ABSTRACT
Dengue is the most important arbovirus disease throughout the world and it is responsible for more than 500,000 dengue hemorrhagic cases and 22,000 deaths every year. One vaccine was recently licensed for human use in Brazil, Mexico and Philippines and although at least seven candidates have been in clinical trials the results of the most developed CYD vaccine have demonstrated immunization problems, such as uneven protection and interference between serotypes. We constructed a vaccine candidate based on vesicular stomatitis virus (VSV) expression of pre-membrane (prM) and envelope (E) proteins of dengue-2 virus (DENV-2) and tested it in mice to evaluate immunogenicity and protection against DENV-2 infection. VSV has been successfully used as vaccine vectors for several viruses to induce strong humoral and cellular immune responses. The VSV-DENV-2 recombinant was constructed by inserting the DENV-2 structural proteins into a VSV plasmid DNA for recombinant VSV-DENV-2 recovery. Infectious recombinant VSV viruses were plaque purified and prM and E expression were confirmed by immunofluorescence and radiolabeling of proteins of infected cells. Forty Balb/C mice were inoculated through subcutaneous (s.c.) route with VSV-DENV-2 vaccine in a two doses schedule 15 d apart and 29 d after first inoculation, sera were collected and the mice were challenged with 50 lethal doses (LD50) of a neurovirulent DENV-2. The VSV-DENV-2 induced anti-DENV-2 antibodies and protected animals in the challenge experiment comparable to DENV-2 immunization control group. We conclude that VSV is a promising platform to test as a DENV vaccine and perhaps against others Flaviviridae.
KEYWORDS: dengue 2, dengue vaccine candidate, mouse challenge, neutralizing antibodies, recombinant vesicular stomatitis virus
Introduction
Dengue, a mosquito-borne disease caused by any of the four antigenically distinct dengue virus serotypes (DENV-1 to −4), is a major public health problem in the tropics and subtropics. The clinical spectrum of the disease ranges from asymptomatic or mild infection to severe organ involvement, including a dengue shock syndrome (DSS). The World Health Organization estimates that 500,000 people with severe dengue will require hospitalization each year, a large proportion of those are children and about 2.5% of those affected will die.1 Prevention depends primarily on control of the mosquito vector and has achieved only limited success in reducing dengue virus transmission. The rapid expansion of epidemic throughout the world has demonstrated that vaccines are the best means to prevent dengue disease.
Dengue vaccine development has been a challenge to this field in almost half a century. Sabin & Schlesinger.2 first tested a mouse brain attenuated DENV-1 to immunize army volunteers. Although successful, this vaccine was left behind due to safety concerns regarding to mouse brain products.3 Currently, there are seven vaccine candidates being evaluated in clinical trials,4,5 two live attenuated vaccines (LAV) are in the pipeline of development and the results of the only vaccine evaluated in phase 3 controlled clinical trials, the tetravalent chimeric yellow fever dengue vaccine (CYD-TV) in Asia-Pacific and Americas countries during the years of 2012 and 2015 have shown uneven immunogenicity probably due to viral interference/immunodominance among the four serotypes in tetravalent formulations.6-9 To overcome this problem purified inactivated virus, recombinant subunits, virus like particles and viral vectors are attractive approaches to induce balanced antibody response to all four serotypes.7 Due to the pathophysiology of the severe disease manifestations, a dengue vaccine has to be tetravalent and protect against all four serotypes. Failure to do so may lead to increased risk of severe disease. Furthermore, the risk of antibody-dependent enhancement (ADE) in individuals partially protected, the limited understanding of protective immune responses against dengue and the lack of an appropriate animal model has hampered advancement of dengue vaccine candidates.10
Vesicular stomatitis virus (VSV) is a negative-strand RNA virus of the Rhabdoviridae family (Vesiculovirus genus). It is a promising expression vector that was already used in experimental vaccines such as Chikungunya, Influenza, Papilloma and Ebola viruses11-14 and it is currently being used in HIV vaccine clinical trials (Clinicaltrials.gov, trials NCT01438606). The virus genome is stable, and although rare in humans, VSV infection usually presents itself as a mild or asymptomatic infection that makes it attractive candidate for DENV vaccines.
A live attenuated recombinant VSV-DENV-2 virus was constructed and tested in BALB/c mouse as a vaccine candidate to DENV. This live vaccine expresses the immunogenic pre-membrane (prM) and envelope (E) proteins of DENV-2 as vaccine targets. The humoral immune response and animal protection against lethal challenge were evaluated.
Results
VSV-DENV-2 construction and characterization
To generate the recombinant VSV-DENV-2 expressing prM/E proteins, a RT-PCR fragment of 1982 bp of prM/E of DENV-2, New Guinea C strain, was inserted into pVSV-XN2 plasmid. The insertion of the 1.9 kb fragment was confirmed by enzyme restriction analysis with XhoI, NheI and PstI and sequencing (data not shown). The recombinant plasmid was then transfected into BHK-21 cells for recombinant VSV-DENV-2 recovery. Recombinant viruses were plaque purified and titrated in BHK-21 cells. After 48 hpi, more than 60% of cells were detached and the chosen recombinant virus titer was 7 × 107 PFU/mL.
Expression of DENV-2 prM/E by VSV-DENV-2
To determine if VSV-DENV-2 was expressing DENV-2 prM/E proteins, infected cells were metabolically labeled with [35S] methionine and the cellular extracts were resolved by 4–12% SDS-PAGE (Fig. 1). A 18 KDa and 53 KDa bands on the gel confirm the expression of DENV-2 prM and E proteins respectively, and are expressed together with VSV L, N, G, P and M proteins.
Figure 1.
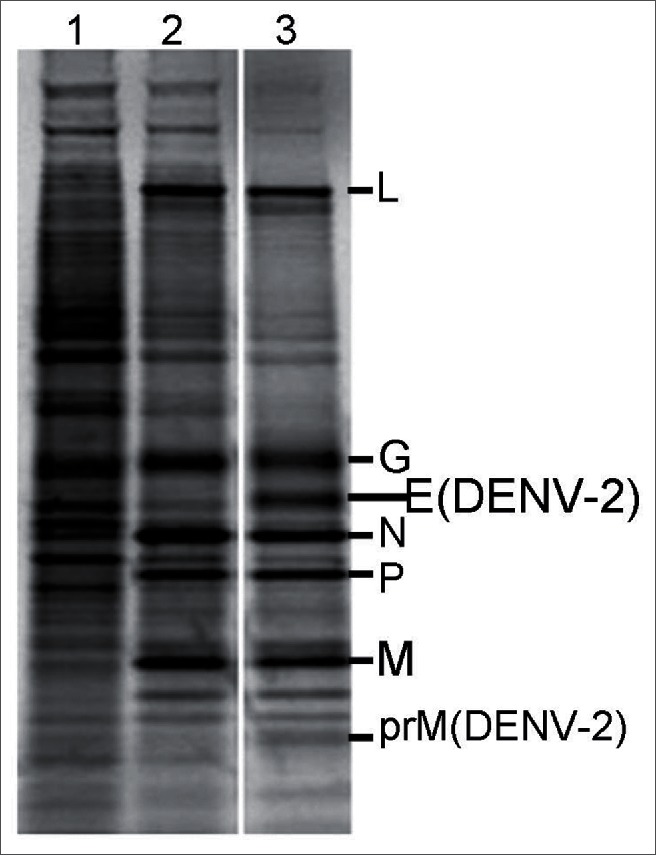
Metabolic label of rVSV-DENV-2 proteins and prM/E expression. SDS-PAGE analyses of crude extract of BHK-21 infected cells labeled 30 minutes with [35S] methionine after four to six hours post infection with: (1) mock-infected, (2) rwtVSV, (5) VSV-DENV-2. The two extra bands in lane (5) with 18 and 53 KDa are DENV-2 prM and E proteins, respectively.
The prM/E expression by VSV-DENV-2 was also demonstrated in BHK-21 cells infected cells stained with the flavivirus mouse immune ascitic fluid (MIAF) (Fig. 2C). DENV proteins are evidenced by a specific intracellular reticular pattern of fluorescence in VSV-DENV-2 but not in recombinant wild-type (rwtVSV) or mock-infected cells.
Figure 2.
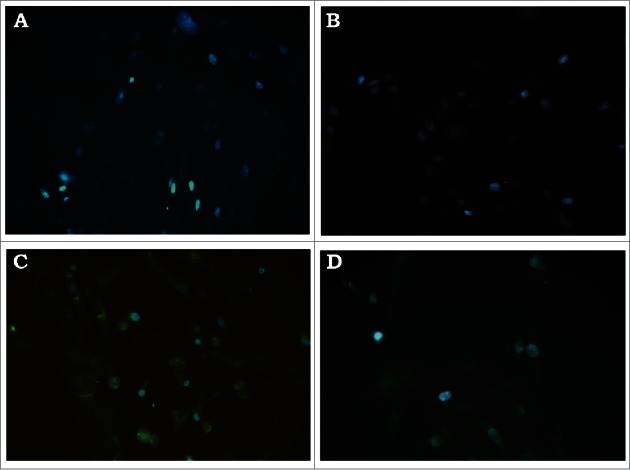
Immunofluorescence of BHK-21 cells infected with recombinant VSV-DENV-2. BHK-21 cells were infected with: (A) mock-infected, (B) rwtVSV, (C) VSV-DENV-2 and (D) control wtDENV-2 stained with MIAF broadly reacting with flaviviruses.
VSV-DENV-2 vaccine candidate induces antibodies against DENV-2
As demonstrated in the Table 1, inoculations with VSV-DENV-2 and DENV-2 induced a specific antibody response. To investigate serum conversion in immunized mice, the enzyme linked immunosorbent assay (ELISA) and plaque reduction neutralization test (PRNT) were used in sera collected 30 d after the first inoculation. Results shown here represents the mean values acquired in three independent experiments. Animals immunized with VSV-DENV-2 developed the highest geometrical mean titer (GMT) of 1:448, while the control DENV-2 immunized mice developed GMT titer of 1:366, both were significantly different from mock-infected mice (p < 0 .05). Slight cross-reactivity was observed in serum from animals immunized with DENV-2 and rwtVSV. The background in ELISA test was reduced with prior incubation of serum dilutions with 2 × 105 PFU of rwtVSV for 30 min at 37°C and the cutoff was set as the mean optical density plus three standard derivations of DMEM control sera.
Table 1.
Geometrical mean titer of IgG and neutralizing antibodies in mouse after immunization with VSV-DENV-2 vaccine analyzed by ELISA and PRNT.
| Vaccine group | ELISA | PRNT50 |
|---|---|---|
| VSV-DENV-2 | 1:448 | 1:100 |
| DENV-2 | 1:366 | 1:133 |
| rwtVSV | <1:100 | <1:10 |
| Mock | NR# | NR# |
#No reactivity
Pooled sera were collected 15 d after second dose by the time of challenge experiment. The cutoff value for seropositivity in ELISA was the mean optical density plus three standard deviations of mock control sera. Due to cross reactivity between rwtVSV and DENV-2 in ELISA, all mouse sera were adsorbed with 2 × 105 PFU of rwtVSV for 30 min before ELISA assay. The plaque reduction neutralization titer for 50% inhibition (PRNT50) was determined by probit analysis. Serum dilution represents the geometric mean titer (GMT) obtained in three independent experiments with 10 animals per group (n = 40).
Neutralizing antibodies (NAbs), measured by plaque assay, were also generated in animals immunized with VSV-DENV-2 vaccine candidate and their GMT is comparable to DENV-2. Control animals immunized either with rwtVSV or DMEM did not produce NAbs. The GMT of VSV-DENV-2 vaccine and DENV-2 was 1:100 and 1:133, respectively.
The vaccine protects animals against lethal challenge
Thirty days after the first inoculation, mice were challenged with 50 LD50 of a neurovirulent DENV-2 and followed for signals of paralysis or death for 21 d. As demonstrated in the Figure 3A, the first deaths occurred in the control animal groups within seven to ten post-challenge. On the other hand, the mortality of wtDENV-2 and VSV-DENV-2 immunized animals began on days 13 and 16, and at this time, 90% of wtDENV-2 and VSV-DENV-2 immunized animals were alive against only 30% and 20% of rwtVSV and DMEM inoculated mice (p < 0 .01). By the end of experiment, 90% of wtDENV-2 and 80% of VSV-DENV-2 immunized mice survived the lethal challenge and without significant differences in both groups. In contrast, the rwtVSV and DMEM groups were severely affected by DENV-2 infection and only 20 % and 10 % of animals survived to the challenge, respectively (p < 0 .01).
Figure 3.
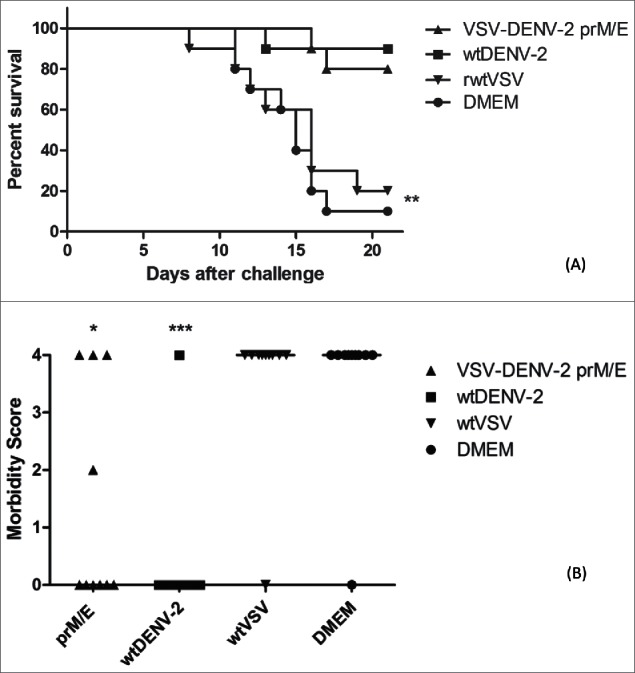
Mouse protection experiments against challenge with 50LD50 of DENV-2. Groups of ten animals were immunized with 1 × 106 PFU of DENV-2 TR1751/animal, VSV-DENV-2 vaccine candidate, recombinant wild type VSV vector or only DMEM, in a two doses schedule 15 d apart, and challenged two weeks after the second inoculation. Mortality (A) and morbidity (B) were observed for 21 d. 80% and 90% of animals immunized with VSV-DENV-2 candidate vaccine and the control DENV-2 respectively are protected after lethal challenge. Two of surviving animals in the VSV-DENV-2 group had mild and severe paralysis, but the morbidity were statistically different of DMEM or wtVSV group. Asterisks indicate statistically significant differences between vaccinated animals and mice inoculated with the control rwtVSV or DMEM (*p < 0 .05 and ***p < 0 .001). Data represent one of three independent experiments with 10 animals per group (n = 40).
The signs of encephalitis were also reduced in the animals immunized with the VSV-DENV-2 vaccine candidate as demonstrated in the Figure 3B. By the end of experiment on day 21 after challenge, out of the eight surviving animals in the VSV-DENV-2 group only two showed signs of encephalitis, one mild and other severe, in contrast, none of wtDENV-2 control group had signs of encephalitis, but both groups were statistically different from rwtVSV and DMEM control groups (p < 0 .05 and p < 0 .001), respectively.
Discussion
The development of a dengue vaccine has faced unique challenges. The results of a phase 3 clinical trial of the most promising dengue vaccine candidate in children of the Asia-Pacific and Latin America regions, the Sanofi Pasteur yellow fever/dengue chimeric vaccine failed to protect against DENV-2 and showed limited protection against DENV-1, including the increased risk of hospitalization of children under 9 y old, indicating the need to pursue new strategies to reach a broader protection against dengue infections.3,9,15,16 Among the strategies used to construct dengue vaccines, the recombinant live attenuated viruses are attractive because attenuated viruses are able to induce strong cellular and humoral responses that mimic natural infection, moreover inactivated and subunit vaccines require multiple inoculations, elicit short-term immunity and fail to induce robust MHC class I T cell immunity.7,17
In this study, we describe, for the first time, the use VSV to construct a dengue candidate vaccine. Our chimeric VSV-DENV-2 expresses prM and E proteins of DENV-2 and induces humoral response in BALB/c mice. Because dengue virus-induced NAb can bind these proteins and prevent it from binding to host cell receptors, inducing NAb is particularly important to block virus entry into target cells. These proteins are the major flavivirus structural proteins, they are involved in virus attachment and are the main target of NAb. Therefore, they are often explored for the construction of dengue vaccines.3,7,18,19
No animal model mimics natural human DENV infection. The interferon (INF) deficient AG-129 mouse provides consistent clinical manifestations of disease similar to human cases and is suitable for vaccine studies, but VSV vector is lethal to INF deficient mouse, so we decided to use immunocompetent BALB/c mice to test VSV-DENV-2 candidate vaccine.20 Because mice become naturally resistant to intracerebral (i.c.) challenge the majority of pre-clinical development of vaccines is performed in young mice, no older than 6 weeks and neutralizing antibodies is correlated with protection.21-23 Immunocompetent mice inoculated with wild type dengue virus (wtDENV) through i.p. or i.m. route do not develop disease, but rather develops immunity and resistance to neurological disease resulted from i.c. challenge, for this reason we used the wtDENV-2 as control of protection. Even thought the experimental approach we used were similar to the successful dengue vaccines tested in mice that are current in clinical trials, the protection and the magnitude of NAb responses cannot be compared between studies due to the difference in the neutralizing antibody assay methodologies and animal models used. In preclinical studies with a monovalent chimeric yellow fever/dengue vaccine against DENV-2, BALB/c mice immunized subcutaneously with 105 PFU developed high titers of NAb (PRNT50 = 1:422) 34 d after second dose, but a poor antibody response (PRNT50 = 1:19) with a single inoculation even though mice were 100% protected against 100 LD50 challenge.22 Another CYD vaccine constructed with prM/E of DENV-2 NGC strain, developed by Caufour et al.,23 demonstrated that Swiss mice immunized with an IP dose of 105 PFU in a three dose schedule developed high titers of NAb (PRNT50 = 1:426 to 1:4265) against homologous DENV-2 NCG, two weeks after the last dose and 85% the animals were protected against challenge with 220LD50. By the time of challenge, immunization with our recombinant VSV-DENV-2 induced NAb titers of PRNT50 = 100 and protected 80% of the animals challenged with 50LD50 of TR1751 DENV-2 strain. Although the NAb titer is lower than previous reports with CYD vaccine tested in mice, the magnitude of the response of VSV-DENV-2 was comparable to wtDENV-2 immunization (PRNT50 = 100 and 133) respectively, while the CYD vaccine was 10 times lower than wilt type DENV-2 immunization. The NAb titer is probably lower than CYD vaccine because of more complete immunological response to a Flavivirus than a Vesiculovirus vector. It is also expected a higher NAb titer to homologous than heterologous DENV-2 strain. In the CYD vaccine the NAb and challenge were with homologous genotype DENV-2 NGC strain, while in our experiment the prM and E of VSV-DENV-2 belongs to DENV-2 NGC Asian genotype and the NAb and the challenge were against DENV-2 TR-1751 American genotype. We have to bear in mind that mouse species, number of doses and vaccine titer are different among works, but the most relevant is that VSV-DENV-2 induce NAb and is capable to protect against lethal challenge. Despite the high rates of protection, two animals immunized with VSV-DENV-2 showed evidence of infection, but the scores of morbidity were comparable to wtDENV-2. In contrast, neither the animals immunized with rwtVSV nor mock-infected were protected against lethal challenge or developed NAb response.
The current strategy for developing dengue vaccines is based on monotypic protective immunity, it is the assumption that a neutralizing immune response to a single strain will protect against most of DENV within the serotype. But, it has been demonstrated that dengue neutralization by antibodies could vary up to 10 times among different genotypes of the same serotype, this is one of the hypothesis of the reduced CYD 23 vaccine efficacy and might have an impact on the future of dengue vaccines.8,17,24-27 The prM/E expressed in VSV-DENV-2 belongs to the NGC Asian genotype 2 and the DENV-2 TR1751 used on the neutralization and challenge experiments is an American genotype. Although they belong to different genotypes and share 93% of identity,28 the immunity raised by VSV-DENV-2 NGC was sufficient to neutralize the heterologous American genotype TR1751 infection in vivo and in vitro. Our results are in agreement with the magnitude of neutralization among the same serotype strains and extend these data to animal protection.
Thus, this report on DENV-2 recombinant VSV vaccines shows that VSV-vectored vaccines are good candidates for developing a live-attenuated dengue vaccine. Furthermore, vaccine development approaches should aim at the production of live-attenuated dengue vaccines, such as the ones shown here, because different than inactivated or subunit vaccines they tend to develop broad and long-lasting cellular and humoral immune response, no adjuvant is needed and usually a limited number of doses.
Material and methods
Viruses and cell lines
Mouse-adapted DENV-2 strains New Guinea C and TR1751/Trinidad, kindly provided by Dr Robert B. Tesh, University of Texas Medical Branch, were grown in C6/36 Aedes albopictus cells (ATCC CRL-1660), propagated in Leibovitz L-15 medium supplemented with 1% L-glutamine, 1% penicillin/streptomycin/amphotericin B and 10 % tryptose and fetal calf serum (FCS). VSVs were grown in Baby Hamster Kidney (BHK-21, ATCC CCL-10) cells, maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum FCS. Vero cells (ATCC CCL-81), derived from African green monkey kidney, were maintained in DMEM containing 1% L-glutamine, 10% FCS and antibiotics.
Construction of recombinant VSV-DENV encoding the DENV-2 prM and E proteins
The prM and E sequences of DENV-2 strain New Guinea C (GeneBank: KM2014118.1), nucleotides 439 to 2421 were RT-PCR amplified using forward 5′-GATCGATCCTCGAGAACATGAGAACTGCAGGCATGATCATTATG-3′ and reverse 5′-CCGGGGGGATCGGCTAGCTTAGGCCTGCACCATAACTCCCAAATAC-3′ primers, flanked by XhoI and NheI restriction sites at 5′ and 3′ end (boldfaced), respectively. The amplified sequence was inserted into the pVSV-XN2 plasmid, between the G and L genes of VSV genome to generate pVSV-DV2 prM/E. Infectious recombinant VSV-DENV-2 was recovered in BHK-21 cells as described elsewhere.29,30 Briefly, 10 plates with 1×106 cells were infected at a multiplicity of infection (moi) of 20 with a recombinant vaccinia virus expressing T7 polymerase (vTF7-3). After 1 h, vTF7-3 was removed and the cells were transfected with pVSV-DV2 prM/E along with support plasmids pBS-L, -N and -P, encoding the necessary VSV proteins to initiate VSV replication. Transfected cell supernatants were collected after 48 h post transfection, filtered through 0.1µm filter to remove vaccinia virus and then transferred onto fresh BHK-21 cells. Supernatant containing the recombinant virus (VSV-DENV-2) was collected after 48 h and plaque purified to prepare virus stock in the same cell line.
Radiolabeling and SDS-PAGE of VSV-DENV-2 recombinant proteins
To metabolically label VSV-DENV-2 proteins, 0.5 × 106 BHK-21 cells were infected at moi of 10 with recombinant VSV-DENV-2, rwtVSV or mock-infected. At 5 h post-infection, cells were labeled with 100 μCi of [35S] methionine in 1.0 mL of methionine-free DMEM for 30 min at 37°C. Cells were then washed twice with PBS and lysed with detergent lysis buffer (1% Nonidet P-40, 0.4% deoxycholate, 50 mM Tris-HCl, pH 8.0, 62.5 mM EDTA) on ice for 5 min. Cellular extracts were clarified at 16,000 x g for 2 min at 4°C and cell lysates were analyzed on a 4 to 12% Bis-Tris NuPAGE gel (Invitrogen, Inc.), and protein bands were imaged using a Fujifilm BAS 1800 imaging system.
Indirect immunofluorescence microscopy
Approximately 2 ×105 BHK-21 cells were infected at moi of 10 with rwtVSV or VSV-DENV-2. Four to 9 hours post-infection (hpi), cells were fixed for 20 min with 3% paraformaldehyde in PBS, washed three times with PBS and incubated with a 1:400 dilution of MIAF broadly reactive to flavivirus at 37°C for 30 min. The cells were washed as described previously and incubated with 1:400 dilution of goat anti-mouse FITC-conjugate (Sigma-Aldrich, Co). After 30 min at 37°C, the secondary antibody was removed and the cells were washed and counterstained with DAPI (Sigma-Aldrich, Co). Stained cells were observed in Olympus BX40 microscope at 400 X magnification.
Ethics statement
All experiments were carried out at the animal facility of Virology Research Center – School of Medicine of Ribeirão Preto – University of São Paulo - Brazil and approved by the institutional ethics committee for animal experimentation and care “CEUA FMRP-USP – Comissão de Ética no Uso de Animais da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo” (protocol number 021/2011) and in accordance with the recommendations of the guidelines for the care and use of laboratory animals of the Brazilian College of Animal Experimentation.
Animal experiments
Where anesthesia was used, a mixture of ketamine and xylazine was administered at approximately 100 mg/kg body weight for Ketamine and 5 mg/kg body weight for Xylazine injected intraperitoneally (i.p.) and euthanasia was performed by cervical dislocation. Forty female BALB/c mice, 4–6 weeks old were immunized either through subcutaneous route with approximately 1.0 × 106 plaque formation units (PFU) per animal of VSV-DENV-2, rwtVSV, DENV-2 strain TR1751 or mock-infected. After 15 d post inoculation, the animals received a second dose of homologous virus, and on day 30 they were anesthetized with a mixture of ketamine and xylazine and challenged with intracranial inoculation with 50 LD50 of neurovirulent DENV-2 TR1751. One day before challenge, sera were collected from submandibular vein, the complement was inactivated at 56°C for 40 min and the sera were stored at −70°C. The animals were followed for 21 d to score signs of paralysis or death. The degree of morbidity was scored using a scale ranging between 0 to 4 (0: no symptoms; 1: ruffling fur or change in the behavior; 2: mild paralysis in one hind leg or alteration of the spine characterized by a small hump; 3: severe paralysis in one or both hind legs; 4: two severe hind leg paralysis and deformed spinal column or death).
Antibodies against DENV
To detect IgG antibodies against DENV in the serum of immunized mice, the indirect ELISA kit (Panbio-Alere) was used with few modifications. All reagents and dilutions were used according to the manufacturer's recommendations, except for the anti-human peroxidase antibody that was substituted by a goat anti-mouse IgG conjugate antibody (Cell Technologies, Inc.). Because there was a slight cross reactivity between VSV and DENV antibodies using a Panbio ELISA plate, all serum were previously adsorbed with 2 × 105 PFU of rwtVSV for 30 min at 37°C before the ELISA assay. Briefly, 100 uL of mice serum was serially two-fold diluted and pipetted into microwells coated with DENV antigens. The plate was covered and incubated for 30 min at 37°C. After incubation, the plate was washed three times with washing buffer and 100 uL of a 1:5000 dilution of goat anti-mouse peroxidase antibody was added and incubated for 30 min at 37° C. The plate was then washed as described previously and 100 uL of substrate reagent was added and incubated for 10 min at room temperature and then, stopped with 100 uL of stop solution. The plate was read at 450 nm and 650 nm filter with Multiskan Ascent microplate reader (MTX Lab Systems, Inc.) and the cut-off calculated as the average of optical density of mock-infected mice serum plus three times the standard deviation.
Neutralizing antibodies against DENV
To determine the neutralizing antibodies titer in the mice serum, the PRNT50 was used.31 Briefly, about 100 PFU of DENV-2 TR1751 were incubated either with DMEM only or a serial two-fold dilution of mouse sera in serum-free DMEM. The mixture was incubated for 1 h at 37°C and inoculated onto confluent monolayers of Vero cells for 1 h at 37°C and 5%CO2. The inocula were removed and a semisolid DMEM overlay (2% carboximethilcelulose, 2% FCS, 1% antibiotics and 1% L-glutamin) was added. The cells were incubated at the same conditions described above for 7 days, the overlay removed and cells fixed for 1 h with 10% buffered formaldehyde solution at room temperature. The monolayer was stained with 1% crystal violet solution, the plaques counted, and the 50 percent PRNT titers (PRNT50) were determined by probit analysis of two-fold dilution of mouse sera from 1:10 to 1:1280.
Statistical analysis
Prism software (version 5, GraphPad Software, Inc.) was used to analyze all data. The comparisons of serum antibody titers and score of morbidity were analyzed with Mann-Whitney test. The Kaplan-Meier analysis was performed to generate survival curves, and a p < 0 .05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Luis Tadeu Moraes Figueiredo, University of Sao Paulo, for the kind gift of mouse immune ascitic fluid (MIAF) broadly reactive to flavivirus.
Funding
This work was supported in part by National Counsel of Technological and Scientific Development (CNPq), grant 307703/2014-2 and U.S. National Institute of Allergy and Infectious Disease (NIAID/NIH), grant U54AI057158. Flávio Lauretti was supported by Posdoctoral fellowship of Coordination for the Improvement of Higher Education Personnel (CAPES), grant PNPD 07171532828. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- [1].Dengue and severe dengue WHO media centre. Fact sheet N 117 2015; http://www.who.int/mediacentre/factsheets/fs117/en/ [Google Scholar]
- [2].Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science 1945; 101:640-2; PMID:17844088; http://dx.doi.org/ 10.1126/science.101.2634.640 [DOI] [PubMed] [Google Scholar]
- [3].Innis B, Eckels KH. Progress in development of a live-attenuated, tetravalent dengue virus vaccine by the United States Army Medical Research and Material Command. Am J Trop Med Hyg 2003; 69(6):1-4; PMID:14756126 [DOI] [PubMed] [Google Scholar]
- [4].Norrby R. Outlook for a dengue vaccine. Clin Microbiol Infect 2014; 20(5):92-4; PMID:24438016; http://dx.doi.org/ 10.1111/1469-0691.12442 [DOI] [PubMed] [Google Scholar]
- [5].Guzman E. Harris Dengue. Lancet 2015; 385(9966):453-65; PMID:25230594; http://dx.doi.org/ 10.1016/S0140-6736(14)60572-9 [DOI] [PubMed] [Google Scholar]
- [6].Blaney JE, Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol 2005; 79(9):5516-28; PMID:15827166; http://dx.doi.org/ 10.1128/JVI.79.9.5516-5528.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wan SW, Lin CF, Wang S, Chen YH, Yeh TM, Liu HS, Anderson R, Lin YS. Current progress in dengue vaccines. J Biomed Sci 2013; 20:37; PMID:23758699; http://dx.doi.org/ 10.1186/1423-0127-20-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Halstead SB. Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 2013; 31:4501-7; PMID:23896423; http://dx.doi.org/ 10.1016/j.vaccine.2013.06.079 [DOI] [PubMed] [Google Scholar]
- [9].Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al.. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195-206; PMID:26214039; http://dx.doi.org/ 10.1056/NEJMoa1506223 [DOI] [PubMed] [Google Scholar]
- [10].World Health Organization Meeting of the Strategic Advisory Group of Experts on immunization – conclusions and recommendations. Weekly Epidemiological Record 2013; 20(88):205-6, http://www.who.int/wer/2013/wer8820.pdf?ua=1 [Google Scholar]
- [11].Geisbert TW, Daddario-DiCaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, Paragas J, Matthias L, Smith MA, Jones SM, et al.. Vesicular Stomatitis Virus-Based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog 2008; 4(11):e1000225; PMID:19043556; http://dx.doi.org/ 10.1371/journal.ppat.1000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reuter JD, Vivas-Gonzalez BE, Gomez D, Wilson JH, Brandsma JL, Greenstone HL, Rose JK, Roberts A. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J Virol 2002; 76(17):8900-9; PMID:12163609; http://dx.doi.org/ 10.1128/JVI.76.17.8900-8909.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schwartz JA, Buonocore L, Suguitan A, Hunter M, Marx MA, Subbarao K, Rose JK. Vesicular Stomatitis Virus-based H5N1 avian Influenza vaccines induce potent cross-clade neutralizing antibodies in rhesus macaques. J Virol 2011; 85(9):4602-5; PMID:21325423; http://dx.doi.org/ 10.1128/JVI.02491-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chattopadhyay A, Wang E, Seymour R, Weaver R, Rose JK. A chimeric Vesiculo/Alphavirus is an effective Alphavirus vaccine. J Virol 2013; 87(1):395-402; PMID:23077320; http://dx.doi.org/ 10.1128/JVI.01860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al.. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358-65; PMID:25018116; http://dx.doi.org/ 10.1016/S0140-6736(14)61060-6 [DOI] [PubMed] [Google Scholar]
- [16].Hernández-Ávila M, Santos-Preciado JI. Análisis de la evidencia sobre eficacia y seguridad de la vacuna de dengue CYD-TDV y su potencial registro e implementación en el Programa de Vacunación Universal de México. Salud Publica Mex 2016; 58(1):71-83 [PubMed] [Google Scholar]
- [17].Edelman R. Dengue vaccines approach the finish line. Clin Infect Dis 2007; 45:S56-60; PMID:17582571; http://dx.doi.org/ 10.1086/518148 [DOI] [PubMed] [Google Scholar]
- [18].Wahala M, Wahala PB, Silva AM. The human antibody response to Dengue Virus infection. Viruses 2011; 3:2374-95; PMID:22355444; http://dx.doi.org/ 10.3390/v3122374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, et al.. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci 2012; 109:7439-44; PMID:22499787; http://dx.doi.org/ 10.1073/pnas.1200566109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in α/β interferon receptor-deficient mice. J Virol 1995; 69:2153-8; PMID:7884863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Putnak R, Barvir DA, Burrous JM, Dubois DR, D'Andrea VM, Hoke CH, Sadoff JC, Eckels KH. Development of a purified, inactivated, dengue-2 virus vaccine prototype in vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infec Dis 1996; 174:1176-84; http://dx.doi.org/ 10.1093/infdis/174.6.1176 [DOI] [PubMed] [Google Scholar]
- [22].Van der Most RG, Murali-Krishna K, Ahmed R, Strauss JH. Chimeric yellow fever/dengue virus as a candidate dengue vaccine: quantitation of the dengue virus-specific CD8 T-cell response. J Virol 2000; 74:8094-101; PMID:10933719; http://dx.doi.org/ 10.1128/JVI.74.17.8094-8101.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caufour PS, Motta MC, Yamamura AM, Vazquez S, Ferreira II, Jabor AV, Bonaldo MC, Freire MS, Galler R. Construction, characterization and immunogenicity of recombinant yellow fever 17D-dengue type 2 viruses. Virus Res 2001; 79:1-14; PMID:11551641; http://dx.doi.org/ 10.1016/S0168-1702(01)00273-8 [DOI] [PubMed] [Google Scholar]
- [24].Barban V, Munoz-Jordan JL, Santiago GS, Mantel N, Girerd Y, Giulia S, Claude JB, Lang J. Broad neutralization of wild-type dengue virus isolates following immunization in monkeys with a tetravalent dengue vaccine based on chimeric yellow Fever 17D/dengue viruses. Virology 2012; 429(2):91-8; PMID:22542002; http://dx.doi.org/ 10.1016/j.virol.2012.03.007 [DOI] [PubMed] [Google Scholar]
- [25].Messer WB, Yount B, Hacker KE, Donaldnos EF, Huynh JP, de Silva AM, Baric RS. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl Trop Dis 2012; 6(2):e1486; PMID:22389731; http://dx.doi.org/ 10.1371/journal.pntd.0001486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wahala WM, Donaldson EF, de Alwis R, Accavitti-Loper MA, Baric RS, de Silva AM. Natural strain variation and antibody neutralization of dengue serotype 3 viruses. PLoS Pathog 2010. 19; 6(3):e1000821; PMID:20333252; http://dx.doi.org/ 10.1371/journal.ppat.1000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, Diamond MS. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 2010; 84(20):10630-43; PMID:20702644; http://dx.doi.org/ 10.1128/JVI.01190-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lewis JA, Chang GJ, Lanciotti RS, Kinney RM, Mayer LW, Trent DW. Phylogenetic relationships of dengue-2 viruses. Virology 1993; 197(1):216-24; PMID:8212556; http://dx.doi.org/ 10.1006/viro.1993.1582 [DOI] [PubMed] [Google Scholar]
- [29].Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant Vesicular Stomatitis Viruses from DNA. Proc Natl Acad Sci 1995; 92:4477-81; PMID:7753828; http://dx.doi.org/ 10.1073/pnas.92.10.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schenell JM, Buonocore L, Kretzschmar E, Johnson E, Rose JK. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci 1996; 93:11359-65; PMID:8876140; http://dx.doi.org/ 10.1073/pnas.93.21.11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99:285-90; PMID:6031202 [PubMed] [Google Scholar]


