ABSTRACT
Influenza has a major impact on healthcare systems and society, but can be prevented using vaccination. The World Health Organization (WHO) currently recommends that influenza vaccines should include at least two virus A and one virus B lineage (trivalent vaccine; TIV). A new quadrivalent vaccine (QIV), which includes an additional B virus strain, received regulatory approval and is now recommended by several countries. The present study estimates the cost-effectiveness of replacing TIVs with QIV for risk groups and elderly population in Spain. A static, lifetime, multi-cohort Markov model with a one-year cycle time was adapted to assess the costs and health outcomes associated with a switch from TIV to QIV. The model followed a cohort vaccinated each year according to health authority recommendations, for the duration of their lives. National epidemiological data allowed the determination of whether the B strain included in TIVs matched the circulating one. Societal perspective was considered, costs and outcomes were discounted at 3% and one-way and probabilistic sensitivity analyses were performed. Compared to TIVs, QIV reduced more influenza cases and influenza-related complications and deaths during periods of B-mismatch strains in the TIV. The incremental cost-effectiveness ratio (ICER) was 8,748€/quality-adjusted life year (QALY). One-way sensitivity analysis showed mismatch with the B lineage included in the TIV was the main driver for ICER. Probabilistic sensitivity analysis shows ICER below 30,000€/QALY in 96% of simulations. Replacing TIVs with QIV in Spain could improve influenza prevention by avoiding B virus mismatch and provide a cost-effective healthcare intervention.
KEYWORDS: costs and cost analysis, cost-effectiveness analysis, human, healthcare costs, influenza, influenza B virus, influenza vaccines, QIV, vaccines
Introduction
Seasonal influenza is an acute viral infection that circulates worldwide and spreads easily from person to person. It can affect any age group, cause annual epidemics and represents a serious public health problem, due to the severity of the illness and the number of deaths in high risk populations. Furthermore, influenza can have a huge economic impact through reduced workforce productivity and overwhelmed health services during peaks in infection.1
There are three types of seasonal influenza viruses: A, B and C. Type C influenza cases occur much less frequently than types A and B1 but type A influenza viruses cause most influenza infections. Nevertheless, type B infection is also frequent in children and young adults and is the predominant virus to cause epidemics every 2–4 y.2 Type A and B infections produce similar clinical symptoms, hospitalization rates and rates of admission to Intensive Care Units.3,4
Influenza vaccination is the most effective way to prevent infection and thereby disease development and potential severe outcomes. A number of safe and effective vaccines are available and have been used for more than 60 y.1 Current available trivalent vaccines (TIVs) protect against two A subtypes (H1, H3) and one B lineage. However, as global co-circulation of two B lineages has occurred in the past, there remains a gap to be filled.2,5 The proportion of influenza infections that are not covered with TIVs varies from year to year due to B type mismatch between vaccine and circulating B lineages. In Spain, in seven out of eight seasons since 2005/2006 (excluding the pandemic influenza season in 2009/2010), two distinct B lineages have co-circulated. As a result of this mismatch, the TIVs have not been fully adequate during the last eight seasons. A quadrivalent inactivated influenza vaccine (QIV) has therefore recently been developed to address the unmet need of adequate protection in case of mismatch between circulating B viruses.
The National Immunization Schedule in Spain is the calendar that defines the antigens and schedules (including recommended number of doses and ages) for the systematic vaccination of the entire population. There is a framework for the systematic assessment of any changes to the schedule (e.g. the inclusion of new antigens or modifications to current regimens). This considers five assessment criteria: burden of disease, efficacy and safety, impact of change in the vaccination schedule (i.e. co-administration issues), ethical considerations and economic evaluation.6
The economic evaluation of health technologies is defined as the comparative analysis of alternative courses of action in terms of both their costs and their consequences.7 Together with other relevant criteria, such evaluations provide useful knowledge to facilitate informed healthcare resource allocation decisions.
The aim of the present study was to compare the cost-effectiveness of vaccination programs in Spain with either TIV or QIV in preventing seasonal influenza in elderly (≥ 65 y old) and at risk individuals (≥ 3 y old).
Results
Base case analysis (lifetime horizon)
Over a lifetime horizon (100 y) evaluation of influenza vaccinated age-cohorts, using QIV would result in 40,000 additional quality-adjusted life years (QALYs) gained compared to the use of TIV, with an increased cost of 350 million €. The incremental cost-effectiveness ratio (ICER) of QIV over TIV was 8,748€/QALY gained (Table 1). From the National Health System (NHS) perspective, costs related to the use of QIV are higher mainly because of the difference in vaccine price versus TIV. That difference is however offset when considering societal costs, due to the lower QIV indirect costs (less productivity loss and absenteeism).
Table 1.
Cost-effectiveness of quadrivalent influenza vaccination (QIV) compared to trivalent vaccine (TIV): base case scenario with lifetime horizon.
| TIV | QIV | Difference | |
|---|---|---|---|
| Spanish Healthcare System costs | 11,901,394,637 € | 12,348,949,428 € | 447,554,791 € |
| Societal costs | 34,462,064,137 € | 34,364,483,970 € | − 97,580,167 € |
| Total costs | 46,363,458,774 € | 46,713,433,398 € | 349,974,624 € |
| Life years | 1,143,182,206 | 1,143,233,538 | 51,332 |
| QALYs |
1,038,585,055 |
1,038,625,059 |
40,005 |
| ICER (€/QALY) NHS perspective | 11,188 € | ||
| ICER (€/QALY) Societal perspective | 8,748 € |
TIV: Trivalent influenza vaccine; QIV: Quadrivalent influenza vaccine; QALY: Quality-adjusted life year; ICER: Incremental cost-effectiveness ratio; NHS: National Health System.
One-year time horizon results
Although the lifetime horizon allows a holistic evaluation of the value of QIV in the long term, a short term analysis would be better for estimating the potential health impact of QIV.
Using a one-year scenario (year after administration was initiated), and average seasonal matching data, the preventive strategy of influenza vaccination with QIV would deliver significant reductions in both disease-related morbidity and mortality: 18,565 influenza cases; 2,577 influenza-related complications; 407 influenza-related hospitalizations and 181 deaths compared to TIV during the first year; with an incremental cost of 11,203,359€ due to the incremental vaccine cost (17.7 million €) partially offset by cost savings in absenteeism (−3.7 million €); influenza complications (−2.2 million €) and uncomplicated influenza (−0.4 million €).
If we consider a mismatched season, the outcomes were more greatly improved. Figure 1 shows the annual outcomes of QIV depending on the match achieved by the vaccine.
Figure 1.
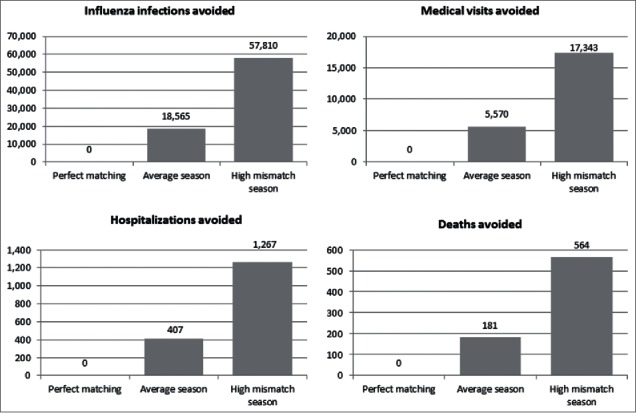
Additional outcomes of quadrivalent influenza vaccine (QIV) versus trivalent influenza vaccine (TIV) at one-year time horizon Legend Perfect matching: 100% matching between B strain circulating and B strain included in TIV; Average season: mean B strain matching over the last 8/10 seasons; High mismatch season: data from 2007–2008 season, with high mismatch between B strain included in trivalent influenza vaccine and B strain circulating.
Sensitivity analysis
The one-way sensitivity analysis found that the parameters most affecting the cost-effectiveness of QIV were: the circulation of type A influenza (higher circulation of A virus resulting in a lower differential benefit for QIV) and the potential mismatch between the circulating type B lineage and the one included in TIV (higher mismatch leading to better cost-effectiveness for QIV). Obviously, if there was almost insignificant type B circulating influenza or if lineage B matching was perfect, QIV would not offer any additional benefit over TIV. None of the other tested parameters produced meaningful changes on the results.
The probabilistic sensitivity analysis, including a total of 1,000 simulations, showed that in 3.6% of simulations, QIV achieved better health outcomes than TIV with lower total costs (dominant). At a willingness to pay threshold of 20,000€/QALY, QIV had an 87% probability to be a cost-effective alternative for influenza prevention as compared to TIV; this rose to 96% at a willingness to pay threshold of 30,000€/QALY (Fig. 2).
Figure 2.

Probabilistic sensitivity analysis, cost-effectiveness acceptability curve Legend QALY: Quality-adjusted life year.
Discussion
Cost-effectiveness analysis should be an additional tool that, together with epidemiological and clinical evidence, contributes for discussing about inclusion of new antigens or expand vaccination programs.
This analysis used a lifetime, multi-cohort Markov model to compare the potential effects of QIV and TIV on the disease burden of influenza in Spain from the societal perspective. Our base case results indicated that the QIV would deliver substantial health benefits from the NHS perspective by further reducing the number of symptomatic influenza cases, of medical visits, of hospitalizations for complications, and of deaths as well as of work absenteeism, compared to TIV. The estimated reduction in influenza cases with QIV would also reduce the costs of treating influenza and indirect costs resulting from time lost from work due to influenza, partially offsetting the increased costs of QIV compared to TIV. Overall, QIV was estimated to be a cost-effective intervention compared to TIV, with an ICER estimated at 8,748€/QALY.
The results of the study are consistent with findings from studies in the USA and the UK, which indicated that QIV would be expected to reduce influenza cases, hospitalizations and deaths, more than TIV.8,9 It is also aligned with other vaccines cost-effectiveness analyses targeting adult and elderly, like pneumococcal and zoster vaccination, with the difference that influenza needs to be addressed every year while the others require one-time vaccination and no recurrent changes in vaccine composition.
A lifetime model, such as the one reported in this article, can follow a cohort of individuals over a lifetime of influenza seasons and repeated vaccination and/or other interventions. A lifetime cohort model is a better option than a one-year model to answer research questions about the cost-effectiveness of a particular vaccination policy when applied to today's eligible population cohorts, who will then age over time. Due to differences in modeling approaches, the results of this model are not directly comparable with previously published results from one-year models.10,11
A multi-cohort model could reflect population heterogeneity. Different age groups may vary in their probability of infection, baseline utility, mortality risk and other factors. However, this capacity for heterogeneity was not completely taken advantage of, because detailed age-specific data were not available. However, some inputs, such as length of stay for the complications that required hospitalization, exploited this granularity. This model structure allows granular information to be included as soon as it becomes available. However we recommend that a model selection should be based on the research question to answer rather than being determined by data availability.12
A number of limitations arise from this study. Firstly, as the model adopted the Ministry of Health recommendations for influenza vaccination,13 the population targets for vaccination were the elderly over 65 y and the at-risk groups below 65 y. At a regional level, there could be different target populations for influenza vaccination and the model could not therefore represent an exact picture across Spain. Nevertheless, the Spanish Health Authorities are now trying to introduce a unique vaccination calendar for the whole country, unifying recommendations, and therefore the information used in the model would be more representative.
Secondly, herd effect is not included in this analysis. Several studies have shown how indirect herd effect from vaccination of children offers potential for improving the effectiveness of influenza prevention in the remaining unvaccinated population. For example, a study in Canada found that vaccination against influenza of children and adolescents up to the age of 15 y achieved a protection of 61% against influenza infection in unvaccinated individuals.14 However, that effect cannot be captured by static models like the present Markov model. Further dynamic modeling approaches are needed to explore herd effect impact of influenza vaccination. Not including herd effect in the present study was nevertheless a conservative assumption against QIV, which would be expected to have a higher herd effect due to its additional B virus strain. Including a herd effect, as estimated from influenza studies,15 would result in a more favorable ICER for QIV.
Obviously it is not possible to predict influenza virus circulation over the next 100 years, it is already difficult enough to predict the circulation for the next season. We used an average from the latest seasons of type B influenza circulation and matching B lineage to model future circulation over a 100 y time horizon. Seasonal influenza variations and the unpredictability of the influenza virus in the future may prove that estimations from historical data could be incorrect. However, at the time of the analysis, they were considered the best available estimates.
Finally, there were limitations in the data available to populate the model. Often there was a lack of data regarding the differences between healthy and at risk groups. Consequently, some data included in the model were taken from studies conducted outside Spain. Spanish data were preferred over studies conducted abroad, but if no Spanish data were available or they were considered to be unrepresentative due to small sample sizes, other European sources were chosen.
In conclusion, this economic evaluation of QIV compared to TIV in elderly and clinical risk groups in Spain, using a multi-cohort Markov model, estimated that QIV will further reduce influenza cases, complications, hospitalizations, and deaths compared to TIV. With a cost of 8,748€/QALY gained, it would provide a cost-effective intervention at the 30,000€/QALY threshold in Spain.16,17 Including QIV within national immunization programs could therefore contribute to reduce the burden of disease and alleviate the huge healthcare demand that occurs every year during the influenza season.
Methods
A static, lifetime, multi-cohort state transition model with a one-year cycle time, which had been previously used to assess QIV in the UK,8 was adapted to the Spanish setting. Nine age groups were considered: 0–4, 5–17, 18–49, 50–64, 65–69, 70–74, 75–79, 80–84 and ≥ 85 years, which were split in 2 groups according to risk: the first group included the population who was at risk of serious complication from influenza due to other conditions and chronic diseases (“at-risk group”). The second group included a healthy population for whom the influenza vaccine is also recommended, for example individuals aged over 65 y (“healthy group”). The at-risk group was defined according to the Spanish Ministry of Health recommendations for influenza vaccination,13 and included patients with chronic cardiovascular or lung disease, metabolic disease, morbid obesity, chronic renal disease, hemoglobin disorders and anaemia, asplenia, chronic liver disease, severe neuromuscular diseases, immunosuppressed, cochlear implanted, cognitive dysfunction, people living in closed institutions, pregnant women and children from 6 months to 18 y receiving long-term treatment with acetylsalicylic acid. For the age group 0–4 y, the proportion of vaccinated subjects was adjusted according to QIV approved indication (children >3 y old only are vaccinated).18
The vaccine coverage rate was adjusted for each age and risk group to calculate the number of vaccinated individuals. A lifetime horizon (100 y) was considered to allow a comprehensive analysis of the clinical benefits in terms of accrued life years (LYs) and QALYs. Hence, the model allowed cases in the youngest age group to be followed up through all age groups. Once an age cohort reached the starting age of the next age cohort, all probabilities of the corresponding age group were applied.
Influenza infection was split according to virus type A (H1N1 and H3N2 together) and type B (Victoria and Yamagata separately) to ensure that additional QIV protection benefit could be captured.
As influenza causes annual epidemics and yearly vaccination is recommended, a one-year cycle was chosen. A number of events could happen during each cycle, with any subject having a differential probability of the following events: vaccination; suffer influenza infection; seek medical advice for influenza (Primary Care or Emergency Room); suffer influenza-related complications; need hospitalization for complication; death. All survivors from each cycle would begin a new annual cycle.
Two prevention strategies were analyzed: influenza vaccination with either QIV or TIV.
The economic evaluation of QIV was conducted from a societal perspective, which allowed the costs associated with sick leave to be included, and also from the NHS perspective.
The model was developed in Microsoft Excel 2010. A discount rate of 3% was used for costs and health outcomes as recommended.19 All costs were in 2014 euros.
Inputs of the Economic Model
Demographic data
Details on the Spanish population (46,727,891 individuals), and their distribution by age, risk groups and all-causes mortality rates were obtained from the National Statistics Institute and National Health Survey 2011–2012.20-23 Several assumptions were required due to a lack of information. Based on UK data,24 the model assumed that all-cause mortality in the at-risk group was 10-fold the all-cause mortality in the healthy group. The probability of moving to the at-risk group was independent of influenza exposure or vaccination status, and was calculated from all-cause mortality data and the age distribution of the at-risk group. Once individuals moved to the at-risk group, they remained in this group for the remainder of their life.
Vaccine efficacy
Vaccine efficacy against influenza type A was assumed to be the same for QIV and TIV, and was estimated differentially for age groups based on three Cochrane systematic reviews in healthy children,25 healthy adults,26 and the elderly.27 Efficacy in the at-risk groups was assumed to be identical to that of healthy groups.
The efficacy of TIV against influenza type B was estimated from a meta-analysis in adults which considered the case of perfect matching and total mismatching.28 A reduction of efficacy in children and the elderly was applied.25,28,29 Notably, TIV does have some cross-protection against type B influenza in the case of mismatching.28 Efficacy against type B influenza is the main difference between TIV and QIV, due to the inclusion of the second type B lineage in QIV, which increases the probability of matching with the circulating type B influenza. It is assumed that TIV efficacy against influenza B is proportional to the percentage of matching with circulating type B influenza. Hence, QIV efficacy would be equal to the efficacy of TIV in the event of optimal matching.
The proportion of circulating type B influenza within all influenza cases (type A + type B), which varies by year, also has an effect on the incremental health benefit of QIV over TIV. The model estimates type A and type B circulation based on the average of the last ten influenza seasons in Spain, excluding the season of pandemic influenza 2009–2010, provided by Spanish National Epidemiology Center.30-39 This source was also used to determine the average matching between circulating type B lineage and the one included in TIV, over the last eight seasons, excluding the pandemic season. Table 2 summarizes influenza circulation and matching data in Spain.
Table 2.
Influenza circulation, lineage and matching in Spain.30-39
| B lineage circulation |
Proportion of predominant virus |
|||||
|---|---|---|---|---|---|---|
| Season | Victoria | Yamagata | B lineage in trivalent vaccine | Mismatching B | Type A | Type B |
| 2003–2004 | 99.75% | 0.25% | ||||
| 2004–2005 | 83.78% | 16.22% | ||||
| 2005–2006 | 86.4% | 13.6% | Yamagata | 86.4% | 59.61% | 40.39% |
| 2006–2007 | 11.1% | 88.9% | Victoria | 88.9% | 90.92% | 9.08% |
| 2007–2008 | 3.0% | 97.0% | Victoria | 97.0% | 47.00% | 53.00% |
| 2008–2009 | 100.0% | 0.0% | Yamagata | 100.0% | 73.00% | 27.00% |
| 2010–2011 | 95.6% | 4.4% | Victoria | 4.4% | 72.12% | 27.88% |
| 2011–2012 | 15.4% | 84.6% | Victoria | 84.6% | 92.38% | 7.62% |
| 2012–2013 | 17.2% | 82.8% | Yamagata | 17.2% | 25.23% | 74.77% |
| 2013–2014 | 35.3% | 64.7% | Yamagata | 35.3% | 99.10% | 0.90% |
| Average | 45.5% | 54.5% | 64.2% | 74.29% | 25.71% | |
Probabilities
Vaccination coverage was estimated from the 2011–2012 National Health Survey for adults over 18 y,23 and from a regional population study for children.40 It was assumed that vaccination followed Ministry of Health recommendations. Other assumptions were: equal vaccination coverage would be reached with TIV and QIV, and QIV was used according to the label age indications (≥ 3 y).18
The annual probability of developing symptomatic influenza without prophylaxis is reportedly higher in children (19.21%) than in adults (6.55%) or the elderly (6.17%).5 The probability of seeking medical advice (Primary Care or Emergency Room) for influenza symptoms was estimated at 30% in Spain, based on a volunteer internet-registry to monitor the activity of influenza-like illness.41 The proportion of population seeking advice at the Primary Care (81.67%) or Emergency Room level was established according to clinical expert opinion.
Neuraminidase inhibitors are not reimbursed in outpatient settings in Spain, and they are not usually prescribed for post-exposure prophylaxis or influenza treatment. This Spanish economic evaluation does not therefore include the use of neuraminidase inhibitors.
The probabilities of developing influenza complications, type of complication, hospitalization, and death related to influenza, were taken from a large UK study.42,43 This source was used because the information available in Spain came from small studies not representatives of the whole population.44,45
Table 3 shows a summary of demographic, efficacy and probability inputs.
Table 3.
Demographic, efficacy and probability inputs used to populate the model.
| Age group (years) |
0–4 |
5–17 |
18–49 |
50–64 |
65–69 |
70–74 |
75–79 |
80–84 |
85+ |
| Population distribution | 5.18% | 12.73% | 46.04% | 18.37% | 4.86% | 3.67% | 3.69% | 2.93% | 2.53% |
| Proportion healthy in each age group | 81.18% | 78.63% | 68.63% | 36.18% | 21.21% | 12.72% | 11.60% | 10.13% | 9.13% |
| Population at-risk in each age group | 18.82% | 21.37% | 31.37% | 63.82% | 78.79% | 87.28% | 88.40% | 89.87% | 90.87% |
| Vaccine efficacy against influenza A, trivalent and quadrivalent | 59.00% | 59.00% | 60.00% | 60.00% | 58.00% | 58.00% | 58.00% | 58.00% | 58.00% |
| Trivalent vaccine efficacy against influenza B, match | 66.00% | 77.00% | 77.00% | 73.00% | 69.00% | 69.00% | 66.00% | 66.00% | 66.00% |
| Trivalent vaccine efficacy against influenza B, mismatch | 44.00% | 52.00% | 52.00% | 49.00% | 47.00% | 47.00% | 44.00% | 44.00% | 44.00% |
| Trivalent vaccine efficacy against influenza, base case | 51.87% | 60.95% | 60.95% | 57.59% | 54.87% | 54.87% | 51.87% | 51.87% | 51.87% |
| Quadrivalent vaccine efficacy against influenza B | 66.00% | 77.00% | 77.00% | 73.00% | 69.00% | 69.00% | 66.00% | 66.00% | 66.00% |
| Influenza vaccine coverage, healthy | 0.00% | 0.00% | 0.00% | 0.00% | 28.38% | 49.55% | 48.18% | 64.56% | 57.63% |
| Influenza vaccine coverage, at-risk | 24.16% | 24.24% | 9.26% | 24.54% | 47.00% | 54.40% | 63.85% | 72.47% | 67.59% |
| Influenza-related complication, healthy | 14.05% | 14.05% | 7.61% | 7.95% | 10.34% | 10.34% | 10.34% | 10.34% | 10.34% |
| Influenza-related complication, at risk | 18.29% | 18.29% | 12.32% | 12.59% | 13.76% | 13.76% | 13.76% | 13.76% | 13.76% |
| Hospitalization due to complication, healthy | 10.87% | 10.87% | 10.87% | 10.87% | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% |
| Hospitalization due to complication, at risk | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% | 15.79% |
| Death after influenza complication, healthy | 0.00% | 0.00% | 0.405% | 0.96% | 11.21% | 11.21% | 11.21% | 11.21% | 11.21% |
| Death after influenza complication, at risk | 0.15% | 0.15% | 0.34% | 1.64% | 12.18% | 12.18% | 12.18% | 12.18% | 12.18% |
Costs
Unitary costs applied to health resource consumption were retrieved from Spanish cost databases and official tariffs published by health authorities46-52 and from previously published studies of indirect costs associated with influenza.53 When the same costs were found from different sources, an average cost was considered. The wholesale price of the vaccines was considered and the administration costs were taken from regional tariffs.46-52 The remaining unitary costs were equal for both alternatives.
The analysis was conducted from a societal perspective and therefore included both direct medical costs and costs incurred by the patient or society. Absenteeism costs and productivity loss caused by influenza in the working-age population (18–65 y) was estimated based on National Statistics Institute data. A complete list of all unitary costs included in the model is available in Table 4.
Table 4.
Unitary costs.46-53
| Age group (years) |
0–4 |
5–17 |
18–49 |
50–64 |
65–69 |
70–74 |
75–79 |
80–84 |
85+ |
| National Health System Costs | |||||||||
| Quadrivalent vaccine | 9.50 € | 9.50 € | 9.50 € | 9.50 € | 9.50 € | 9.50 € | 9.50 € | 9.50 € | 9.50 € |
| Trivalent vaccine | 7.00 € | 7.00 € | 7.00 € | 7.00 € | 7.00 € | 7.00 € | 7.00 € | 7.00 € | 7.00 € |
| Vaccine administration | 11.00 € | 11.00 € | 11.00 € | 11.00 € | 11.00 € | 11.00 € | 11.00 € | 11.00 € | 11.00 € |
| Primary Care visit | 52.73 € | 52.73 € | 52.73 € | 52.73 € | 52.73 € | 52.73 € | 52.73 € | 52.73 € | 52.73 € |
| Emergency Room | 127.35 € | 127.35 € | 146.10 € | 146.10 € | 146.10 € | 146.10 € | 146.10 € | 146.10 € | 146.10 € |
| Outpatient complication | 35.18 € | 35.18 € | 35.74 € | 35.74 € | 35.74 € | 35.74 € | 35.74 € | 35.74 € | 35.74 € |
| Bronchitis hospitalization | 3,261.84 € | 3,274.08 € | 2,933.41 € | 2,877.61 € | 2,786.57 € | 2,724.34 € | 2,662.10 € | 2,680.25 € | 2,690.46 € |
| Pneumonia hospitalization | 3,373.31 € | 4,082.09 € | 5,730.34 € | 9,377.09 € | 9,241.09 € | 9,120.06 € | 9,489.30 € | 4,723.53 € | 4,018.12 € |
| URTI hospitalization | 3,373.31 € | 4,082.09 € | 5,730.34 € | 9,377.09 € | 9,241.09 € | 9,120.06 € | 9,489.30 € | 4,723.53 € | 4,018.12 € |
| Hospitalization for cardiac complication | 4,283.24 € | 3,530.25 € | 6,660.38 € | 5,106.92 € | 4,886.06 € | 4,758.99 € | 4,501.25 € | 4,078.34 € | 3,994.09 € |
| Hospitalization for renal complication | 5,344.98 € | 4,893.87 € | 4,471.18 € | 4,150.43 € | 3,720.80 € | 4,044.61 € | 5,219.13 € | 3,985.55 € | 4,074.83 € |
| CNS hospitalization | 2,602.31 € | 2,746.39 € | 3,344.55 € | 3,131.91 € | 4,111.21 € | 3,211.37 € | 3,808.75 € | 3,284.81 € | 3,492.01 € |
| OM hospitalization | 2,511.82 € | 2,728.14 € | 2,648.37 € | 2,755.52 € | 2,762.39 € | 2,879.33 € | 2,483.54 € | 2,318.71 € | 2,196.13 € |
|
Society cots | |||||||||
| Productivity loss, influenza | – | – | 623.96 € | 623.96 € | – | – | – | – | – |
| Productivity loss, hospitalization | – | – | 1,482.70 € | 1,482.70 € | – | – | – | – | – |
| Productivity loss, outpatient complication | – | – | 623.96 € | 623.96 € | – | – | – | – | – |
URTI: upper respiratory tract infection; CNS: central nervous system; OM: otitis media.
Health outcomes / utilities
The utilities and disutilities are used for estimating quality of life of subjects suffering from influenza and are needed to calculate QALYs gained through vaccination and thus establish the corresponding cost-effectiveness. Utilities data used in the model were taken from 2011–2012 National Health Survey and were estimated from the EQ-5D54 according to age and risk group.23 The EQ-5D is a standardized generic instrument that comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has one specific question and three levels of response: 1 “no problems,” 2 “some problems” and 3 “severe problems.” The instrument therefore defines distinct health states from all the possible combinations of dimensions and levels of severity. Considering the responses to the descriptive system, each health state is converted into a utility/disutility index by applying the general population preference values. The EQ-5D utility index ranges from 1 (best health status) to negative values (health states valued as worse than death), where 0 is equal to death. This utility index can then be used to calculate QALYs. In the study, disutility caused by influenza infection was retrieved from a large Spanish observational longitudinal study.55
Table 5 summarizes the utilities included in the model and the duration of influenza episodes.
Table 5.
Utility and disutility scores used in the model.54-55
| Age group (years) |
0–4 |
5–17 |
18–49 |
50–64 |
65–69 |
70–74 |
75–79 |
80–84 |
85+ |
| Baseline | |||||||||
| Healthy | 0.99 | 0.99 | 0.97 | 0.96 | 0.97 | 0.94 | 0.93 | 0.85 | 0.73 |
| At risk | 0.96 | 0.96 | 0.94 | 0.87 | 0.85 | 0.82 | 0.78 | 0.69 | 0.54 |
| Influenza | |||||||||
| Disutility | − 0.41 | − 0.41 | − 0.465 | − 0.36 | − 0.32 | − 0.32 | − 0.32 | − 0.32 | − 0.32 |
| Length (days) | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Outpatient complications | |||||||||
| Disutility | − 0.41 | − 0.41 | − 0.465 | − 0.36 | − 0.32 | − 0.32 | − 0.32 | − 0.32 | − 0.32 |
| Length (days) | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 |
| Inpatient complications | |||||||||
| Disutility | − 0.54 | − 0.54 | − 0.60 | − 0.58 | − 0.56 | − 0.56 | − 0.56 | − 0.56 | − 0.56 |
| Bronchitis length | 3.49 | 3.81 | 5.25 | 5.70 | 6.21 | 6.56 | 6.49 | 6.61 | 6.59 |
| Pneumonia length | 4.75 | 5.94 | 8.75 | 10.44 | 13.55 | 12.65 | 14.13 | 9.33 | 9.33 |
| URTI length | 3.93 | 3.94 | 4.50 | 7.89 | 5.89 | 7.85 | 5.29 | 6.90 | 6.62 |
| Cardiac length | 10.64 | 4.75 | 9.07 | 9.14 | 9.07 | 9.07 | 8.86 | 8.06 | 7.28 |
| Renal length | 4.50 | 4.38 | 5.03 | 3.96 | 5.04 | 6.91 | 8.00 | 7.27 | 9.81 |
| CNS length | 3.46 | 3.23 | 4.89 | 5.68 | 5.68 | 6.20 | 7.85 | 6.49 | 6.32 |
| OM length | 3.80 | 2.32 | 1.93 | 2.23 | 2.46 | 4.04 | 4.52 | 5.33 | 7.40 |
URTI: upper respiratory tract infection; CNS: central nervous system; OM: otitis media.
Analysis
The base case analysis included the aforementioned inputs as well as a 100 years' time horizon, societal perspective and cost-utility analysis. The ICER was calculated with the formula:
Although a one-year time horizon does not allow LYs or QALYs gained by alternative interventions to be calculated, first year results were reported in terms of cases avoided and costs, in order to facilitate comparisons with other assessments.
To assess the robustness of the results, two sensitivity analyses were performed: a one-way sensitivity analysis to determine which variable has individually the greatest impact on cost-effectiveness results, and a probabilistic sensitivity analysis which assessed the level of uncertainty of the variables in combination within the model. The probabilistic sensitivity analysis was performed using Monte Carlo simulations with 1,000 iterations, each selecting the input parameter values from a probability distribution. The representation of the probabilistic sensitivity analysis is presented as a cost-effectiveness acceptability curve, showing the probability of QIV being cost-effective.
Abbreviations
- ICER
Incremental cost-effectivess ratio
- LY
Life year
- QALY
Quality-adjusted life year
- QIV
Quadrivalent influenza vaccine
- NHS
National Health System
- TIV
Trivalent influenza vaccine
- WHO
World Health Organization
Disclosure of potential conflicts of interest
At the time of this analysis, DC and JC were employees of BAP Health Outcomes Research S.L. (Oviedo, Spain) which received funding from the GSK group of companies to perform this study. DC is now an employee of IMS Health (Madrid, Spain) and JC is independent freelance consultant. AG, RO and JR received consulting fees from BAP Health Outcomes Research S.L. (Oviedo, Spain) during the conduct of this study. RM is an employee and shareholder of the GSK group of companies.
Acknowledgments
The authors thank Julia Donnelly (Freelance on behalf of GSK Vaccines) for English writing review and Stephanie Garcia (Business & Decision Life Sciences on behalf of GSK Vaccines) for editorial assistance and manuscript coordination as well as Veronique Gochet (Business & Decision Life Sciences on behalf of GSK Vaccines) for editorial assistance.
Funding
GlaxoSmithKline S.A. (Madrid, Spain) funded this study (GSK study identifier: HO-14-15215) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and publication of this manuscript.
Contributorship
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
References
- [1].World Health Organization Influenza (Seasonal). Fact sheet N° 211. http://www.who.int/mediacentre/factsheets/fs211/en/ 2014 [Google Scholar]
- [2].Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010. September 7; 28(Suppl 4):D45-53; PMID:20713260; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.028 [DOI] [PubMed] [Google Scholar]
- [3].Daley AJ, Nallusamy R, Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. J Paediatr Child Health 2000. August; 36(4):332-335; PMID:10940165; http://dx.doi.org/ 10.1046/j.1440-1754.2000.00533.x [DOI] [PubMed] [Google Scholar]
- [4].Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. Int J Infect Dis 2007. January; 11(1):40-47; PMID:16678464; http://dx.doi.org/ 10.1016/j.ijid.2005.10.008 [DOI] [PubMed] [Google Scholar]
- [5].Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003; 7(35):iii-xiii:1 [DOI] [PubMed] [Google Scholar]
- [6].Criterios de evaluación para fundamentar modificaciones en el Programa de Vacunación en España. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad,Politica Social e Igualdad 2011. [Google Scholar]
- [7].Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes Third ed. New York: Oxford University Press; 2005. [Google Scholar]
- [8].Van Bellinghen LA, Meier G, Van VI. The Potential cost-effectiveness of quadrivalent vs. Trivalent Influenza Vaccine in elderly people and clinical risk groups in the UK: a lifetime multi-cohort model. PLoS One 2014; 9(6):e98437; PMID:24905235; http://dx.doi.org/ 10.1371/journal.pone.0098437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012 March 2; 30(11):1993-1998; PMID:22226861; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.098 [DOI] [PubMed] [Google Scholar]
- [10].De Juanes JR, Cisterna R, Sanz J, Magaz S, Badia X. Efficiency of influenza vaccination in the working population in Spain. Gac Sanit 2006 March; 20(2):101-107; http://dx.doi.org/ 10.1157/13087326 [DOI] [PubMed] [Google Scholar]
- [11].Pradas-Velasco R, Antonanzas-Villar F, Martinez-Zarate MP. Dynamic modelling of infectious diseases: an application to the economic evaluation of influenza vaccination. Pharmacoeconomics 2008; 26(1):45-56; PMID:18088158; http://dx.doi.org/ 10.2165/00019053-200826010-00005 [DOI] [PubMed] [Google Scholar]
- [12].Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health 2012 September; 15(6):796-803; PMID:22999128; http://dx.doi.org/ 10.1016/j.jval.2012.06.012 [DOI] [PubMed] [Google Scholar]
- [13].Ministerio de Sanidad SSeI. Prevención de la gripe. Vacunación antigripal ¿Quién se debe vacunar frente a la gripe? http://www.msssi.gob.es/ciudadanos/enfLesiones/enfTransmisibles/gripe/gripe.htm#Prev1 2014 25-2-2015. [Google Scholar]
- [14].Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJ, Aoki F, Horsman G, Van Caeseele P, Chokani K, et al.. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303:943-950; PMID:20215608; http://dx.doi.org/ 10.1001/jama.2010.250 [DOI] [PubMed] [Google Scholar]
- [15].Van VI, Van Bellinghen LA, Meier G, Nautrup BP. An approximation of herd effect due to vaccinating children against seasonal influenza - a potential solution to the incorporation of indirect effects into static models. BMC Infect Dis 2013; 13:25; http://dx.doi.org/ 10.1186/1471-2334-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ortún V. 30.000 euros por AVAC. Economía y Salud 2004; 49:1-2 [Google Scholar]
- [17].Sacristan J, Oliva J, Del Llano J, Prieto L, Pinto J. Qué es una tecnología sanitaria eficiente en España? Gac Sanit 2002; 16(4):334-343; http://dx.doi.org/ 10.1016/S0213-9111(02)71933-X [DOI] [PubMed] [Google Scholar]
- [18].MSSSI, AEMPS. Fluarix Tetra. Product Information. http://www aemps gob es/cima/pdfs/es/ft/78568/FT_78568.pdf 2014 [Google Scholar]
- [19].Lopez-Bastida J, Oliva J, Antonanzas F, Garcia-Altes A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010 October; 11(5):513-520; http://dx.doi.org/ 10.1007/s10198-010-0244-4 [DOI] [PubMed] [Google Scholar]
- [20].Instituto Nacional de Estadística Población en establecimientos colectivos por sexo, edad y tipo de establecimiento. Censos de Población y Viviendas 2011. Resultados Nacionales. http://www.ine.es/censos2011_datos/cen11_datos_caracteristicas_col.htm. 2011:16-5-2014 [Google Scholar]
- [21].Instituto Nacional de Estadística Defunciones según la Causa de Muerte 2012. Resultados nacionales. Tasas de mortalidad por causas, sexo y edad. http://www.ine.es/jaxi/menu.do?type=pcaxis&path=/t15/p417/a2012/&file=pcaxis. 2012:16-5-2014 [Google Scholar]
- [22].Instituto Nacional de Estadística. Cifras de población a 1 de enero de 2013. Resultados nacionales. Población residente por fecha, sexo y edad. http://www.ine.es/jaxi/tabla.do 2014:16-5-2014 [Google Scholar]
- [23].Ministerio de Sanidad SSeI, Instituto Nacional de Estadística. Encuesta Nacional de Salud 2011–2012; 2014:20-5-2014 [Google Scholar]
- [24].Department of Health The seasonal flu immunisation programme 2011/12. http://www dh/gov/uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_128175.pdf 2011 [Google Scholar]
- [25].Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012;. 8:CD004879; PMID:22895945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2014; 3:CD001269; PMID:24623315 [DOI] [PubMed] [Google Scholar]
- [27].Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; 2:CD004876; PMID:20166072 [DOI] [PubMed] [Google Scholar]
- [28].Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; http://dx.doi.org/ 10.1186/1741-7015-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012. January; 12(1):36-44; PMID:22032844; http://dx.doi.org/ 10.1016/S1473-3099(11)70295-X [DOI] [PubMed] [Google Scholar]
- [30].Área de Vigilancia de la Salud Pública, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Resumen de la temporada 2007-2008; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2008:20-5-2014 [Google Scholar]
- [31].Área de Vigilancia de la Salud Pública, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Resumen de la temporada 2008-2009; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2009:20-5-2014 [Google Scholar]
- [32].Área de Vigilancia de la Salud Pública, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Temporada 2010-2011; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2011:20-5-2014 [Google Scholar]
- [33].Área de Vigilancia de la Salud Pública, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Temporada 2011-2012; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2012: 20-5-2014 [Google Scholar]
- [34].Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Sistemas Centinela. Temporada 2006-2007; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2007:20-5-2014 [Google Scholar]
- [35].Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2012-2013; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2013:20-5-2014 [Google Scholar]
- [36].Grupo de Vigilancia de la Gripe en España, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Sistemas Centinela. Resumen de la temporada 2003-2004; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2004:20-5-2014 [Google Scholar]
- [37].Grupo de Vigilancia de la Gripe en España, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Sistemas Centinela. Resumen de la temporada 2004-2005; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2005:20-5-2014 [Google Scholar]
- [38].Grupo de Vigilancia de la Gripe en España, Centro Nacional de Epidemiología, Instituto de Salud Carlos III. Vigilancia de la Gripe en España. Sistemas Centinela. Resumen de la temporada 2005-2006; http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-enfermedades/gripe.shtml. 2006:20-5-2014 [Google Scholar]
- [39].Instituto de Salud Carlos III Sistema de Vigilancia de la Gripe en España. Informe de Vigilancia de la Gripe en España. Temporada 2013-2014 (Desde la semana 40/2013 hasta la semana 20/2014). http://vgripe/isciii/es/gripe/documentos/20132014/InformesAnuales/Informe_Vigilancia_GRIPE_2013-2014_v12092014.pdf 2014 [Google Scholar]
- [40].Gonzalez R, Campins M, Rodrigo JA, Uriona S, Vilca LM. Influenza vaccination coverage in children with risk conditions in Catalonia. Enferm Infecc Microbiol Clin 2015; 33(1):22-26; http://dx.doi.org/ 10.1016/j.eimc.2013.12.010 [DOI] [PubMed] [Google Scholar]
- [41].GripeNet.es. El final de la primera estación gripal de GripeNet.es. https://www.gripenet.es/media/cms_page_media/85/boletin_14_temporada_1_small.pdf Boletín GripeNet temporada 1, 14° entrega. 2013 [Google Scholar]
- [42].Meier CR, Napalkov PN, Wegmuller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis 2000. November; 19(11):834-842; PMID:11152308; http://dx.doi.org/ 10.1007/s100960000376 [DOI] [PubMed] [Google Scholar]
- [43].Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, Nicholson K. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol Assess 2009. February; 13(11):iii, ix-iii:246. [DOI] [PubMed] [Google Scholar]
- [44].Lopez-Medrano F, Aguado JM, Lizasoain M, Folgueira D, Juan RS, Diaz-Pedroche C, Lumbreras C, Morales JM, Delgado JF, Moreno-Gonzalez E. Clinical implications of respiratory virus infections in solid organ transplant recipients: a prospective study. Transplantation 2007. October 15; 84(7):851-856; PMID:17984837; http://dx.doi.org/ 10.1097/01.tp.0000282788.70383.8b [DOI] [PubMed] [Google Scholar]
- [45].van Esso Arbolave DL, Estabanell BA, Fernandez GI, Perez CM, Besora AR, Casanovas Gordo JM, Pumarola ST, Marcos Maeso MA, Martinez MA, Dominguez GA. Clinical and epidemiological characteristics of influenza A virus infection in children aged less than 7 years old in primary care. An Pediatr (Barc) 2006. September; 65(3):211-218; http://dx.doi.org/ 10.1157/13092156 [DOI] [PubMed] [Google Scholar]
- [46].Consejería de Sanidad. ORDEN 731/2013, de 6 de septiembre, del Consejero de Sanidad, por la que se fijan los precios públicos por la prestación de los servicios y actividades de naturaleza sanitaria de la Red de Centros de la Comunidad de Madrid. BOCM 2013 [Google Scholar]
- [47].Consejo General de Colegios Oficiales de Faramacéuticos. Bot PLUS 2.0 https://botplusweb.portalfarma.com. 2015:15-11-2014 [Google Scholar]
- [48].Departamento de Salud. SLT/30/2013, de 20 de febrero, por la que se aprueban los precios públicos del Servicio Catalán de la Salud. DOGC 2013 [Google Scholar]
- [49].Ministerio de Sanidad SSeI. Portal Estadístico. Consulta Interactiva del SNS. http://pestadistico.inteligenciadegestion.msssi.es/publicoSNS/comun/DefaultPublico.aspx 2014. Fifteen-11-2014 [Google Scholar]
- [50].Osakidetza, Eusko Jaurlaritza. Tarifas para facturación de servicios sanitarios y docentes de Osakidetza para el año 2014. 2014 [Google Scholar]
- [51].Servicio Andaluz de Salud. Precios públicos de servicios sanitarios prestados en el SSPA. http://www.juntadeandalucia.es/servicioandaluzdesalud/ordenpreciospublicos. 2014:15-11-2014 [Google Scholar]
- [52].Instituto Nacional de Estadística. Encuesta Anual de Estructura Salarial 2012; http://www.ine.es/jaxi/tabla.do. 2014:16-5-2014 [Google Scholar]
- [53].Galante M, Garin O, Sicuri E, Cots F, Garcia-Altes A, Ferrer M, Dominguez A, Alonso J. Health services utilization, work absenteeism and costs of pandemic influenza A (H1N1) 2009 in Spain: a multicenter-longitudinal study. PLoS One 2012; 7(2):e31696; PMID:22348122; http://dx.doi.org/ 10.1371/journal.pone.0031696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Badia X, Roset M, Montserrat S, Herdman M, Segura A. La versión española del EuroQol: descripción y aplicaciones. Medicina Clínica (Barc) 1999; 112(1):79-86 [PubMed] [Google Scholar]
- [55].Hollmann M, Garin O, Galante M, Ferrer M, Dominguez A, Alonso J. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS One 2013; 8(3):e60477; PMID:23555979; http://dx.doi.org/ 10.1371/journal.pone.0060477 [DOI] [PMC free article] [PubMed] [Google Scholar]


