ABSTRACT
Rotavirus (RV) is worldwide considered as the most important viral agent of acute gastroenteritis in children less than 5 y. Since 2006, the availability of anti-RV vaccines has deeply modified the incidence and economic burden of RV infection. In Europe, some countries have introduced an anti-RV vaccination program in the last 10 y. Although community acquired RV (CARV) disease is the most studied condition of RV infection, recently some authors have highlighted the importance of nosocomial RV (nRV) disease as an emerging public health issue. The aim of this review is to summarize the epidemiology of both CARV and nRV, in order to discuss the difficulty of a clear evaluation of the burden of the disease in absence of comparable data. In particular, we focused our attention to European studies regarding nRV in terms of divergences related to definition, report of incidence rate and methodological issues.
KEYWORDS: hospital length of stay, nosocomial RV, rotavirus
Introduction
Rotavirus (RV) is worldwide recognized as the leading cause of acute gastro-enteritis (AGE) in children <5 years of age (<5 yrs). In a recent sentinel surveillance report from 6 regions of World Health Organization (WHO) with different economic levels, about 40% of hospitalizations for diarrhea among children <5 yrs are attributed to community-acquired RV-infection (CARV). According to the last WHO report, about 450,000 cases of deaths in the world are annually related to CARV, with more than 90% of fatal cases among children. Specifically, more than 300 death per 100,000 cases are estimated in low-income countries of Africa and Asia, and around 1 death per 100,000 in European countries.1,2
The virus presents an ubiquitous distribution, and a transmission occurring via fecal-oral route with direct or indirect contact through infected person or contaminated fomites, respectively. Child susceptibility to RV infection is the same in both developed and developing countries, with about 95% of children <5 yrs worldwide having met the virus at least once in their life. The age of first presentation in industrialized countries is around 6–24 months, which is relatively late when compared to developing countries, where the infants are most affected.1 RV infection is characterized by a single winter peak in high-income countries with temperate climates and by more than one seasonal peak in low-income countries of Asia and Africa.2 RV presents a high transmissibility, due to the large amount of viral particles eliminated in acute phases and a low infective dose (<100 viral particles). Moreover, it presents a long environmental persistence and a relatively high resistance to disinfectants.2,3 Generally, the first infection is the most severe and presents a more unfavorable outcome in developing countries.4
In the last 2 decades, 3 conditions have deeply increased the differences between developed and developing countries. Specifically, the availability of rehydrating therapy for severe-moderate AGE, the improvement of health conditions and the administration of a RV vaccine have modified RV epidemiology, hospitalization rate and mortality.4 Since 2006, a monovalent and a pentavalent live, attenuated oral vaccines are internationally administered.2
Although CARV represents the main manifestation of RV infection, the frequent hospital admission of children <5 yrs increases the risk of acquiring RV in healthcare departments, especially in emergency department (ED) and pediatric wards.3,5 After its introduction from the community, RV was isolated in 25–55% stool samples of hospitalized children.3,6-8 Moreover, nosocomial RV (nRV) infection seems to be responsible for around 25% of all RV-related hospitalizations, especially in immunocompromised children.2,9,10 Nosocomial RV is an important emerging issue; however, the lack of comparable data among studies limits the availability of consistent epidemiological figures for socio-economic evaluation.7
Aim of this review is to summarize the epidemiology of CARV in pre- and post-vaccine era, and divergences about incidence, hospitalization rate and length of nRV in European countries.
Results
CARV: Hospitalization rate and ED visits
In Europe, the hospitalization rates for CARV in pre-vaccine era have been counted by the Pediatric ROTavirus European Committee (PROTECT) study.11 The authors of this systematic review highlight a median annual incidence rate in children <5 yrs around 3/1,000 (range 0.3–11.9/1,000), with a higher prevalence (60–80%) for children <2 years of age; admission for CARV determines a median hospital-stay length of 4.8 days, ranging from 2 to 9.5 d. These data have confirmed through many prospective and retrospective studies.12-15 The most robust results have come from the REVEAL study,16 a prospective, multicenter, observational study of pediatric AGE in 7 European countries. According to this study, CARV represents the primary diagnosis of 50% of hospital admissions and ED visits for RV related gastroenteritis, affecting mainly children 6–23 months of age. Furthermore, a difference in the hospitalization rates has been observed, that was related to the different epidemiological features of CARV in both eastern and western countries, and in a different attitude for hospital access of children.11,17,18 In the post-vaccine era, a reduced burden of CARV was observed in terms of hospitalization rate and ED visits. Giaquinto et al.,19 in 2011, observed about 94.5% decrease in hospitalizations and ED visits. Zlamy et al.,20 in 2013 in Austria, reported a reduction in hospitalization rate of 74%, and the mean hospital stay for CARV was significantly lower compared to the pre-vaccine era (p < 0.001), and the overall direct costs reduction was 72.7%.
In those European countries that have adopted RV vaccination, the reduction was observed not only in RV vaccinated age groups. The results of these studies pointed out the possibility of achieving herd immunity, with an overall reduction of incidence and hospitalization rates around 41% and 24% respectively, particularly related to mild cases.11
Nosocomial RV
An AGE related to RV could be defined as nosocomial every time the symptoms appear from 48 hours after the admission in hospital to 72 hours after hospital discharge.21 In particular, some studies have pointed out a 15–30% of cases of nRV after hospital discharge. This amount makes the difference for an additional 0.8–1.0/100 cases in the seasonal incidence in infants and toddlers, although their relevance in increasing direct health care costs is minimal because they are not associated with an increased hospitalization length or a readmission.22
A real nRV estimate is hard to obtain, as the largest studies on nosocomial diseases have focused their attention on adult population. Nonetheless, viruses are the most putative agents for nosocomial disease in pediatric ward (from 23 to 34% of infections). Nosocomial diarrhea is caused by virus in 91–94% of all cases (65–90% of pediatric hospital-acquired infection), with RV responsible for 31–87% of cases.23
We analyzed 23 papers (both original articles and reviews) about nRV,11,18,20-40 and we found extreme divergences in incidence and extra length of hospitalization stay estimations. Table 1 shows the 23 European studies selected.
Table 1.
List of selected European studies on nRV.
| Author | Year of Publication | Study design | Country | Ref |
|---|---|---|---|---|
| Gleizes et al. | 2006 | Review | ES-FR-GB-IT-PL | 21 |
| PROTECT | 2006 | Review | EU | 11 |
| Johansen et al. | 2008 | RS | SW | 22 |
| Stefkovicova et al. | 2008 | RS | SK | 23 |
| Forster et al. | 2009 | PS | ES-DE-FR-GB-IT | 24 |
| Gil-Prieto et al. | 2009 | RS | ES | 25 |
| Muhsen et al. | 2009 | PS | IL | 26 |
| Waisbourd-Zinman et al. | 2009 | PS | IL | 27 |
| Wildi-Runge et al. | 2009 | RS | CH | 28 |
| Cunliffe NA et al. | 2010 | PS | GB | 29 |
| Festini et al. | 2010 | PS | IT | 30 |
| Gutiérrez-Gimeno et al. | 2010 | PS | ES | 31 |
| García-Basteiro et al. | 2011 | RS | ES | 32 |
| Panatto et al. | 2011 | RS | IT | 33 |
| Bruijning-Verhagen et al. | 2012 | Review | West EU | 34 |
| Ogilvie et al. | 2012 | Review | West EU | 18 |
| Nitsch-Osuch et al. | 2013 | RS | PL | 35 |
| Zlamy et al. | 2013 | RS | AU | 20 |
| Konstantopoulos et al. | 2013 | PS | GR | 36 |
| Anca et al. | 2014 | PS | RO | 37 |
| Rinder et al. | 2014 | PS | SW | 38 |
| Stefcovicova et al. | 2015 | PS | SK | 39 |
| Redondo-Gonzalez | 2015 | RS | ES | 40 |
Legend:
PS = Prospective Study
RS = Retrospective Study
AU = Austria
CH = Switzerland
DE = Germany
ES = Spain
EU = Europe
FR = France
GB = Great Britain
GR = Greece
IL = Israel
IT = Italy
PL = Poland
RO=Romania
SK = Slovakia
SW = Sweden
Incidence of nRV was calculated in the 23 selected papers, with a sample size from 134 to 355,339, and an age range from 0 to 18 years, with particular focus on the 0–5 y age group. A median of 29 (range 3–277) nRV/1,000 hospitalizations, 198 (30–1030) nRV/100,000 children <5 yrs, 0.32 (0.03–0.96) nRV/hospitalized CARV, 4.8 (0.46–13) nRV/1,000 d of hospitalization was calculated. Table 2 shows 10/23 articles with data from different European countries. Median values from these 10 papers are: 61.5 (range 3–277) for nRV/1,000 hospitalizations; 291.5 (range 160–1030) for nRV/100,000 children <5 yrs; 0.32 (range 0.03–0.96) for nRV/hospitalized CARV; 5.25 (range 0.52–13) for nRV/1,000 hospitalization-days. In Austria, a comparison of nRV incidence pre- and post-antiRV vaccine introduction showed a significant reduction of the rate nRV/CARV from 11.5% to 3.1%.20
Table 2.
Incidence of nosocomial RV infection.
| nRV/1,000 Hospitalizations | nRV/100,000 children < 5 yrs | nRV/CARV hospitalized | nRV/1,000 d of hospitalization | Age range | Sample size | Ref | |
|---|---|---|---|---|---|---|---|
| Europe, 2006 (FR, IT, PL, ES, GB) | 53 | — | 0.61 | 8.1 | 0–5 y | 5,470 | 21 |
| 277 | — | — | — | 0–18 m | 220 | ||
| — | 198 | 0.64 | — | 0–5 y | 757 | ||
| 70 | 160 | 0.96 | 13 | 0–2 y | 666 | ||
| 3 | 333 | 0.76 | — | 0–15 y | 295 | ||
| Israel, 2009 | 10 | — | — | 2.4 | 0–18 y | 35,833 | 27 |
| United Kindom, 2010 | — | — | 0.63* | — | 0–16 y | 576 | 29 |
| Spain, 2011 | — | 250 | 0.38 | — | 0–5 y | 355,339 | 32 |
| Austria, 2013 | — | — | 0.12* | — | 0–18 y | 1,026 | 20 |
| — | — | 0.09* | — | 372 | |||
| — | — | 0.03* | — | 134 | |||
| Poland, 2013 | 9.1 | — | 0.32 | — | 0–18 y | 63,173 | 35 |
| Romania, 2014 | — | — | 0.20 | 0.52 | 0–5 y | 1,290 | 37 |
| Sweden, 2014 | 81 | — | — | — | 0–5 y | 604 | 38 |
| Slovakia, 2015 | 74 | 1030 | 0.30 | — | 0–5 y | 10,356 | 39 |
| Spain, 2015 | 6 | 500 | 0.17 | — | 0–5 y | 9,602 | 40 |
Extrapolated from displayed data
Prolongation of hospital stay when RV contagion occurs in hospitalized children is very hard to define. Extra-day of hospitalization was reported only in 12/23 papers selected for this review, shown in Table 3. A median 4.7 d (range 1.7–10) more than normal hospital stay has been calculated for nRV. However, the estimates are deeply different because of the extremely high divergences related to: unclear methodological tools to define extra-days hospitalization for nRV, and the lack of precise and specified referral disease to compare the nRV hospitalization. Once again, Zlamy20 compared the estimation of hospitalization in pre- and post-vaccine era with an increased prolongation of hospital stay for post-vaccine era, although it did not reach the statistical significance mainly due to the small sample size for nRV cases (n = 4) in the post-vaccine period (Table 3).
Table 3.
Extra-days length of hospitalization.
| nRV hospitalization (mean days ± SD) | Extra Length of stay (days) | nRV (Sample) | Sample size (tot) | Age range | Ref | |
|---|---|---|---|---|---|---|
| Gleizes et al. | — | 1.7—5.9a | — | 70—5,470a | <18 y | 21 |
| Stefkovicova M et al. | 7.32 | 3.4b | 62 | 1,635 | <5 y | 23 |
| Forster et al. | — | 3.0 | 117 | 3,734 | <5 y | 24 |
| Festini et al. | 8.1 ± 5.4 | 1.7 | 28 | 608 | <30 m | 30 |
| Gutiérrez-Gimeno et al. | 7.5 ± 3.7 | 1.7 | 69 | 1,576 | 1—23 m | 31 |
| García-Basteiro et al. | 9.7 ± 13.6 | 6b | 892 | 355,339 | <5 y | 32 |
| Panatto et al. | — | 4.4 (SD: ±2.7) | 22 | 20,690 | <5 y | 33 |
| Nitsch-Osuch et al. | 11.6 ± 0.4 | 7b | 575 | 63,173 | <18 y | 35 |
| Zlamy et al. | 8.0 | 4.2b | 106 | 652,557c | <18 y | 20 |
| 13.3 | 10b | 4 | 305,393d | |||
| Konstantopoulos et al. | — | 5 (range: 4—7) | 8 | 22,963 | <5 y | 36 |
| Anca et al. | — | 5 (range: 1—10) | 137 | 53,445 | <5 y | 37 |
| Redondo-Gonzalez et al. | 9 | 5b | 49 | 9,602 | <18 y | 40 |
range from a review of studies in 6 European countries
Extrapolated
pre-vaccine era (2002—2005)
post-vaccine era (2007—2008)
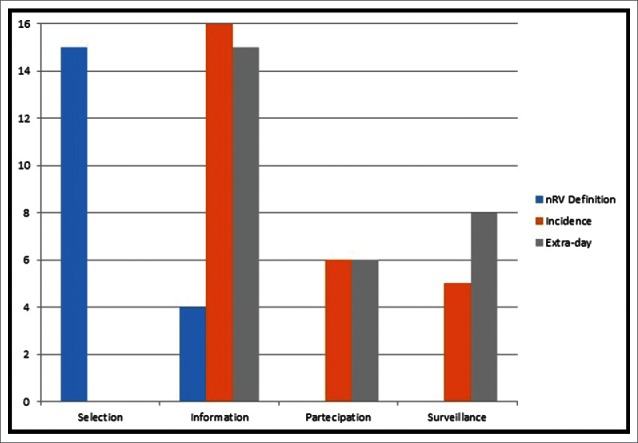
Besides a wide variety of incidence rates, we found a high variability of data in particular for nRV definition and epidemiological figures; this variability was formally named as “divergence.” The selected papers presented a high level of divergences related to 3 study features (i.e. nRV definition, incidence and extra-days rate), as shown in Fig. 1. The most important was the information divergence reported for all the selected papers; in particular, 16/23 showed divergence for incidence rate, 15/23 for extra-day evaluation, and 4/23 for nRV definition. Interestingly, information divergences have been observed also for nRV definition in the 4 selected reviews. However, the most important divergences (15/23 studies) related to nRV definition was about selection of cases. Indeed, selection divergences accounted only for nRV definition. Participation divergence was observed in 6/23 paper for both incidence and extra-day evaluation; and finally, surveillance divergence in 5/23 and 8/23 paper for incidence and extra-day estimate respectively.
Figure 1.
Divergences of 23 selected studies.
Discussion
RV is currently the most common cause of severe AGE in children <5 yrs worldwide. RV infection is an important public health issue in developed countries for the highest burden on health care resources.13,41,42 The importance of nRV and the potential of vaccination in its prevention has been highlighted in recent years. RV is generally introduced in the pediatric wards after the hospitalization of children with CARV, especially after their stay in the emergency room before admission. Symptoms of CARV and nRV are similar, with the viral excretion beginning shortly before the clinical starting and persisting up to 57 d after the resolution of diarrhea. The period of transmissibility is around 2 weeks, although it could be longer in immunosuppressed patients. Asymptomatic infection represents 18–39% of all nRV cases with a prevalence in neonates and children <3 months of age. However, some conditions have been associated with an increased risk of nRV infection: hospitalization length, with an increasing nRV rate after the 6th day; young age; insufficient organization in pediatric services; low hygiene procedures or use of non-disposable tools; high presence of non-medical population (e.g. parents and relatives) in the ward; prematurity and low birth weight; severe immunodeficiency; malnutrition; presence of disease that could prolong hospitalization.21,34
The introduction of anti-RV vaccine has deeply modified the burden of RV disease, both in the community and in the hospital. The anti-RV vaccination led to an important reduction in terms of ED visit and hospitalization rates in US as much as in Europe. Indeed, many authors evidenced in US an estimate of 11.26/10,000 ED visits and 16.67/10,000 hospitalizations due to CARV in pre-vaccine era,42-45 with an overall hospitalization rate ranging 13–18/10,000 children <5 yrs.42 A hospitalization and ED visits decline is observed in all US regions, with a range around 62–78% for hospitalization and a mean reduction of 57% for ED visits.41,42,45 These data are similar to results reported by Zlamy20 and Giaquinto19 in Europe.
In particular, the diffusion of virus in pediatric care settings is difficult to control, as it is repeatedly introduced in the ward from the community. However, nRV phenomenon is often underrated, as most of the studies focused their attention on CARV. Inadequate methodological tool, short duration of studies and small sample size are the most important limitations for nRV evaluation.29,34 In our review, studies on nRV present several issues related to a high rate of methodological divergences.
A good nRV estimation is not possible to achieve, caused by the high heterogeneity of several studies as reported by a recent metanalysis.34 A unique definition of nRV was not pointed out, due to the lack of a consensus about international definition of nosocomial infection, as some authors did not recognize that these infections could also appear 48–72 hours after hospital discharge. For this reason, we propose to include the post-discharge onset into nRV definition that could lead to a better methodological evaluation. Moreover, screening of pediatric population admitted to hospital is important to identify some asymptomatic CARV often misdiagnosed with nRV.
Besides the not unique nRV definition, we highlight an important divergence in evaluation of nRV incidence and prolongation of hospital stay. In particular, incidence rates shown in Table 2 were mainly characterized by 4 different ways to assess the denominator. Among these indicators, we suggest that only nRV/Hospitalization and nRV/1000 hospitalization-days improve the nRV phenomenon characterization: the former is important to evaluate the nosocomial infection independently from primary diagnosis of hospitalization; the latter could produce an estimation of risk of nRV related to length of hospital stay.
In addition, studies on prolongation of hospital stay for nRV present an extremely wide variety in displaying results. We think that the best way to present the data is to use central tendency measures about extra-days and to correlate them with the mean pediatric hospitalization stay.
The wide difference in results evidenced in our analysis has important socio-economic implications. Indeed, the absence of precise and comprehensive results could hamper a good evaluation of the economic impact of the disease, often referred only to direct costs of hospitalization.15 Moreover, we supposed that the lack of indirect cost evaluation is also influenced by the absence of adequate post-discharge follow-up of children with nRV.
Our study has some limitations. Firstly, we did not evaluate the different RV serotypes and the presence of specific strains related to nosocomial infection, even if some authors consider the presence of RV variants (e.g., nursery strains) to explain asymptomatic or mild nRV cases.46 The presence of these variants could give an important contribution to the spread of the virus considering the 20–40% asymptomatic children. Another limitation of our study has been not to assess the different healthcare organization among European countries for management of children with acute diarrhea. Indeed, the lack of a consensus for treatment of CARV was reported by Forster, who highlighted a difference in access to participating hospital and ED visits.24 Specifically, clearly defined guidelines for CARV treatment as well as the presence of cultural factors could influence health-seeking behaviors. This condition increases the extreme geographical differences of RV infection already reported by PROTECT study.11
RV related disease is an important healthcare issue, especially in case of nosocomial infection where a prolongation of hospital stay has been observed. A good methodological approach is important to enhance the knowledge of nRV in European countries, where there is still no homogeneity in prevention, management and treatment of RV diseases. Planning a cost-effectiveness evaluation on the introduction of anti-RV vaccine to fight the most important cause of dehydrating diarrhea in children <5 y requires reducing the heterogeneity of studies about nRV.
Methods
An update of the last 10 y for RV epidemiology was made on Pubmed search-engine with following key words: “Rotavirus,” “Rotavirus Epidemiology,” “Community Acquired Rotavirus Infection,” “Rotavirus Hospitalization,” “Nosocomial Rotavirus Infection” and “Rotavirus vaccine.”
From 8,683 papers counted from 2006 up to September 2015 and filtered for human species, we selected 30 papers. Inclusion criteria for our analysis were original articles (prospective and retrospective studies) about hospitalized CARV or nRV or both with a clear evaluation of: hospitalization rate, hospitalization length, extra-day in hospital stay, incidence and prevalence in developed European countries. Reviews or metanalyses have been included if authors had explored the epidemiology of RV infection requiring hospitalization. Articles with sample aged >18 years or specific risk category for RV infection, papers related only to genomic or serological typing, and papers exploring only environmental or individual risk factors have been excluded. Finally, we analyzed 10/30 papers concerning hospitalized CARV, and 23/30 about nRV, all related to RV epidemiology before and/or after introduction of anti-RV vaccination.
Furthermore, a systematic evaluation of divergences was made considering 3 features: definition of nRV disease, incidence rate calculation of nRV, and extra-days length for hospital stay in cases of nRV. Each feature was analyzed under 4 different aspects of divergences, based on bias definition:47
selection of cases, based on different definition of nRV;
information of data, evidenced in different way to calculate rates and summarize data;
participation of selected patients, related to a sub-selection of specific patients;
surveillance methods, due to the presence or the absence of adequate follow-up.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565-72; PMID:12737740; http://dx.doi.org/ 10.3201/eid0905.020562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization Rotavirus vaccines. WHO position paper - January 2013. Weekly Epidemiol Rec Health Sect Secr Leag Nations 2013; 88(5):49-64. [Google Scholar]
- [3].Payne DC, Wikswo M, Parashar UD. Manual for the surveillance of vaccine-preventable diseases. Chapter 13: Rotavirus. Cent Dis Control Prev Atlanta GA 2011; 1-11. [Google Scholar]
- [4].Bernstein DI. Rotavirus overview. Pediatr Infect Dis J 2009; 28:S50-3; PMID:19252423; http://dx.doi.org/ 10.1097/INF.0b013e3181967bee [DOI] [PubMed] [Google Scholar]
- [5].Smith MJ, Clark HF, Lawley D, Bell LM, Hodinka RL, DiStefano DJ, Kulnis G, Zaoutis TE, Coffin SE. The clinical and molecular epidemiology of community- and healthcare-acquired rotavirus gastroenteritis. Pediatr Infect Dis J 2008; 27:54-8; PMID:18162939; http://dx.doi.org/ 10.1097/INF.0b013e31814b279d [DOI] [PubMed] [Google Scholar]
- [6].Raymond J, Aujard Y. Nosocomial infections in pediatric patients: a European, multicenter prospective study. European Study Group. Infect Control Hosp Epidemiol 2000; 21:260-3; http://dx.doi.org/ 10.1086/501755 [DOI] [PubMed] [Google Scholar]
- [7].Chandran A, Heinzen RR, Santosham M, Siberry GK. Nosocomial rotavirus infections: a systematic review. J Pediatr 2006; 149:441-7; PMID:17011311; http://dx.doi.org/ 10.1016/j.jpeds.2006.04.054 [DOI] [PubMed] [Google Scholar]
- [8].Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson M-A, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet Lond Engl 2006; 368:323-32; http://dx.doi.org/ 10.1016/S0140-6736(06)68815-6 [DOI] [PubMed] [Google Scholar]
- [9].Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine 2004; 22:S49-54; PMID:15576202; http://dx.doi.org/ 10.1016/j.vaccine.2004.08.017 [DOI] [PubMed] [Google Scholar]
- [10].Clark HF, Offit PA, Glass RI, Ward RL. Rotavirus vaccines In: Plotkin S, Orenstein W, Offit P, eds. Vaccines 6th Ed. Elsevier, Saunders: 2013; 669-287. [Google Scholar]
- [11].Pediatric ROTavirus European CommitTee (PROTECT) . The paediatric burden of rotavirus disease in Europe. Epidemiol Infect 2006; 134:908-16; PMID:16650331; http://dx.doi.org/ 10.1017/S0950268806006091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:S7-11; http://dx.doi.org/ 10.1097/01.inf.0000197622.98559.01 [DOI] [PubMed] [Google Scholar]
- [13].Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12:304-6; PMID:16494759; http://dx.doi.org/ 10.3201/eid1202.050006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect 2009; 137:607-16; PMID:19134232; http://dx.doi.org/ 10.1017/S0950268808001714 [DOI] [PubMed] [Google Scholar]
- [15].Aballéa S, Millier A, Quilici S, Caroll S, Petrou S, Toumi M. A critical literature review of health economic evaluations of rotavirus vaccination. Hum Vaccines Immunother 2013; 9:1272-88; http://dx.doi.org/ 10.4161/hv.24253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M, REVEAL Study Group . Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis 2007; 195:S4-16; PMID:17387650; http://dx.doi.org/ 10.1086/516714 [DOI] [PubMed] [Google Scholar]
- [17].Ogilvie I, Khoury H, El Khoury AC, Goetghebeur MM. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: serotype distribution and burden of illness. Hum Vaccin 2011; 7:523-33; PMID:21422818; http://dx.doi.org/ 10.4161/hv.7.5.14819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis 2012; 12:62-76; PMID:22429601; http://dx.doi.org/ 10.1186/1471-2334-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TTH, Maldonado YA, Spoulou V, Mast TC, Staat MA. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin 2011; 7:734-48; PMID:21734466; http://dx.doi.org/ 10.4161/hv.7.7.15511 [DOI] [PubMed] [Google Scholar]
- [20].Zlamy M, Kofler S, Orth D, Würzner R, Heinz-Erian P, Streng A, Prelog M. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis 2013; 13:112-122; PMID:23452879; http://dx.doi.org/ 10.1186/1471-2334-13-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, Giaquinto C, Grimprel E. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J 2006; 25:S12-21; PMID:16397425; http://dx.doi.org/ 10.1097/01.inf.0000197563.03895.91 [DOI] [PubMed] [Google Scholar]
- [22].Johansen K, Hedlund K-O, Zweygberg-Wirgart B, Bennet R. Complications attributable to rotavirus-induced diarrhoea in a Swedish paediatric population: report from an 11-year surveillance. Scand J Infect Dis 2008; 40:958-64; PMID:18777248; http://dx.doi.org/ 10.1080/00365540802415509 [DOI] [PubMed] [Google Scholar]
- [23].Stefkovicová M, Simurka P, Juracková L, Hudecková H, Mad'ar R. Nosocomial rotaviral gastroenteritis in paediatric departments. Cent Eur J Public Health 2008; 16:12-6 [DOI] [PubMed] [Google Scholar]
- [24].Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al.. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393-400; PMID:19254975; http://dx.doi.org/ 10.1542/peds.2008-2088 [DOI] [PubMed] [Google Scholar]
- [25].Gil-Prieto R, San Martín M, de Andrés AL, Alvaro-Meca A, González A, de Miguel AG. Hospital-acquired rotavirus infections in Spain over a ten-year period (1998–2007). Hum Vaccin 2009; 5:748-53; PMID:19829053; http://dx.doi.org/ 10.4161/hv.5.11.9792 [DOI] [PubMed] [Google Scholar]
- [26].Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D, et al.. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of israeli children <5 years of age, 2007–2008. J Infect Dis 2009; 200:S254-63; PMID:19817606; http://dx.doi.org/ 10.1086/605425 [DOI] [PubMed] [Google Scholar]
- [27].Waisbourd-Zinman O, Ben-Ziony S, Solter E, Scherf E, Samra Z, Ashkenazi S. Hospitalizations for nosocomial rotavirus gastroenteritis in a tertiary pediatric center: a 4-year prospective study. Am J Infect Control 2009; 37:465-9; PMID:19155098; http://dx.doi.org/ 10.1016/j.ajic.2008.09.017 [DOI] [PubMed] [Google Scholar]
- [28].Wildi-Runge S, Allemann S, Schaad UB, Heininger U. A 4-year study on clinical characteristics of children hospitalized with rotavirus gastroenteritis. Eur J Pediatr 2009; 168:1343-8; PMID:19205732; http://dx.doi.org/ 10.1007/s00431-009-0934-z [DOI] [PubMed] [Google Scholar]
- [29].Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, Kitchin N, Nakagomi O, Nakagomi T, Hart CA, Regan M. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis 2010; 16:55-62; PMID:20031043; http://dx.doi.org/ 10.3201/eid1601.090401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Festini F, Cocchi P, Mambretti D, Tagliabue B, Carotti M, Ciofi D, Biermann KP, Schiatti R, Ruggeri FM, De Benedictis FM, et al.. Nosocomial Rotavirus Gastroenteritis in pediatric patients: a multi-center prospective cohort study. BMC Infect Dis 2010; 10:235-43; PMID:20696065; http://dx.doi.org/ 10.1186/1471-2334-10-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gutiérrez-Gimeno MV, Martin-Moreno JM, Díez-Domingo J, Asensi-Botet F, Hernández-Marco R, Correcher-Medina P, Sánchez-Fauquier A. Nosocomial rotavirus gastroenteritis in Spain: a multicenter prospective study. Pediatr Infect Dis J 2010; 29:23-7; http://dx.doi.org/ 10.1097/INF.0b013e3181b3603a [DOI] [PubMed] [Google Scholar]
- [32].García-Basteiro AL, Bosch A, Sicuri E, Bayas JM, Trilla A, Hayes EB. Hospitalizations due to rotavirus gastroenteritis in Catalonia, Spain, 2003–2008. BMC Res Notes 2011; 4:429-36; http://dx.doi.org/ 10.1186/1756-0500-4-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Panatto D, Amicizia D, Giacchino R, Tacchella A, Natalizia AR, Melioli G, Bandettini R, Di Pietro P, Diana MC, Gasparini R. Burden of rotavirus infections in Liguria, Northern Italy: hospitalisations and potential savings by vaccination. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 2011; 30:957-64; http://dx.doi.org/ 10.1007/s10096-011-1180-7 [DOI] [PubMed] [Google Scholar]
- [34].Bruijning-Verhagen P, Quach C, Bonten M. Nosocomial rotavirus infections: a meta-analysis. Pediatrics 2012; 129:e1011-9; PMID:22392170; http://dx.doi.org/ 10.1542/peds.2011-2779 [DOI] [PubMed] [Google Scholar]
- [35].Nitsch-Osuch A, Kuchar E, Kosmala A, Zycinska K, Wardyn K. Nosocomial rotavirus gastroenterocolitis in a large tertiary paediatric hospital in Warsaw, 2006–2010. Arch Med Sci AMS 20 2013; 9:493-8; http://dx.doi.org/ 10.5114/aoms.2013.33177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Konstantopoulos A, Tragiannidis A, Fouzas S, Kavaliotis I, Tsiatsou O, Michailidou E, Spanaki A, Mantagos S, Kafetzis D, Papaevangelou V, et al.. Burden of rotavirus gastroenteritis in children <5 years of age in Greece: hospital-based prospective surveillance (2008–2010). BMJ Open 2013; 3:e003570-003577; PMID:24334153; http://dx.doi.org/ 10.1136/bmjopen-2013-003570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anca IA, Furtunescu FL, Pleşca D, Streinu-Cercel A, Rugină S, Holl K. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children below five years of age in Romania. Germs 2014; 4:30-40; PMID:24967217; http://dx.doi.org/ 10.11599/germs.2014.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rinder M, Tran AN, Bennet R, Brytting M, Cassel T, Eriksson M, Frithiof D, Gothefors L, Storsaeter J, Trollfors B, et al.. Burden of severe rotavirus disease leading to hospitalization assessed in a prospective cohort study in Sweden. Scand J Infect Dis 2014; 46:294-302; PMID:24484415; http://dx.doi.org/ 10.3109/00365548.2013.876511 [DOI] [PubMed] [Google Scholar]
- [39].Štefkovičová M, Litvová S, Šimurka P, Göczeová J, Gajdošíková A, Krištúfková Z. Rotavirus type profile in nosocomial and community infections in Western Slovakia. Folia Microbiol (Praha) 2015; 60:177-81 [DOI] [PubMed] [Google Scholar]
- [40].Redondo-González O. Validity and reliability of the minimum basic data set in estimating nosocomial acute gastroenteritis caused by rotavirus. Rev Esp Enfermedades Dig Organo Of Soc Esp Patol Dig 2015; 107:152-61 [PubMed] [Google Scholar]
- [41].Desai R, Curns AT, Steiner CA, Tate JE, Patel MM, Parashar UD. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis Off Publ Infect Dis Soc Am 2012; 55:e28-34; http://dx.doi.org/ 10.1093/cid/cis443 [DOI] [PubMed] [Google Scholar]
- [42].Krishnarajah G, Demissie K, Lefebvre P, Gaur S, Sheng Duh M. Clinical and cost burden of rotavirus infection before and after introduction of rotavirus vaccines among commercially and Medicaid insured children in the United States. Hum Vaccines Immunother 2014; 10:2255-66; http://dx.doi.org/ 10.4161/hv.29511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malek MA, Curns AT, Holman RC, Fischer TK, Bresee JS, Glass RI, Steiner CA, Parashar UD. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics 2006; 117:1887-92; PMID:16740827; http://dx.doi.org/ 10.1542/peds.2005-2351 [DOI] [PubMed] [Google Scholar]
- [44].Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J 2006; 25:489-93; PMID:16732145; http://dx.doi.org/ 10.1097/01.inf.0000215234.91997.21 [DOI] [PubMed] [Google Scholar]
- [45].Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, Zhou F, Parashar UD. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med 2011; 365:1108-17; PMID:21992123; http://dx.doi.org/ 10.1056/NEJMoa1000446 [DOI] [PubMed] [Google Scholar]
- [46].Usonis V, Ivaskeviciene I, Desselberger U, Rodrigo C, Pediatric ROTavirus European CommiTtee (PROTECT) . The unpredictable diversity of co-circulating rotavirus types in Europe and the possible impact of universal mass vaccination programmes on rotavirus genotype incidence. Vaccine 2012; 30:4596-605; PMID:22579864; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.097 [DOI] [PubMed] [Google Scholar]
- [47].Iain Lang. Inference, causality and interpretation In: Guest Charles, Ricciardi Walter, Kawachi Ichiro, Lang Iain. Oxford Handbook of Public Health Practice. Third Edition. Oxford University Press; 2013; 2(6):120-129 [Google Scholar]


