 8) and jugular vein catheterized male Sprague-Dawley rats (n = 16) were purchased from Charles River Laboratories (Wilmington, MA). Studies for the determination of METH dose vs. locomotor response were performed in the non-catheterized rats. The catheterized rats were used for the vehicle- and mAb-treatment studies. Rat care was as previously described, except that non-catheterized rats were housed 2 per cage.10 Rat acclimation to the experimental environment and pre-conditioning with 0.56 mg/kg METH doses (to stabilize the possible effects of tolerance and sensitization) was performed as previously described.10 All experiments were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals, and with the approval of the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Behavioral studies
Behavioral studies were performed as previously reported.10 Briefly, for studies of locomotor activity following increasing METH doses (0.56–5.6 mg/kg), rats were placed in the open-top 60 × 45 × 40 cm behavioral chambers for one hr prior to METH administration. Rats were administered a saline dose and video recorded for 4 hrs to obtain baseline locomotor activity measurements with Ethovision 8 software (Noldus Information Technology, Inc.). After weekly s.c. METH injections, rat movement was recorded for 4 hrs after the 0.56 mg/kg METH dose, 8 hrs after the 1.68 mg/kg and 3.0 mg/kg doses, and 10 hrs after the 5.6 mg/kg dose.
In the mAb7F9-mAb4G9 treatment studies, each mAb was administered separately due to different protein formulation requirements (pH 7.3 for mAb7F9 and 6.5 for mAb4G9). The 141 mg/kg mAb7F9 i.v. loading dose was administered first, followed immediately by the 141 mg/kg i.v. mAb4G9 loading dose. Each mAb was administered over 0.75 min. The subsequent 70.5 mg/kg maintenance doses for mAb7F9 and mAb4G9 were administered on days 7 and 14 in a similar manner over 0.4 min. Animals were matched into groups based on baseline day −4 measurements. The 0.56 mg/kg METH dose was used in Phase 1 because it produced reproducible activity in preliminary studies with limited initial suppression of locomotion. It also allowed us to increase the dose 3-fold on days 24 and 28 and measure the majority of the activity during the 4 hr study period.
The mAb7F9-mAb4G9 study started with 16 rats (8/group). All METH doses were administered i.v. unless otherwise noted. A single rat in the mAb7F9-mAb4G9 treatment group was excluded from the study due to catheter failure prior to the final mAb administration. Due to catheter failure later in the studies, 2 rats in each group were administered s.c. (instead of i.v.) METH toward the end of Phase 1 of the study (which lasted 21 days), and on day 24 in Phase 2 of the studies. Four animals in each group required s.c. METH administration on day 28. We included data from these animals in the final analysis since we have previously determined that the results from s.c. and i.v. METH dosing produce a similar pattern and magnitude of locomotor effects.10,27
We justified the use of sc METH after catheter failure based on 3 factors. First, data from Gentry et al. (2004)27 shows METH-induced duration of activity data resulting from i.v. or s.c. routes in Sprague Dawley rat are not statistically different at 0.3 and 1 mg/kg METH doses, but are different at the 3 mg/kg. Second, we re-analyzed the rat locomotor activity data on days 0, 24, and 28 by grouping results from vehicle treated rats that received i.v. and s.c. administrations, and rats that received only i.v. administration. Based on these data, there appeared to be 2 clusters of rat locomotor responses. We found that the low responders on days 24 and 28 were already the low responders on day 0, and the high responders on days 24 and 28 were already the high responders on day 0. Thus these data strongly suggested the inherent activity of the individual rats was the main cause of the difference in response, and not the route of administration. Third, the METH brain concentrations on day 28 were not significantly different between different routes of administration.
METH and AMP metabolite concentrations
Blood was collected from the tail vein 5 hrs after METH administration on days 0–24. On day 28, rats were decapitated under isoflurane anesthesia for collection of trunk blood and brain tissue 5 hrs after METH administration. Paw pinch and respiration frequency were used to assess depth of anesthesia as a measure of adequate pain relief. Collected tissues were stored at −80°C.
Analysis of METH and AMP serum and brain concentrations was performed by liquid chromatography coupled to tandem mass spectrometry (LC/MS-MS).13 For the current studies, the initial steps of the extraction procedure were modified to assure maximum recovery of METH and AMP in the presence of high mAb concentrations. This involved equilibrating each 25 µl aliquot of tissue sample with 25 µl of 100 ng/ml METH-D5 internal standard prior to the addition of 50 µl of ice cold 20% trichloroacetic acid, and then mixing under refrigeration to precipitate proteins. Afterward, 1 ml of LC/MS-MS loading buffer (100 mM NaPO4, pH 8.1) was added. This mixture was then transferred to a Strata X-C 33 µM Polymeric Strong Cation solid phase extraction column (Phenomenex). The remaining steps for LC/MS-MS analysis were as previously described. The lower and upper limit of quantification for METH and AMP was 1 and 1000 ng/ml, respectively. All measurements of METH and AMP standards were within ± 20% of the predicted values.
Statistical analysis
Comparisons of total distance traveled and duration of action measurements between 0.56, 1.68, 3.0, and 5.6 mg/kg METH doses were determined with a one-way repeated measures ANOVA using Bonferroni's post-hoc test using Graphpad Prism 6.0 (Graphpad Software). As described previously,10 comparisons of serum concentrations and behavioral measurements between the mAb7F9-mAb4G9- and vehicle-treated groups were performed using a 2-way repeated measures ANOVA and a Bonferroni adjustment of P-values with the Stepdown Bonferroni method in proc multtest using SAS 9.3 (SAS Institute). Data from this analysis was presented as the mean ± standard deviation. Comparisons of brain concentrations between mAb7F9-mAb4G9- or mAb7F9- and vehicle-treated groups were made with a student's t-test. A significance level of P < 0.05 was used for all statistical analyses.
Disclosure of potential conflicts of interest
S.M.O. is Chief Scientific Officer of and has financial interests in InterveXion Therapeutics, LLC, a pharmaceutical biotechnology company, whose main interest is the development of antibody medications for the treatment of human diseases, including drug abuse.
Acknowledgments
The authors thank Sherri Wood, Yingni Che, C. Michael West, and Anna Mazur for technical assistance.
Funding
Funding for this work was provided by the NIH National Institute on Drug Abuse (Grants DA11560, and T32DA022981); the National Center for Advancing Translational Sciences (Grant ULITR000039) and the Arkansas Biosciences Institute (the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000).
References
- [1].Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, et al.. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47-53; PMID:25171469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al.. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 2012; 492:118-22; PMID:23103874; http://dx.doi.org/ 10.1038/nature11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zakhari JS, Zorrilla EP, Zhou B, Mayorov AV, Janda KD. Oligoclonal antibody targeting ghrelin increases energy expenditure and reduces food intake in fasted mice. Mol Pharm 2012; 9:281-9; PMID:22149064; http://dx.doi.org/ 10.1021/mp200376c [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gentry WB, Rüedi-Bettschen D, Owens SM. Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin 2009; 5:206-13; PMID:19276653; http://dx.doi.org/ 10.4161/hv.5.4.7456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Owens SM, Atchley WT, Hambuchen MD, Peterson EC, Gentry WB. Monoclonal antibodies as pharmacokinetic antagonists for the treatment of (+)-methamphetamine addiction. CNS Neurol Disord Drug Targets 2011; 10:892-8; PMID:22229314; http://dx.doi.org/ 10.2174/187152711799219370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Celikel R, Peterson EC, Owens SM, Varughese KI. Crystal structures of a therapeutic single chain antibody in complex with two drugs of abuse-Methamphetamine and 3,4-methylenedioxymethamphetamine. Protein Sci 2009; 18:2336-45; PMID:19760665; http://dx.doi.org/ 10.1002/pro.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sull TJ, Chass GA, Varro A, Papp JG. A comparative conformational analysis of selected central nervous system stimulants. Journal of Molecular Structure: THEOCHEM 2003; 623:51-62; http://dx.doi.org/ 10.1016/S0166-1280(02)00661-9 [DOI] [Google Scholar]
- [8].Tubergen MJ, Lavrich RJ, Plusquellic DF, Suenram RD. Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone. J Phys Chem A 2006; 110:13188-94; PMID:17149832; http://dx.doi.org/ 10.1021/jp064810u [DOI] [PubMed] [Google Scholar]
- [9].Moreno AY, Mayorov AV, Janda KD. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc 2011; 133:6587-95; PMID:21473576; http://dx.doi.org/ 10.1021/ja108807j [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hambuchen MD, Rüedi-Bettschen D, Williams DK, Hendrickson H, Owens SM. Treatment of rats with an anti-(+)-methamphetamine monoclonal antibody shortens the duration of action of repeated (+)-methamphetamine challenges over a one month period. Vaccine 2014; 32:6213-9; PMID:25252196; http://dx.doi.org/ 10.1016/j.vaccine.2014.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stevens MW, Tawney RL, West CM, Kight AD, Henry RL, Owens SM, Gentry WB. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. MAbs 2014; 6:547-55; PMID:24492290; http://dx.doi.org/ 10.4161/mabs.27620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Laurenzana EM, Stevens MW, Frank JC, Hambuchen MD, Hendrickson HP, White SJ, Williams DK, Owens SM, Gentry WB. Pharmacological effects of two anti-methamphetamine monoclonal antibodies. Supporting data for lead candidate selection for clinical development. Hum Vaccin Immunother 2014; 10:2638-47; PMID:25483484; http://dx.doi.org/ 10.4161/hv.29707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hambuchen MD, Carroll FI, Rüedi-Bettschen D, Hendrickson HP, Hennings LJ, Blough BE, Brieaddy LE, Pidaparthi RR, Owens SM. Combining Active Immunization with Monoclonal Antibody Therapy To Facilitate Early Initiation of a Long-Acting Anti-Methamphetamine Antibody Response. J Med Chem 2015; 58:4665-77; PMID:25973614; http://dx.doi.org/ 10.1021/acs.jmedchem.5b00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carroll FI, Abraham P, Gong PK, Pidaparthi RR, Blough BE, Che Y, Hampton A, Gunnell M, Lay JO, Peterson EC, et al.. The synthesis of haptens and their use for the development of monoclonal antibodies for treating methamphetamine abuse. J Med Chem 2009; 52:7301-9; PMID:19877685; http://dx.doi.org/ 10.1021/jm901134w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu K, Lin H, Miyamoto Y, Wu C, Yang J, Uno K, Nitta A. Pseudoginsenoside-F11 inhibits methamphetamine-induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens. Psychopharmacology (Berl) 2016; 233:831-40; PMID:26621348; http://dx.doi.org/ 10.1007/s00213-015-4159-8 [DOI] [PubMed] [Google Scholar]
- [16].Cotter R, Pei Y, Mus L, Harmeier A, Gainetdinov RR, Hoener MC, Canales JJ. The trace amine-associated receptor 1 modulates methamphetamine's neurochemical and behavioral effects. Front Neurosci 2015; 9:39; PMID:25762894; http://dx.doi.org/ 10.3389/fnins.2015.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia 1967; 11:300-10; PMID:4968376; http://dx.doi.org/ 10.1007/BF00404607 [DOI] [PubMed] [Google Scholar]
- [18].Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 1974; 2:249-55; PMID:4857295; http://dx.doi.org/ 10.1016/0091-3057(74)90060-4 [DOI] [PubMed] [Google Scholar]
- [19].Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther 1987; 242:917-26; PMID:3656119 [PubMed] [Google Scholar]
- [20].Rivière GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther 1999; 291:1220-6. [PubMed] [Google Scholar]
- [21].Rivière GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther 2000; 292:1042-7. [PubMed] [Google Scholar]
- [22].Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos 1993; 21:717-23; PMID:8104133 [PubMed] [Google Scholar]
- [23].Stevens MW, Henry RL, Owens SM, Schutz R, Gentry WB. First human study of a chimeric anti-methamphetamine monoclonal antibody in healthy volunteers. MAbs 2014; 6:1649-56; PMID:25484042; http://dx.doi.org/ 10.4161/19420862.2014.976431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol 2003; 461:119-28; PMID:12586207; http://dx.doi.org/ 10.1016/S0014-2999(03)01313-X [DOI] [PubMed] [Google Scholar]
- [25].Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol Exp Ther 2007; 322:30-9; PMID:17452421; http://dx.doi.org/ 10.1124/jpet.106.117150 [DOI] [PubMed] [Google Scholar]
- [26].Laurenzana EM, Hendrickson HP, Carpenter D, Peterson EC, Gentry WB, West M, Che Y, Carroll FI, Owens SM. Functional and biological determinants affecting the duration of action and efficacy of anti-(+)-methamphetamine monoclonal antibodies in rats. Vaccine 2009; 27:7011-20; PMID:19800446; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav 2004; 79:751-60; PMID:15582684; http://dx.doi.org/ 10.1016/j.pbb.2004.10.006 [DOI] [PubMed] [Google Scholar]


