ABSTRACT
For many cancers the use of conventional chemotherapy has been maximized, and further intensification of chemotherapy generally results in excess toxicity with little long-term benefit for cure. Many tumors become resistant to chemotherapy, making the investigation of novel approaches such as immunotherapy of interest. Because the tumor microenvironment is known to promote immune tolerance and down regulate the body's natural defense mechanisms, modulating the immune system with the use of dendritic cell (DC) therapy is an attractive approach. Thousands of patients with diverse tumor types have been treated with DC vaccines. While antigen specific immune responses have been reported, the duration and magnitude of these responses are typically weak, and objective clinical responses have been limited. DC vaccine generation and administration is a multi-step process with opportunities for improvement in source of DC for vaccine, selection of target antigen, and boosting effector cell response via administration of vaccine adjuvant or concomitant pharmacologic immunomodulation. In this review we will discuss recent developments in each of these areas and highlight elements that could be moved into pediatric clinical trials.
KEYWORDS: cancer immunotherapy, dendritic cell, dendritic cell vaccine, vaccine adjuvant
Approximately 15,780 children age 0–19 y are diagnosed with cancer in the US each year.1 While improved surgical, chemotherapeutic, and radiation based approaches have increasingly resulted in cure for many of these diseases, almost 2000 children die of cancer every year in the US and current treatment regimens are associated with significant toxicity. In addition, children with relapsed cancer often have limited curative options, making development of immune-based strategies of interest. Further improvements in cure rate are likely to come from adjuvant therapies. Because the tumor microenvironment is known in many cases to promote immune tolerance, one attractive approach is modulating the immune system to stimulate antitumor immune response.
Dendritic Cells (DC) are the most powerful antigen presenting cells of the immune system, capable of stimulating naïve and memory CD8+ T-cells as well as B-cells and CD4+ helper T-cells. In the immature state DC are present in blood and tissues, processing foreign antigens for presentation to the immune system. The uptake of presentable antigen stimulates maturation of DC and promotes DC migration to lymph nodes, where these cells can directly interact with immune effector cells. Mature DC are capable of stimulating T helper type-1 immune responses and antigen specific CD8+ cytotoxic T-lymphocytes (CTL), but within the tumor microenvironment DC promote tumor tolerance, facilitating T helper type-2 responses. Therefore DC can exert both strong positive and negative influences on the acquisition of tumor specific cellular immune responses.
DC vaccines have generally consisted of autologous monocytes that are matured in vitro and pulsed with antigen before injection. These vaccines have been given to thousands of patients of all ages with diverse tumor types and have been generally well-tolerated with little toxicity beyond local skin reactions.2,3 While antigen specific immune responses have been reported in a number of these trials, the duration and magnitude of these responses are typically weak, and objective clinical responses have been limited. Sipuleucel-T, an autologous dendritic cell vaccine primed with a recombinant antigen composed of prostatic acid phosphatase linked to GM-CSF as an adjuvant, is the only DC vaccine which has shown sufficient efficacy in a Phase III clinical trial to gain FDA approval.4 While this vaccine is targeted to an adult malignancy, its success does offer hope that an effective DC vaccine can be developed for pediatric tumors. Clinical responses to DC vaccines in children with malignant solid tumors have been disappointing to date, with excellent tolerability but poor efficacy both in high grade CNS tumors5-7 and in a more diverse group of recurrent solid tumors.3,8-14
Each step of DC vaccine production (see Fig. 1), DC generation, antigen loading, in vitro maturation, and inoculation with or without adjuvant is an opportunity to enhance efficacy. DC vaccine research has therefore focused on expanding the available sources of DC and improving DC immunogenicity, optimizing the source and presentation of antigen, developing new immune adjuvants, and investigation of concomitant immunomodulation or chemotherapy. In this review we will discuss developments made in the last 5 y in each of these categories.
Figure 1.
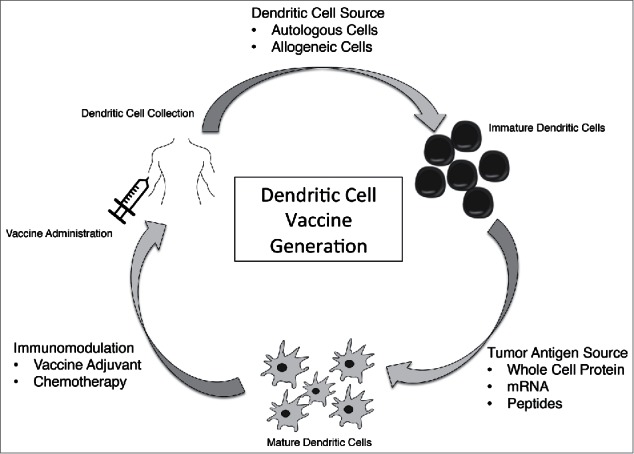
Dendritic Cell Vaccine Generation. DCV generation and administration is a multi-step process. A cell source for DC must be selected and DC generated, target antigen must be selected and dendritic cells exposed to the antigen for maturation, and finally DCV must be administered which can be done with concurrent immune modulators or vaccine adjuvants.
Source of dendritic cells
In a majority of immunotherapy clinical trials, DC are generated from peripheral blood mononuclear cells (PBMC) collected by leukapheresis or phlebotomy. This usually results in consistent vaccine generation, but for patients who have recently received chemotherapy or those with CNS tumors who may require steroid therapy, generation of DC from a PBMC collection may not be feasible.3,15 Because of the difficulty in generating DC from some patients, alternative sources of DC have been explored. Three studies have recently reported the generation of DC from novel cell sources in the pediatric population. A single patient with residual active leukemia following haematopoietic stem cell transplant (HSCT) was reported to receive an allogeneic DC vaccine derived from PBMC collected from her stem cell donor.16 Our group reported a single patient with neuroblastoma whose DC were generated from a cryopreserved, G-CSF mobilized peripheral blood stem cell (PBSC) product,3 and Nair reported the feasibility of generating DC from cryopreserved autologous PBSC products in patients with medulloblastoma.17 This group was able to generate phenotypic DC from 3/5 samples and functional DC from 2/5 samples.17 While this study met metrics for feasibility of DC generation, results are in line with previous data that the yield of functional DC may be lower from children with active tumors than from healthy adult donors. This is likely multifactorial and due to immunosuppression or tolerization by the tumor mass as well as previous myelo- and/or immunosuppressive therapy.18,19
PBSC could be an attractive source of DC because they can be collected prior to the onset of chemotherapy or even induced from an allogeneic source, bypassing the need to culture these cells from an immunocompromised host.20 However, PBSC are also a problematic source of DC because GCSF mobilization can potentially skew DC to a DC-2/tolerogenic phenotype21,22 making them a poor choice for an immunotherapy product. Several groups have generated DC from pluripotent stem cell lines,23 induced pluripotent stem cells,20,24 or embryonic stem cells.25,26 In all cases, these DC were able to induce antigen specific cytotoxic T lymphocyte (CTL) or natural killer (NK) cell responses in vitro, and in 2 studies murine tumor models were used to show in vivo efficacy, defined by shrinkage of tumors and prolonged survival.24,25 No human studies have used DC derived from pluripotent or embryonic stem cells. Finally, de Haar and group have reported a protocol for generating DC from a portion of a cord blood unit used for HSCT, such that patients could be vaccinated with allogeneic DC from their cord blood HSCT donor.27 These alternative cell sources warrant further investigation, particularly regarding in vivo survival and immune response stimulation, because they could expand therapeutic options for these patients.
Antigen selection and loading
The selection of tumor antigen for loading onto DC is critical to maximize the likelihood of achieving an immune response. It is attractive to use a tumor specific antigen if one is known, but for some tumors no consistent tumor associated antigen (TAA) has been identified. Choices for antigen include HLA restricted epitopes, whole tumor lysate, or mixes of peptides from whole antigen. Each of these antigen sources have associated advantages and pitfalls and much research is done regarding the optimal antigen to stimulate the immune system for a given tumor.
Individual tumor antigen epitopes have the advantage of being well-characterized, immunogenic, and available from non-autologous sources. Nevertheless the use of individual epitopes limits immunotherapy to individuals with a specific HLA background. This problem can be overcome by using multiple overlapping peptides from a single protein, thereby providing an epitope library in a non-HLA restricted manner. Peptide mixes for some TAA are commercially available, although differences in composition of various lots could impact vaccine immunogenicity. Examples of recently studied tumor specific antigens include the use of an HLA restricted Wilms' tumor 1 epitope in recurrent high grade glioma patients 28 and a non-HLA restricted pancreatic bile salt protein in pancreatic adenocarcinoma.29 A group in Japan pulsed autologous DC with a mix of WT-I and WT-II to expand the applicability of this approach and demonstrated increased EFS and OS in patients who had positive DTH skin testing to either antigen after administration.30 Our group has published a Phase I trial of DC vaccine targeting the cancer germline antigens (CGA) MAGE-A1, MAGE-A3, and NY-ESO-1 in children with solid tumors which were previously shown to upregulate these antigens in response to the demethylating chemotherapy agent decitabine (DAC). Patients were treated with DAC to upregulate CGA followed by injection of an autologous DC-vaccine pulsed with commercially obtained overlapping peptides derived from each of these 3 antigens,3 permitting the enrollment of patients irrespective of their HLA background. We were able to demonstrate some immune responses and one heavily pretreated patient had a durable complete response.
If an immunogenic TAA has not been identified, one option is treatment with DC that have been pulsed with either whole cell protein or whole cell mRNA. Autologous tumor protein lysates have been used widely in pediatric DC immunotherapy.5,7-11,13,14 Strengths of this approach include that fact that lysate is a reliable source of tumor antigen, particularly in tumors for which antigens have been poorly characterized. However, tumor tissue must be obtained, which limits this approach to those with measurable and resectable disease. This method also necessitates individualized vaccine production which takes time and may limit accessibility of therapy. Obtaining viable cells from autologous tumors may allow for some manipulation of these cells before lysate is generated. One possible manipulation is short term culture to isolate a cancer stem cell (CSC) population for selective CSC lysate pulsing. Because CSC are recognized to be capable of escaping traditional chemotherapy, the use of DC-based immunotherapy targeting antigens expressed by CSC could lead to better long term tumor control. However, there are concerns regarding theoretical generation of autoreactive T cells directed against stem cells in the tissue of tumor origin or in other stem cell populations. CSC antigen pulsed DC have been generated in a mouse model of breast cancer and re-inoculated with good efficacy and no evidence of toxicity to other stem cell populations.31 Another group planned to study feasibility of producing autologous tumor cell lysates from patients with hepatocellular carcinoma. Vaccine production relied on short-term tumor cell culture. Unexpectedly, the success rate of tumor cell culture in their model was 100%, which they speculate may have been due to growth media selecting and propagating cells with stem cell properties. This feasibility trial showed no hepatotoxicity of DC vaccine with these hepatic “stem-like” cell lysates used to load DC.32
Another potential source of antigen is autologous tumor whole-cell mRNA. Electroporated mRNA drives expression of TAA in DC resulting in antigen presentation through MHC Class I molecules and could potentially result in more persistent presentation of tumor antigens than with protein pulsing. This antigen source, similar to whole cell lysate, offers the advantage of presenting a full range of tumor-relevant antigens. A Phase I trial in glioblastoma patients utilized mRNA isolated from autologous sphere-forming CSCs expanded in short term culture and demonstrated tumor specific T-cell proliferation after vaccination as well as increased progression free survival compared to historical controls.33 Importantly, there was not any observed auto-immunity, particularly in eye (neural) tissue or myeloid stem cells.33 In 2015, long-term follow-up of 30 melanoma patients who were treated with autologous mRNA-DC vaccine was reported. These patients had micrometastases but no measurable disease at the time of vaccination. At 6 y from the end of therapy, median survival had not yet been reached, and observed 2 y and 4 y survival rates of 93% and 70%, were at least 10% higher than historical controls at 4 y.34 Interestingly, the EFS was not improved, but relapses were all early and these patients were effectively salvaged, leading to the excellent OS. Similar findings regarding lag time to vaccine response have been reported4 and may indicate that time is needed for DC driven anti-tumor immunity to develop.
Since autologous tumor tissue may not be available in all cases, another source of tumor antigen being explored is allogeneic tumor cell lines. Although there are differences within cell lines for the same tumor type, the similarities to a patient tumor are likely to be greater than the differences, so it may be possible that DC loaded with lysates from a cell line would yield results similar to loading with autologous tumor, therefore bypassing the need for surgery. This approach was safe in a study of 8 vaccinated patients with recurrent brain tumors including 3 pediatric patients.35 In addition, a post-hoc analysis in these patients indicated that patients with stable disease post-vaccine had an increase in IL-17α production, natural killer cells, and CD8+ memory T-cells and a decrease in myeloid derived suppressor cells (MDSC), providing evidence for immune responses to common antigens as a possible explanation for disease stabilization in these patients. Though it did not reach significance, there were also lower CTLA-4 levels in patients with stable disease suggesting that this may be a potential target of immune modulation in future studies.
Some investigators have reported using multiple novel modalities in which an alternative source of DC and ubiquitous tumor antigens were used in combination. DC have been generated from pluripotent stem cells and since they can be passaged in culture, these DC can be directly transduced with tumor antigen DNA for stable expression, which can be presented on MHC class I and II molecules. This combination strategy utilizing a novel source of DC and stable transduction of common tumor antigen DNA was used in 3 recent studies23-25 with demonstration of efficient and prolonged CTL stimulation. This strategy is a step toward generation of a generic, non-autologous vaccine, with DC possibly induced from human stem cells, transduced with common tumor antigen, and cultured and packaged for immediate availability. If feasible, this could ultimately expand the population for whom vaccine is available as well as shorten the time to availability. More study is required before it is ready for human trials.
Adjuvant for DC vaccines
For DCV efficacy, DC must migrate to a lymph node and activate effector cells, either CD8+ T-cells or B-cells. In the right milieu, DC can also activate CD4+ helper T-cells and induce tolerance. Therefore, one very active area of research in DC immunotherapy is the development of adjuvants to stimulate DC function and/or specific effector populations in vivo. DC rely in part on signaling through toll like receptors (TLR) for maturation, resulting in expression of MHC Class I and II molecules and the secretion of pro-inflammatory cytokines.36-38 Stimulation of TLR on DC can also facilitate the induction of Th1 immune responses.39,40 In a review of several vaccine studies for malignant melanoma, Engel-Noerregaard reported significantly higher response rates in patients who received adjuvants as a part of their vaccine regimen.41 Poly-ICLC (Hiltonol) is an inosine and cytidine-rich double stranded RNA that stimulates TLR3 and activates DC through a TLR-domain containing adapter inducing interferon-β (TRIF). It also stimulates CD8+ T cells and NK cells and increases interferon production. While it has primarily been used in combination with peptide vaccines, and not with autologous dendritic cells, it has been well-tolerated as a vaccine adjuvant in both children and adults42-45 and due to its stimulatory effects on DC as well as effector cells, this agent is currently being used by our group in our ongoing DC vaccine trials in children with malignant solid tumors.
Other recently published work has focused on the use of novel adjuvants from natural sources,46-49 known recall antigens,50 tumor derived immunogenic proteins,51 or proprietary costimulatory mixtures.52,53 In one study of naturally occurring plant polysaccharides used in ancient Chinese medicine, intraperitoneal injections of extracts from Antrodia cinnamomea were shown to enhance DC activation in vivo, with increased TH1 T cells as well as increased native CD11c+ DC's in tumor draining lymph nodes.47 In another study, vaccination with DC that were stimulated in vitro with a combination of extracts from Astragalus membranaceous and Colonopsis pilosulae resulted in increased tumor control in an in vivo murine breast cancer model.46 Three additional studies showed that in vitro pre-treatment of tumor antigen pulsed DC with uric acid,48 γ-glutamic acid,49 or pancreatic adenocarcinoma upregulated factor (PAUF), a protein naturally secreted by human pancreatic carcinomas,51 resulted in tumor shrinkage in murine tumor models. Co-treatment which removed or inactivated CTL abrogated tumor responses to these adjuvants, demonstrating that the mechanism of tumor death was dependent on pulsed DC activation of CTL. In three of these cases, adjuvant activity was definitively shown to activate the TLR pathway in DC.47,49,51 More study is needed, however, because the role of TLR in DC activation has been extensively and recently reviewed40,54 and one theme that has emerged is that TLR activation in murine models cannot be easily extrapolated to human models because murine and human DC constitutively express a different overlapping set of TLR.
DC, exposed to antigen can be matured in vitro or in vivo. Recent studies regarding in vitro maturation and DC activation include publications by a group in Brussels regarding clinical effectiveness in melanoma of DC that are electroporated with TriMix, a proprietary mixture of mRNA for CD40L, CD70, and a constitutively active TLR4, prior to antigen loading.52,53
A final area of study for vaccine adjuvants is co-injection of immunostimulatory compounds with the DC vaccine. Mitchell and group reported results of a randomized trial for adults with newly diagnosed glioblastoma who were treated with DC vaccine loaded with Cytomegalovirus phosphoprotein 65 (pp65) with or without pre-treatment with tetanus toxoid (Td).50 They found that pretreated patients had increased migration of DC to vaccine draining lymph nodes as well as increased interferon- γ production in post-vaccine ELISPOT assays. In addition, they showed significantly improved progression free survival, with 3 patients alive and disease free at 3 y post-vaccine. Utilizing a mouse model that compared wild type to CCL3 knock-out mice, they showed increased DC migration to be dependent on CCL3. In addition, they showed the CCL3 to be produced by CD4+ T-cells specifically activated by the Td recall response. This approach has not been tested in children but could be relevant to the pediatric population because tetanus vaccination is initiated at 2 months of age.
Because patients with cancer are often immunosuppressed from the tumor or their therapy these developments in adjuvant therapy to maximize DC function and effector cell activation are vital.
Concomitant immunomodulation
A consistent feature of DC immunotherapy studies is that in vitro activity of antigen-loaded DC in stimulating antigen-specific CTL responses does not clearly translate to an in vivo anti-tumor response. One possible explanation is that tumors are increasingly understood to generate an immunosuppressive milieu that induces T-cell anergy and an immunotolerant phenotype. Immunomodulators may have a role in changing this milieu to allow tumor infiltration by CTL and DC activation within the tumor itself. One attractive target for this approach is the PD-1/PD-ligand system. PD-1, when stimulated on the surface of T-cells induces antigen specific anergy or apoptosis.55,56 Many tumor types as well as mature DC have been shown to express PD-ligand (PD-L1) on the cell surface.55 DC found in tumor draining lymph nodes in an ovarian carcinoma model express high levels of PD-L1, and blockade of PD-L1 enhanced activation of CTL by DC and shifted the cytokines produced from a predominantly IL-10 producing TH2 response to an IL-12 TH1 response.57 A study by Zhang et al looked at the effect of treatment with antibody against PD-L1 at various points in the DC vaccine process. This group demonstrated that treatment with α-PD-L1 antibody during DC culture increased proliferation and IL-12 expression of DC and that T-cells stimulated in the presence of α-PD-L1 secreted increased levels of interferon-γ.58 In addition, they demonstrated that DC-vaccine against a PD-L1 expressing breast cancer model showed a statistically significant increase in tumor shrinkage when mice were co-treated with α-PD-L1.58 While this group did not find any evidence of auto-immunity in treated mice, systemic inhibition of the PD-1/PD-L1 system is a concern because of the potential to block important mechanisms for self-tolerance in the normal immune system. This concern has led some groups to investigate ways of achieving a more focused silencing of PD-1/PD-L1.59,60 In one study, DC from healthy volunteers were infected with a lentiviral vector encoding short hairpin RNA (shRNA) for PD-L1 which resulted in abrogation of PD-L1 production. They found no change in the standard DC phenotype except loss of expression of surface PD-L1, but cells treated in this way had increased ability to stimulate T-cell proliferation, secretion of IL-12 and interferon-γ, and in vitro tumor cell killing.60 A study by van der Waart, et al. investigated this further using an in vivo murine AML model.59 In this study DC derived from PBMC were treated with short interfering RNA (siRNA) against PD-L1 and or PD-L2 and were then shown to increase in vitro proliferation of antigen specific CD8+ T-cells by as much as 20-fold. Further, infusion of these Ag-specific CTL simultaneously with vaccination of PD-L1 silenced DC induced an increased and sustained antigen-specific CTL response.59 These modifications to PD-1/PD-L1 expression on DC did not result in any systemic toxicity.
Another mechanism to circumvent the immunosuppressive effects of the tumor microenvironment is IL-10 suppression. IL-10 is known to decrease MHC-I expression, inhibit NK cell activity, and decrease important DC costimulatory molecules. An α-IL-10 antibody administered 24 hours before DC vaccine was able to increase NK responses and was associated with a statistically and clinically significant decrease in tumor growth and increase in survival in a murine breast cancer model.61 This protocol also made use of a single low dose of cyclophosphamide to decrease regulatory T-cells, but this intervention alone did not result in a change in tumor growth in the absence of the α-IL-10 antibody.
Dasatinib is a multi-tyrosine kinase inhibitor with known efficacy in BCR-abl fusion driven haematopoietic malignancies but with only modest effects in other tumors. This agent inhibits cKIT and SRC kinases known to play a role in maintenance of MDSC and Tregs respectively. Low dose oral dasatinib in combination with anti-melanoma DC-vaccine significantly increased tumor infiltrating CTL and CD11c+ DC, decreased signaling through hypoxia mediated pathways, increased intratumoral expression of pro-inflammatory cytokines and chemokines, and resulted in a striking decrease in tumor growth.69 Taken as a group these studies show that concomitant modulation of immunosuppressive pathways may enhance DC-mediated anti-tumor immune response.
Tumors are known to induce immunosuppression through other pathways including modifications of L-arginine metabolism62 and induction of indoleamine 2,3 dioxygenase (IDO).63-65 While no recent work has combined modulation of these systems with dendritic cell vaccines in a preclinical or clinical model, one group demonstrated that IL-6 drives the arginase pathway to induce DC dependent CD4+ T cell dysfunction,66 implying that a drug that interferes with IL-6 such as tocilizumab (α-IL-6R) or situxilizumab (α-IL-6) may boost DCV efficacy. In addition, an in vitro model of monocyte derived DC induction of anti-leukemic T-cell activity demonstrated that PGE2 used in DC maturation increased production of IDO and that co-culture with an inhibitor of IDO, Levo-1-methyl-tryptophan (L-1-MT) significantly increased DC driven T-cell proliferation.67 L-1-MT has been used to inhibit IDO in a mouse glioma model,68 but has not been used in humans and is not commercially available as a drug.
Future directions
Many of the studies regarding DC vaccine biology in the last 5 y have been primarily directed at adult malignancies, but most of these strategies could be easily translated into pediatric trials (see Table 1). Because children are small, they are often transplanted with umbilical cord blood, and studies using cord blood derived DC's in transplant patients or in patients who may have their own cord blood banked could be undertaken. Regarding concomitant immunomodulation strategies, while α-PD-L1 medications have pediatric dose finding studies ongoing and are not yet ready to be routinely administered to children, dasatinib has been through Phase I testing in children70 and is currently used in pediatric patients for leukemias with associated BCR-abl translocations.71,72 There are indications that CTLA-4 blockade may be beneficial in high grade gliomas, and ipilumimab, the α-CTLA-4 medication has been used in pediatric Phase I trials.73 Tocilizumab has a defined safety profile in children with rheumatologic disease74,75 and could be moved into cancer clinical trials. All of these drugs could be readily tested in combination with DC vaccines if pre-clinical data show benefit. Because there seems to be a lag between administration of a DC vaccine product and tumor response, and because DCV are so well tolerated, it is reasonable to consider moving the timing of DC vaccine therapy to a relapse prevention strategy in which DC are harvested before chemotherapy and administered at the conclusion of cytotoxic therapy when the tumor is clinically in remission.
Table 1.
Leading Clinical and Preclinical candidates with potential to increase DCV efficacy. Definitions: DC gen - DC generation; DCV prod - DCV production; caTLR4 - constitutively active TLR4; shRNA - short hairpin RNA; siRNA - short interfering RNA; TKI - tyrosine kinase inhibitor.
| AuthorsRef | Preclinical/Clinical | DCV Stage | Target | Key Results | Applicable to Pediatrics |
|---|---|---|---|---|---|
| Nair, et al.17 | Preclinical | DC gen | New DC source | Feasible to create DC from cryopreserved autologous stem cell apheresis product | Yes |
| Benteyn, et al.53 | Preclinical | DCV prod | DC activation | In vivo DC maturation with proprietary co-stimulatory molecules – TriMix (CD40L, CD70, caTLR4) | Unknown |
| Olin, et al.35 | Clinical | DCV prod | Novel Antigen | DC loaded with allogeneic tumor cell line lysates were tolerated, best response SD in 1 patient | Likely |
| Van Nuffel, et al.52 | Clinical | DCV prod | DC activation | Durable T-cell and clinical response in chemorefractory melanoma patient treated with TriMix | Unknown |
| Mitchell, et al.50 | Clinical | Adjuvant | Effector T-cells | ↑ DC migration to draining lymph nodes, ↑ interferon- γ production post-vaccine, improved PFS | Yes |
| Rossowska, et al.61 | Preclinical | Immuno-modulation | IL-10 | Inhibition of IL-10 with DCV ↑ tumor growth inhibition in a murine model | Unknown |
| Ge, et al.58 | Preclinical | Immuno-modulation | PD-1/PD-L1 | PD-L1 blockade during DC generation/ injection ↓ tumor growth and ↑ PFS in murine breast cancer model | Likely |
| Wang, et al.60 | Preclinical | Immuno-modulation | PD-1/PD-L1 | Transduction of shRNA against PD-L1 into DC ↓ PD-L1 expression, ↓ IL-10 production, ↑ IL-12 | Unknown |
| Van der Waart, et al.59 | Preclinical | Immuno-modulation | PD-1/PD-L1 | siRNA silencing PD-L1 and L2 in DC ↑ in vitro T-cell priming and in vivo CD8+ T-cell responses | Unknown |
| Lowe, et al.69 | Preclinical | Immuno-modulation | Effector T-cells | TKI dasatinib combined with DCV ↓ tumor growth and ↑ OS compared to either modality alone | Likely |
In summary, recent advances have been made with novel adjuvants and concomitant immunomodulation increasing immunogenicity and effector cell stimulation by dendritic cell vaccines, and advances in antigen and DC source selection will make this therapy available to more patients. Currently, there are more than 30 open clinical trials using DC cell based immunotherapy in pediatric and adult cancers. Lessons learned from these trials and ongoing clinical and preclinical testing should inform the future evolution of immunotherapy trials.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64:83-103; PMID:24488779 [DOI] [PubMed] [Google Scholar]
- [2].Mitchell DA, Sayour EJ, Reap E, Schmittling R, DeLeon G, Norberg P, Desjardins A, Friedman AH, Friedman HS, Archer G, et al.. Severe adverse immunologic reaction in a patient with glioblastoma receiving autologous dendritic cell vaccines combined with GM-CSF and dose-intensified temozolomide. Cancer Immunol research 2015; 3:320-5; PMID:25387895; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 2015; 64:1251-60; PMID:26105625; http://dx.doi.org/ 10.1007/s00262-015-1731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- [5].Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediat Blood Cancer 2010; 54:519-25; PMID:19852061 [DOI] [PubMed] [Google Scholar]
- [6].Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, Downie P, Hassall TE, Tang ML, Ashley DM. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-oncology 2004; 6:236-46; PMID:15279716; http://dx.doi.org/ 10.1215/S1152851703000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lasky JL 3rd, Panosyan EH, Plant A, Davidson T, Yong WH, Prins RM, Liau LM, Moore TB. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res 2013; 33:2047-56; PMID:23645755 [PMC free article] [PubMed] [Google Scholar]
- [8].Dohnal AM, Witt V, Hugel H, Holter W, Gadner H, Felzmann T. Phase I study of tumor Ag-loaded IL-12 secreting semi-mature DC for the treatment of pediatric cancer. Cytotherapy 2007; 9:755-70; PMID:17917887; http://dx.doi.org/ 10.1080/14653240701589221 [DOI] [PubMed] [Google Scholar]
- [9].Geiger J, Hutchinson R, Hohenkirk L, McKenna E, Chang A, Mule J. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet (London, England) 2000; 356:1163-5; PMID:11030299; http://dx.doi.org/ 10.1016/S0140-6736(00)02762-8 [DOI] [PubMed] [Google Scholar]
- [10].Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001; 61:8513-9; PMID:11731436 [PubMed] [Google Scholar]
- [11].Himoudi N, Wallace R, Parsley KL, Gilmour K, Barrie AU, Howe K, Dong R, Sebire NJ, Michalski A, Thrasher AJ, et al.. Lack of T-cell responses following autologous tumour lysate pulsed dendritic cell vaccination, in patients with relapsed osteosarcoma. Clin Transl Oncol 2012; 14:271-9; PMID:22484634; http://dx.doi.org/ 10.1007/s12094-012-0795-1 [DOI] [PubMed] [Google Scholar]
- [12].Matsuzaki A, Suminoe A, Hattori H, Hoshina T, Hara T. Immunotherapy with autologous dendritic cells and tumor-specific synthetic peptides for synovial sarcoma. J Pediat Hematol Oncol 2002; 24:220-3; PMID:11990310; http://dx.doi.org/ 10.1097/00043426-200203000-00012 [DOI] [PubMed] [Google Scholar]
- [13].Shilyansky J, Jacobs P, Doffek K, Sugg SL. Induction of cytolytic T lymphocytes against pediatric solid tumors in vitro using autologous dendritic cells pulsed with necrotic primary tumor. J Pediat Surgery 2007; 42:54-61; discussion; PMID:17208541; http://dx.doi.org/ 10.1016/j.jpedsurg.2006.09.008 [DOI] [PubMed] [Google Scholar]
- [14].Suminoe A, Matsuzaki A, Hattori H, Koga Y, Hara T. Immunotherapy with autologous dendritic cells and tumor antigens for children with refractory malignant solid tumors. Pediat Transplant 2009; 13:746-53; PMID:19067917; http://dx.doi.org/ 10.1111/j.1399-3046.2008.01066.x [DOI] [PubMed] [Google Scholar]
- [15].Hunn MK, Bauer E, Wood CE, Gasser O, Dzhelali M, Ancelet LR, Mester B, Sharples KJ, Findlay MP, Hamilton DA, et al.. Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. J Neuro-Oncol 2015; 121:319-29; PMID:25366363; http://dx.doi.org/ 10.1007/s11060-014-1635-7 [DOI] [PubMed] [Google Scholar]
- [16].Saito S, Yanagisawa R, Yoshikawa K, Higuchi Y, Koya T, Yoshizawa K, Tanaka M, Sakashita K, Kobayashi T, Kurata T, et al.. Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: a case report and review of the literature. Cytotherapy 2015; 17:330-5; PMID:25484308; http://dx.doi.org/ 10.1016/j.jcyt.2014.10.003 [DOI] [PubMed] [Google Scholar]
- [17].Nair SK, Driscoll T, Boczkowski D, Schmittling R, Reynolds R, Johnson LA, Grant G, Fuchs H, Bigner DD, Sampson JH, et al.. Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J Neuro-oncol 2015; 125:65-74; PMID:26311248; http://dx.doi.org/ 10.1007/s11060-015-1890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jacobs JF, Hoogerbrugge PM, de Rakt MW, Aarntzen EH, Figdor CG, Adema GJ, de Vries IJ. Phenotypic and functional characterization of mature dendritic cells from pediatric cancer patients. Pediat Blood Cancer 2007; 49:924-7; PMID:17486645; http://dx.doi.org/ 10.1002/pbc.21246 [DOI] [PubMed] [Google Scholar]
- [19].Vakkila J, Vettenranta K, Sariola H, Saarinen-Pihkala UM. Poor yield of dendritic cell precursors from untreated pediatric cancer. J Hematother Stem Cell Res 2001; 10:787-93; PMID:11798505; http://dx.doi.org/ 10.1089/152581601317210881 [DOI] [PubMed] [Google Scholar]
- [20].Haruta M, Tomita Y, Yuno A, Matsumura K, Ikeda T, Takamatsu K, Haga E, Koba C, Nishimura Y, Senju S. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther 2013; 20:504-13; PMID:22875043; http://dx.doi.org/ 10.1038/gt.2012.59 [DOI] [PubMed] [Google Scholar]
- [21].Lonial S, Hicks M, Rosenthal H, Langston A, Redei I, Torre C, Duenzl M, Feinstein B, Cherry J, Waller EK. A randomized trial comparing the combination of granulocyte-macrophage colony-stimulating factor plus granulocyte colony-stimulating factor versus granulocyte colony-stimulating factor for mobilization of dendritic cell subsets in hematopoietic progenitor cell products. Biol Blood Marrow Transplant 2004; 10:848-57; PMID:15570253; http://dx.doi.org/ 10.1016/j.bbmt.2004.07.008 [DOI] [PubMed] [Google Scholar]
- [22].Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 2000; 95:2484-90; PMID:10753825 [PubMed] [Google Scholar]
- [23].Zeng J, Wu C, Wang S. Antigenically Modified Human Pluripotent Stem Cells Generate Antigen-Presenting Dendritic Cells. Sci Rep 2015; 5:15262; PMID:26471005; http://dx.doi.org/ 10.1038/srep15262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iwamoto H, Ojima T, Nakamori M, Nakamura M, Hayata K, Katsuda M, Iida T, Miyazawa M, Iwahashi M, Yamaue H. [Cancer vaccine therapy using genetically modified induced pluripotent stem cell-derived dendritic cells expressing the TAA gene]. Gan To kagaku Ryoho Cancer Chemother 2013; 40:1575-7; PMID:24393853 [PubMed] [Google Scholar]
- [25].Fukushima S, Hirata S, Motomura Y, Fukuma D, Matsunaga Y, Ikuta Y, Ikeda T, Kageshita T, Ihn H, Nishimura Y, et al.. Multiple antigen-targeted immunotherapy with alpha-galactosylceramide-loaded and genetically engineered dendritic cells derived from embryonic stem cells. J Immunother 2009; 32:219-31; PMID:19242378; http://dx.doi.org/ 10.1097/CJI.0b013e318194b63b [DOI] [PubMed] [Google Scholar]
- [26].Zeng J, Wang S. Human dendritic cells derived from embryonic stem cells stably modified with CD1d efficiently stimulate antitumor invariant natural killer T cell response. Stem Cell Transl Med 2014; 3:69-80; PMID:24292792; http://dx.doi.org/ 10.5966/sctm.2013-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Haar C, Plantinga M, Blokland NJ, van Til NP, Flinsenberg TW, Van Tendeloo VF, Smits EL, Boon L, Spel L, Boes M, et al.. Generation of a cord blood-derived Wilms Tumor 1 dendritic cell vaccine for AML patients treated with allogeneic cord blood transplantation. Oncoimmunology 2015; 4:e1023973; PMID:26451309; http://dx.doi.org/ 10.1080/2162402X.2015.1023973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sakai K, Shimodaira S, Maejima S, Udagawa N, Sano K, Higuchi Y, Koya T, Ochiai T, Koide M, Uehara S, et al.. Dendritic cell-based immunotherapy targeting Wilms' tumor 1 in patients with recurrent malignant glioma. J Neurosurg 2015; 123:989-97; PMID:26252465; http://dx.doi.org/ 10.3171/2015.1.JNS141554 [DOI] [PubMed] [Google Scholar]
- [29].Collignon A, Perles-Barbacaru AT, Robert S, Silvy F, Martinez E, Crenon I, Germain S, Garcia S, Viola A, Lombardo D, et al.. A pancreatic tumor-specific biomarker characterized in humans and mice as an immunogenic onco-glycoprotein is efficient in dendritic cell vaccination. Oncotarget 2015; 6:23462-79; PMID:26405163; http://dx.doi.org/ 10.18632/oncotarget.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Okamoto M, Kobayashi M, Yonemitsu Y, Koido S, Homma S. Dendritic cell-based vaccine for pancreatic cancer in Japan. World J Gastrointest Pharmacol Ther 2016; 7:133-8; PMID:26855819; http://dx.doi.org/ 10.4292/wjgpt.v7.i1.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nguyen ST, Nguyen HL, Pham VQ, Nguyen GT, Tran CD, Phan NK, Pham PV. Targeting specificity of dendritic cells on breast cancer stem cells: in vitro and in vivo evaluations. Onco Targets Ther 2015; 8:323-34; PMID:25674007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang X, Bayer ME, Chen X, Fredrickson C, Cornforth AN, Liang G, Cannon J, He J, Fu Q, Liu J, et al.. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J Surg Oncol 2015; 111:862-7; PMID:25873455; http://dx.doi.org/ 10.1002/jso.23897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, Saeboe-Larssen S, Sandberg C, Brinchmann JE, Helseth E, et al.. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 2013; 62:1499-509; PMID:23817721; http://dx.doi.org/ 10.1007/s00262-013-1453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wilgenhof S, Corthals J, Van Nuffel AM, Benteyn D, Heirman C, Bonehill A, Thielemans K, Neyns B. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother 2015; 64:381-8; PMID:25548092; http://dx.doi.org/ 10.1007/s00262-014-1642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, Gustafson MP, Dietz AB, Clark HB, Chen W, et al.. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer 2014; 2:4; PMID:24829761; http://dx.doi.org/ 10.1186/2051-1426-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kabelitz D, Medzhitov R. Innate immunity–cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol 2007; 19:1-3; PMID:17157490; http://dx.doi.org/ 10.1016/j.coi.2006.11.018 [DOI] [PubMed] [Google Scholar]
- [37].Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol 2004; 16:27-34; PMID:14751761; http://dx.doi.org/ 10.1016/j.smim.2003.10.004 [DOI] [PubMed] [Google Scholar]
- [38].Shen HTB, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol 2008; 181:1849-58; PMID:18641322; http://dx.doi.org/ 10.4049/jimmunol.181.3.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Napolitani G RA, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunol 2005; 6:769-76; PMID:15995707; http://dx.doi.org/ 10.1038/ni1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baxevanis CN, Voutsas IF, Tsitsilonis OE. Toll-like receptor agonists: current status and future perspective on their utility as adjuvants in improving anticancer vaccination strategies. Immunotherapy 2013; 5:497-511; PMID:23638745; http://dx.doi.org/ 10.2217/imt.13.24 [DOI] [PubMed] [Google Scholar]
- [41].Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother 2009; 58:1-14; PMID:18719915; http://dx.doi.org/ 10.1007/s00262-008-0568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, et al.. Phase I Trial of Overlapping Long Peptides from a Tumor Self-Antigen and Poly-ICLC Shows Rapid Induction of Integrated Immune Response in Ovarian Cancer Patients. Clin Cancer Res 2012; 18:6497-508; PMID:23032745; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2189 [DOI] [PubMed] [Google Scholar]
- [43].Matthews KCN, Klasse PJ, Moutaftsi M, Carter D, Salazar AM, Reed SG, Sanders RW, Moore JP. Clinical adjuvant combinations stimulate potent B-cell responses in vitro by activating dermal dendritic cells. PLoS One 2013; 8:e63785; PMID:23700434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pollack IF JR, Butterfield LH, Okada H. Peptide vaccine therapy for childhood gliomas. Neurosurgery 2013; 60:113-9; PMID:23839362; http://dx.doi.org/ 10.1227/01.neu.0000430769.33467.68 [DOI] [PubMed] [Google Scholar]
- [45].Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al.. Induction of CD8+ T-Cell Responses Against Novel Glioma–Associated Antigen Peptides and Clinical Activity by Vaccinations With a-Type 1 Polarized Dendritic Cells and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Patients With Recurrent Malignant Glioma. J Clin Oncol 2011; 29:330-6; PMID:21149657; http://dx.doi.org/ 10.1200/JCO.2010.30.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang WT, Lai TH, Chyan YJ, Yin SY, Chen YH, Wei WC, Yang NS. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PloS One 2015; 10:e0122374; PMID:25825910; http://dx.doi.org/ 10.1371/journal.pone.0122374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lin CC, Pan IH, Li YR, Pan YG, Lin MK, Lu YH, Wu HC, Chu CL. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PloS One 2015; 10:e0116191; PMID:25723174; http://dx.doi.org/ 10.1371/journal.pone.0116191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Y, Ma X, Su C, Peng B, Du J, Jia H, Luo M, Fang C, Wei Y. Uric acid enhances the antitumor immunity of dendritic cell-based vaccine. Sci Rep 2015; 5:16427; PMID:26553557; http://dx.doi.org/ 10.1038/srep16427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Seth A, Heo MB, Sung MH, Lim YT. Infection-mimicking poly(gamma-glutamic acid) as adjuvant material for effective anti-tumor immune response. Int J Biol Macromol 2015; 75:495-504; PMID:25709015; http://dx.doi.org/ 10.1016/j.ijbiomac.2015.02.013 [DOI] [PubMed] [Google Scholar]
- [50].Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A, et al.. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015; 519:366-9; PMID:25762141; http://dx.doi.org/ 10.1038/nature14320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kang TH, Kim YS, Kim S, Yang B, Lee JJ, Lee HJ, Lee J, Jung ID, Han HD, Lee SH, et al.. Pancreatic adenocarcinoma upregulated factor serves as adjuvant by activating dendritic cells through stimulation of TLR4. Oncotarget 2015; 6:27751-62; PMID:26336989; http://dx.doi.org/ 10.18632/oncotarget.4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Van Nuffel AM, Benteyn D, Wilgenhof S, Corthals J, Heirman C, Neyns B, Thielemans K, Bonehill A. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother 2012; 61:1033-43; PMID:22159452; http://dx.doi.org/ 10.1007/s00262-011-1176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Benteyn D, Van Nuffel AM, Wilgenhof S, Bonehill A. Single-step antigen loading and maturation of dendritic cells through mRNA electroporation of a tumor-associated antigen and a TriMix of costimulatory molecules. Methods Mol Biol 2014; 1139:3-15; PMID:24619665; http://dx.doi.org/ 10.1007/978-1-4939-0345-0_1 [DOI] [PubMed] [Google Scholar]
- [54].Seya T, Shime H, Takeda Y, Tatematsu M, Takashima K, Matsumoto M. Adjuvant for vaccine immunotherapy of cancer - focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity. Cancer Sci 2015; 106:1659-68; PMID:26395101; http://dx.doi.org/ 10.1111/cas.12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/ 10.1038/nm0902-1039c [DOI] [PubMed] [Google Scholar]
- [56].Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/ 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- [57].Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al.. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature Med 2003; 9:562-7; PMID:12704383; http://dx.doi.org/ 10.1038/nm863 [DOI] [PubMed] [Google Scholar]
- [58].Ge Y, Xi H, Ju S, Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett 2013; 336:253-9; PMID:23523609; http://dx.doi.org/ 10.1016/j.canlet.2013.03.010 [DOI] [PubMed] [Google Scholar]
- [59].van der Waart AB, Fredrix H, van der Voort R, Schaap N, Hobo W, Dolstra H. siRNA silencing of PD-1 ligands on dendritic cell vaccines boosts the expansion of minor histocompatibility antigen-specific CD8(+) T cells in NOD/SCID/IL2Rg(null) mice. Cancer Immunol Immunother 2015; 64:645-54; PMID:25724840; http://dx.doi.org/ 10.1007/s00262-015-1668-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang S, Wang Y, Liu J, Shao S, Li X, Gao J, Niu H, Wang X. Silencing B7-H1 enhances the anti-tumor effect of bladder cancer antigen-loaded dendritic cell vaccine in vitro. Onco Targets Ther 2014; 7:1389-96; PMID:25120371; http://dx.doi.org/ 10.2147/OTT.S65367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rossowska J, Anger N, Kicielinska J, Pajtasz-Piasecka E, Bielawska-Pohl A, Wojas-Turek J, Dus D. Temporary elimination of IL-10 enhanced the effectiveness of cyclophosphamide and BMDC-based therapy by decrease of the suppressor activity of MDSCs and activation of antitumour immune response. Immunobiology 2015; 220:389-98; PMID:25454807; http://dx.doi.org/ 10.1016/j.imbio.2014.10.009 [DOI] [PubMed] [Google Scholar]
- [62].Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 2005; 5:641-54; PMID:16056256; http://dx.doi.org/ 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- [63].Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 2009; 114:555-63; PMID:19465693; http://dx.doi.org/ 10.1182/blood-2008-11-191197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnson TS, Munn DH. Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance. Immunol Invest 2012; 41:765-97; PMID:23017145; http://dx.doi.org/ 10.3109/08820139.2012.689405 [DOI] [PubMed] [Google Scholar]
- [65].Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 2007; 117:2570-82; PMID:17710230; http://dx.doi.org/ 10.1172/JCI31911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Narita Y, Kitamura H, Wakita D, Sumida K, Masuko K, Terada S, Nakano K, Nishimura T. The key role of IL-6-arginase cascade for inducing dendritic cell-dependent CD4(+) T cell dysfunction in tumor-bearing mice. J Immunol 2013; 190:812-20; PMID:23248265; http://dx.doi.org/ 10.4049/jimmunol.1103797 [DOI] [PubMed] [Google Scholar]
- [67].Trabanelli S, Lecciso M, Salvestrini V, Cavo M, Ocadlikova D, Lemoli RM, Curti A. PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J Immunol Res 2015; 2015:253191; PMID:25815345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L, et al.. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 2014; 20:5290-301; PMID:24691018; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lowe DB, Bose A, Taylor JL, Tawbi H, Lin Y, Kirkwood JM, Storkus WJ. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology 2014; 3:e27589; PMID:24734217; http://dx.doi.org/ 10.4161/onci.27589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zwaan CM, Rizzari C, Mechinaud F, Lancaster DL, Lehrnbecher T, van der Velden VH, Beverloo BB, den Boer ML, Pieters R, Reinhardt D, et al.. Dasatinib in children and adolescents with relapsed or refractory leukemia: results of the CA180-018 phase I dose-escalation study of the Innovative Therapies for Children with Cancer Consortium. J Clin Oncol 2013; 31:2460-8; PMID:23715577; http://dx.doi.org/ 10.1200/JCO.2012.46.8280 [DOI] [PubMed] [Google Scholar]
- [71].Kobayashi K, Miyagawa N, Mitsui K, Matsuoka M, Kojima Y, Takahashi H, Ootsubo K, Nagai J, Ueno H, Ishibashi T, et al.. TKI dasatinib monotherapy for a patient with Ph-like ALL bearing ATF7IP/PDGFRB translocation. Pediat Blood Cancer 2015; 62:1058-60; PMID:25400122; http://dx.doi.org/ 10.1002/pbc.25327 [DOI] [PubMed] [Google Scholar]
- [72].Wu KH, Wu HP, Weng T, Peng CT, Chao YH. Dasatinib for a child with Philadelphia chromosome-positive acute lymphoblastic leukemia and persistently elevated minimal residual disease during imatinib therapy. Curr Oncol 2015; 22:303-6; PMID:26300669; http://dx.doi.org/ 10.3747/co.22.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, Delbrook C, Lodish M, Bishop R, Wolchok JD, et al.. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin Cancer Res 2016; 22:1364-70; PMID:26534966; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tarp S, Amarilyo G, Foeldvari I, Christensen R, Woo JM, Cohen N, Pope TD, Furst DE. Efficacy and safety of biological agents for systemic juvenile idiopathic arthritis: a systematic review and meta-analysis of randomized trials. Rheumatology (Oxford, England) 2016; 55:669-79; PMID:26628580; http://dx.doi.org/ 10.1093/rheumatology/kev382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yokota S, Itoh Y, Morio T, Origasa H, Sumitomo N, Tomobe M, Tanaka K, Minota S. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann Rheum Dis 2015; PMID:26644233 [DOI] [PMC free article] [PubMed] [Google Scholar]


