ABSTRACT
To determine if a hypersensitive-type lung pathology might occur when mice were given an inactivated MERS-CoV vaccine and challenged with infectious virus as was seen with SARS-CoV vaccines, we prepared and vaccinated mice with an inactivated MERS-CoV vaccine. Neutralizing antibody was induced by vaccine with and without adjuvant and lung virus was reduced in vaccinated mice after challenge. Lung mononuclear infiltrates occurred in all groups after virus challenge but with increased infiltrates that contained eosinophils and increases in the eosinophil promoting IL-5 and IL-13 cytokines only in the vaccine groups. Inactivated MERS-CoV vaccine appears to carry a hypersensitive-type lung pathology risk from MERS-CoV infection that is similar to that found with inactivated SARS-CoV vaccines from SARS-CoV infection.
Keywords: coronavirus; Eosinophils; immunopathology; Middle East Respiratory Syndrome; vaccination
Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) emerged in 2002 and 2012 respectively and were shown to be caused by a new coronavirus (CoV), now designated as SARS-CoV and MERS-CoV, respectively.1,2 The SARS epidemic was brought under control by using infection control methods. Because of continued outbreaks, vaccines for MERS are urgently needed.
Preclinical evaluations of inactivated subunit and whole-virus vaccines for SARS have elicited serum neutralizing antibody and protection against infection in monkeys, ferrets and mice challenged with infectious SARS-CoV. However, challenged animals exhibited an immunopathologic-type lung reaction, and these results led to safety concerns relative to SARS-CoV vaccines.3,4 Moreover, due to the apparent epidemiologic control of SARS, along with these findings, clinical trials of SARS-CoV vaccines were placed on hold.
With the rise of MERS, we decided to revisit the vaccines prepared for SARS studies and initially prepared a small batch of whole inactivated virus (WIV) for evaluation. We then began tests to determine if vaccination with inactivated MERS-CoV vaccine would result in immunopathology in vaccinated hosts similar to that seen with SARS-CoV. A mouse model for studies of MERS-CoV was not initially available since mice and other small animals lack the MERS-CoV receptor. For this reason, we developed a transgenic mouse model containing the human DPP4 receptor.5,6 Availability of this model provided the opportunity to assess whether an inactivated MERS-CoV vaccine would induce protection against MERS-CoV infection but also induce an eosinophil-containing pulmonary immunopathology as had similar SARS-CoV vaccines. This is the first report that a similar risk for immunopathology appears to exist for MERS-CoV-inactivated vaccines despite an ability to protect against infection.
The WIV stock was prepared by gamma (γ) irradiating (5 mega-rads, cobalt-60) aliquots of Vero E6-derived, cell-free MERS-CoV (∼1.2 × 108 TCID50/ml). Inactivated supernatants, negative in rigorous isolation tests, were subjected to polyethylene glycol/salt precipitation, purified by sucrose density centrifugation,7 and diluted in PBS to an equivalent of ∼1 × 107 TCID50/ml. Western blot analysis by using a rabbit anti-MERS-CoV antibody demonstrated virus structural proteins including surface protein (S) and nucleoprotein (data not shown).5,8
For assessing the immunogenicity and protective efficacy of this WIV as well as its potential to elicit immunopathology upon live virus challenge of vaccinated animals, groups of six hCD26/DPP4 transgenic mice were immunized intramuscularly (I.M.) twice, three weeks apart. Mice received 100 µl of WIV only, WIV adjuvanted with alhydrogel 2% (alum) or with MF59 (Invivogen), or alum or MF59 only, according to protocols approved by the IACUC committee at the University of Texas Medical Branch. Sera were collected 21 days after the second immunization for micro-neutralization antibody tests; mice were then challenged intranasally (I.N.) with 103 TCID50 (100 LD50) of MERS-CoV,6 and sacrificed on days 3 or 6 (3 from each group on each day) for assessing lung viral loads by Vero E6-based infection and quantitative (q) PCR assays targeting the UpE gene of MERS-CoV, and lung cytokines transcriptional profiling of TH1 (IFN-γ) and TH2 (IL-5 and IL-13) in qRT-PCR assays as previously described.5,6 Additionally, de-paraffinized sections were stained with either routine hematoxylin-and-eosin (H&E) for histopathologic evaluations or an antibody specific to eosinophil major basic protein (MBP), provided by the Lee Laboratory, for confirming eosinophil infiltrations, as described.3,9
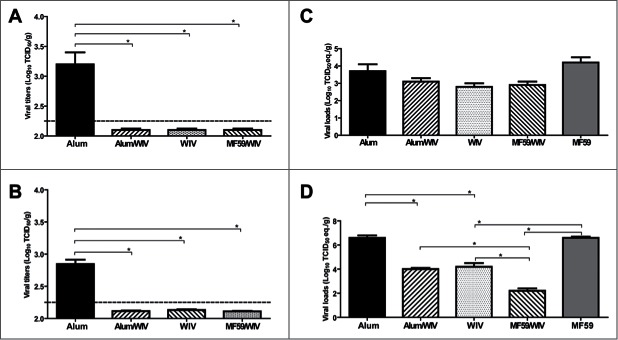
Figure 1 shows that neither adjuvant alone group developed detectable neutralizing antibodies, whereas all vaccine groups developed significantly greater neutralizing antibody responses than the group with adjuvant alone (P < 0.01). Further, the mean titer for WIV/MF59 was higher than that for the WIV/Alum group (P < 0.01). Consistent with the absence of specific antibody response, infectious virus was readily detected in the lungs of three infected animals immunized with alum only (MF59 group not available) on days 3 and 6, with an average of 103.1 and 102.8 TCID50/gm, respectively, in which the limit of detection (LOD) in the Vero E6 cell-based infectivity assay was ∼101.8 TCID50/gm. In contrast, infectious virus remained undetectable in all of the vaccine groups at either time (Fig. 2A–B). Titers of viral RNA, as revealed by qRT-PCR assays and expressed as TCID50 equivalents, were also compared among the groups. All groups exhibited detectable viral RNAs (Fig. 2C–D). The titers were lower in all vaccine groups on day 3 but none were significantly lower than those of the controls (Fig. 2C); however, the titer for each vaccine group on day 6 was significantly lower than those of either adjuvant only group (P < 0.01) (Fig. 2D). The titers in the WIV/MF59 group were also significantly lower than those in either of the other 2 vaccine groups (P < 0.01).
Figure 1.
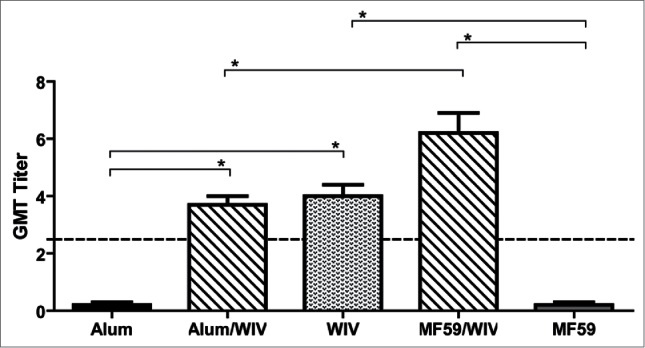
Mean serum-neutralizing antibody titers to MERS-CoV of vaccinated mice 3 weeks after the second immunization. Alum and MF59 are adjuvant only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum adjuvant, MF59/WIV is WIV formulated with MF59 adjuvant. The serum neutralizing antibody titers are expressed as Geometric Mean Titer (GMT) based on a 2-fold dilution sequence beginning at 1:2 (Log2). * Significantly different (P < 0.01) after correcting for multiple comparisons.
Figure 2.
Mean viral titers of MERS-CoV on days 3 and 6 after intranasal challenge of vaccinated mice with 100 LD50 of MERS-CoV. Lung homogenates and total RNAs extracted from tissues of vaccinated mice at days 3 and 6 post challenge with MERS-CoV were subjected to Vero E6 cell-based infectivity assay and one-step real-time RT-PCR analyses targeting the upE gene of MERS-CoV for assessing viral loads, as previously described (5,6). A serial 10-fold diluted MERS-CoV stock with a titer of 107 TCID50/ml was included in parallel during the quantitative PCR assays to calculate and express the levels of upE gene expression in individual specimens as log10 TCID50 equivalents per gram of tissue. Alum and MF59 are adjuvant-only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum, MF59/WIV is WIV formulated with MF59. A: Vero E6-based infectious viral titers at Day 3, B: Vero E6-based infectious viral titers at Day 6, C: RT-PCR-based viral load at Day 3, and D: RT-PCR-based viral load at Day 6. * Significantly different (P < 0.01) after correcting for multiple comparisons.
No gross pathology was noted on either day 3 or 6 (data not shown); however, histopathology was noted in all groups on both days. On a severity scale of 0 to 3 (none, mild, moderate, severe), H&E-stained samples from the Alum and MF59 only groups were graded 1 on both days 3 and 6 for mononuclear cell infiltrations, including lymphocytes, macrophages/monocytes, while each vaccine group was grade 2 on both days (Table 1). Lung sections were similarly scored 0 to 3 for eosinophil infiltrations. As shown in Figure 3 (left), few eosinophils (MBP+ brown) were detected in the peribronchiolar space (Alum, day 3) or alveolar wall (MF59, day 3). This level of eosinophilic infiltration was similar to that revealed in infected mice without prior manipulation, and scored as 0. However, moderate levels (scored 2) of eosinophilic infiltration into peribronchiolar or perivascular spaces could be readily observed at day 3 (Fig. 3, right) and spread to alveoli of mice at day 6 p.i. in each vaccine group (data not shown).
Table 1.
Severity of lung histopathology of vaccinated mice after challenge with MERS-CoV.
| Severity score of lung pathology* |
||
|---|---|---|
| Vaccination Groups | Day 3** | Day 6 |
| Alum only | 1 | 1 |
| MF59 only | 1 | 1 |
| WIV/Alum | 2 | 2 |
| WIV/MF59 | 2 | 2 |
| WIV only | 2 | 2 |
Pathology severity scores (0-3): 0- no pathology, 1- mild, 2- moderate, and 3- severe.
Day post challenge.
Figure 3.
Representative photomicrographs of lung tissue 3 days after challenge of previously vaccinated mice with MERS-CoV. Lung sections were stained with an antibody directed specifically against eosinophilic major basic protein as described (3); eosinophils are brown. The vaccine groups (alum only, MF59 only, WIV only, WIV plus Alum and WIV plus MF59) and the eosinophil infiltration severity score (E0 and E2) are noted on the micrograph; E0 is none, E2 is moderate.
Pulmonary cytokine profiling of vaccinated and challenged mice was performed by using qRT-PCR assays.5 Because of the small number of animals in each test group,3 cytokine assays were performed twice. The day 3 pattern was similar in each test, and there were no significant differences between tests (Fig. 4). Some cytokine activities were seen in the adjuvant only groups but were not significantly different from those in uninfected animals. However, significant increases were seen for all 3 cytokines tested in the vaccine groups. Notable are the increases of IL-5 and IL-13, cytokines associated with hypersensitivity reactions that include eosinophil infiltrations.10,11 Cytokine levels were lower for the vaccine groups for IL-5 and IL-13 at day 6 and not significantly greater than those for the uninfected; however, IFN-γ was significantly increased for the WIV, WIV/MF59 and MF59 only groups (P < 0.01) (data not shown).
Figure 4.
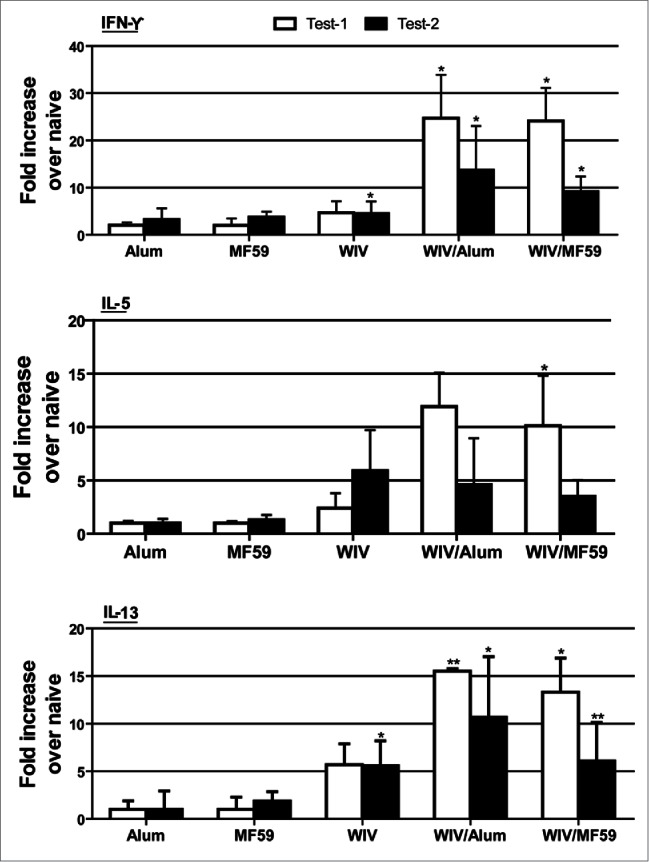
Mean lung cytokine levels on day 3 after challenge of vaccinated mice with MERS-CoV. Alum and MF59 are adjuvant only groups, WIV is whole inactivated vaccine (WIV) only, Alum/WIV is WIV formulated with Alum, MF59/WIV is WIV formulated with MF59. Test 1 and test 2 are separate day tests of the same lung tissue specimen. Results are mean fold increase over naïve transgenic mice based on ΔCt values of each group in reference to those of the internal mouse GAPDH gene. * Significantly greater than for the naïve mouse group (P < 0.01) after correcting for multiple comparisons; ** P = 0.026.
This study was conducted to test whether an inactivated MERS-CoV vaccine would induce neutralizing antibody and protection against MERS-CoV infection and yet lead to a hypersensitivity-type lung immunopathologic reaction with eosinophil infiltrations when challenged with infectious virus, as had been seen with SARS-CoV-inactivated vaccines.3 The results suggest that a similar risk exists for inactivated MERS-CoV vaccines.
The vaccine lot size and requirement for transgenic mice limited the study group sizes. Because of concern for immununogenicity, we included MF59 adjuvant groups since the MF59 adjuvant had been reported to induce superior antibody responses when compared with other adjuvants for a MERS-CoV receptor-binding domain (RBD) protein-based vaccine.12 Serum neutralizing antibody and protection against infection were found in the vaccine alone and both adjuvant groups, but each also exhibited a hypersensitivity-type lung reaction after challenge that included increased pathology with eosinophil infiltrations.
Immune reactions leading to eosinophil infiltrations are considered hypersensitivity reactions and are TH2-type responses mediated via TH2 cytokines.10 Notable cytokines that promote eosinophil infiltrations are IL-5 and IL-13.13 We found this association in the lungs of vaccinated and challenged animals, providing support for the vaccination-related hypersensitivity concept.
Support for attributing the immunopathology to a TH2-type immune response has been provided by immunizations with inactivated SARS-CoV vaccines and TH1-type adjuvants. Studies in mice with inactivated SARS-CoV vaccines given with a TH1 adjuvant did not exhibit a similar immunopathologic reaction after virus challenge.14,15
The finding for MERS-CoV vaccine and the similar findings for SARS-CoV vaccines are reminiscent of those reported in mice given a formalin-inactivated, whole-virus respiratory syncytial virus (RSV) vaccine and challenged with infectious RSV.16-18 Protection against infection occurred despite an increased histopathology. A similar RSV vaccine given to infants had led to increased pulmonary disease severity and 2 deaths.19,20 The component(s) of the vaccine that led to the immunopathology have not been identified, but have been suggested to be viral components as well as contaminants and formalin effects. However, Immunopathology with SARS-CoV vaccines occurred for whole-virus vaccines, subunit vaccines, different inactivation methods, different preparation substrates, and with recombinant surface (S) protein. This experience may indicate that the responsible components(s) for the SARS and MERS-CoV vaccines are viral. Results of studies with vector vaccines point to the nucleoprotein (N) protein as responsible for the immunopathological effects seen and indicate that the S protein might be free of the risk; however, rS protein induced the pathology.3,21,22 Identifying and remedying the specific basis for the immunopathology would facilitate vaccine trials in humans.
The implication of the current study is that application of an inactivated MERS-CoV vaccine for prevention of MERS in humans may carry a risk for lung immunopathology if subsequently exposed to MERS-CoV. The study also leads us to suggest that the extensive background of preclinical experience with inactivated SARS-CoV vaccines may be applicable to inactivated MERS-CoV vaccines.
The major limitation of the current study is the small number of animals in the evaluations that reduces the strength of the findings. Nevertheless, the findings seem clear that a risk similar to that of the SARS-CoV vaccine exists for the MERS-CoV vaccine. Additional data supporting the belief that the immunopathology represents a TH2-biased response and that it also occurs for other MERS-CoV vaccines as it did for SARS-CoV vaccines is contemplated in future studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Heinz Feldmann, National Institute of Health at Hamilton, Montana, and Dr. Ron A. Fouchier, Erasmus Medical Center at Rotterdam, The Netherlands for the MERS-CoV.
Funding
This research was supported in part by a National Institutes of Health Grant, R21AI113206-01 (to C-T.K.T), and pilot grants from the Center for Biodefense and Emerging Infectious Diseases and from the Galveston National Laboratory (Grant Number: 5UC7AI094660-05. Project Title: National Biocontainment Laboratories (NBLs) Operations Support), University of Texas Medical Branch, Galveston, TX (to C-T.K.T.).
References
- [1].Severe acute respiratory syndrome (SARS) Status of the outbreak and lessons for the immediate future; unmasking a new disease. http://www.who.int/csr/media/sars_wha.pdf [Google Scholar]
- [2].Middle East respiratory syndrome coronavirus (MERS-CoV) Summary and risk assessment of the current situation in Korea and China – as of 3 June 2015. http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-3june2015/en/ [Google Scholar]
- [3].Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS One 2012; 7:e35421; PMID:22536382; http://dx.doi.org/ 10.1371/journal.pone.0035421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, Baric RS. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 2011; 85:12201-15; PMID:21937658; http://dx.doi.org/ 10.1128/JVI.06048-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 2015; 89:3659-70; PMID:25589660; http://dx.doi.org/ 10.1128/JVI.03427-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tao X, Garron T, Agrawal AS, Algaissi A, Peng BH, Wakamiya M, Chan TS, Lu L, Du L, Jiang S, Couch RB, Tseng CT. Characterization and demonstration of the value of a lethal mouse model of middle east respiratory syndrome coronavirus infection and disease. J Virol 2015; 90:57-67; PMID:26446606; http://dx.doi.org/ 10.1128/JVI.02009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang C, Peters CJ, Makino S. Severe acute respiratory syndrome coronavirus accessory protein 6 is a virion-associated protein and is released from 6 protein-expressing cells. J Virol 2007; 81:5423-6; PMID:17344286; http://dx.doi.org/ 10.1128/JVI.02307-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tao X, Mei F, Agrawal A, Peters CJ, Ksiazek TG, Cheng X, Tseng CT. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of middle east respiratory syndrome coronavirus. J Virol 2014; 88:3902-10; PMID:24453361; http://dx.doi.org/ 10.1128/JVI.03001-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, et al.. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2009; 7:749-755 e711; PMID:19345285; http://dx.doi.org/ 10.1016/j.cgh.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Locksley RM. Asthma and allergic inflammation. Cell 2010; 140:777-83; PMID:20303868; http://dx.doi.org/ 10.1016/j.cell.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004; 4:583-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang N, Channappanavar R, Ma C, Wang L, Tang J, Garron T, Tao X, Tasneem S, Lu L, Tseng CT, et al.. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol 2015; 13:180-90; PMID:25640653; http://dx.doi.org/ 10.1038/cmi.2015.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nature reviews. Immunol 2015; 15:271-82. [DOI] [PubMed] [Google Scholar]
- [14].Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol 2015; 89:2995-3007; PMID:25520500; http://dx.doi.org/ 10.1128/JVI.02980-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iwata-Yoshikawa N, Uda A, Suzuki T, Tsunetsugu-Yokota Y, Sato Y, Morikawa S, Tashiro M, Sata T, Hasegawa H, Nagata N. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J Virol 2014; 88:8597-614; PMID:24850731; http://dx.doi.org/ 10.1128/JVI.00983-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Teng YW, Graham BS. Anti IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxicT lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest 1994; 94:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson TR, Parker AR, Johnson JE, Graham BS. IL13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J Immunol 2003; 170:2037-45; PMID:12574374; http://dx.doi.org/ 10.4049/jimmunol.170.4.2037 [DOI] [PubMed] [Google Scholar]
- [18].Lee YT, Ko EJ, Kwang HS, Lee JS, Kim KH, Kwon YM, Kang SM. Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages. Int J Nanomed 2015; 10:4491-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiological study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405-21; PMID:4305197. [DOI] [PubMed] [Google Scholar]
- [20].Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratorysyncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422-34; PMID:4305198. [DOI] [PubMed] [Google Scholar]
- [21].Deming D, Sheahan T, Heise M, Yount B, Davis N, Sims A, Suther M, Harkema J, Whitmore A, Piclkes R, et al.. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med 2006; 3:2359-75; http://dx.doi.org/ 10.1371/journal.pmed.0030525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yasui F, Kai C, Kitabatake M, Inoue S, Yoneda M, Yokochi S, Kase R, Sekiguchi S, Morita K, Hishima T, et al.. Prior immunization with severee acute respiraory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol 2008; 181:6337-48; PMID:18941225; http://dx.doi.org/ 10.4049/jimmunol.181.9.6337 [DOI] [PubMed] [Google Scholar]