Abstract
Objectives:
Sepsis is a common cause of morbidity and mortality and is associated with significant costs to the healthcare organizations. We performed a systematic review and meta-analysis to assess whether high or low-dose statin therapy improved mortality in patients with sepsis.
Methods:
The trials analyzed in this study were multicenter or single center randomized control studies using statins for sepsis in a hospital setting. The patients included were adults with suspected or confirmed infection.
Interventions:
This study found eight randomized controlled trials where participants were given either a statin or placebo daily for 14–28 days, the duration of their illness, or until their death or discharge, which ever occurred first.
Primary and Secondary Outcomes Measured:
This meta-analysis measured the effect of statin therapy on in hospital and 28 days mortality.
Results:
In unselected patients, there was no demonstrable difference in the 28 days mortality (relative risk [RR] 0.88 95% confidence interval [CI], 0.70–1.12 and P = 0.16). There was also no significant difference between statin versus placebo for in-hospital mortality (RR 0.98 95% CI, 0.85–1.14 P = 0.36). When the studies where divided into low-dose and high-dose groups, there were no statistically significant differences for in-hospital mortality between low-dose statin versus placebo for (RR 0.81 CI 0.44–1.49 P = 0.27) or high-dose statin versus placebo (RR 0.99 95% CI 0.85–1.16, P = 0.28). There was no significant difference in adverse effects between the high- and low-dose groups.
Conclusions:
In this meta-analysis, we found that the use of statins did not significantly improve either in-hospital mortality or 28-day mortality in patients with sepsis. In the low-dose group, there were fewer quality multicenter studies; hence, conclusions based on the results of this subgroup are limited.
Keywords: High dose, mortality, low dose, sepsis, statin
Introduction
Sepsis is characterized by an exaggerated inflammatory response to infection, resulting in a multisystem physiological, cellular, and organ dysfunction. In a European study, a typical episode of severe sepsis costs the health care organization approximately €25,000.[1] Assuming that we see 100,000 cases of severe sepsis per annum, this equates to a direct current cost to the national health services of England of over 2.5 billion every year. In the USA, the Centers for Disease Control and Prevention's National Center for Health Statistics estimates the number of hospital admissions attributed to sepsis increased from 621,000 in the year 2000 to 1,141,000 in 2008.[2] In 2011, sepsis resulted in an aggregate healthcare cost of $20.3 billion making it the most expensive condition treated in US hospitals.[3]
Mortality from sepsis has improved over the last decade but is still estimated to be 36% in Europe.[1] This warrants a search for novel therapeutic targets and preventative therapies in patient with sepsis. Statins are lipid-lowering drugs that inhibit 3-hydroxy-3 methyglutaryl coenzyme A reductase, with pleotropic mechanisms that may be beneficial in sepsis. They are hypothesized to possess a variety of benefits including anti-inflammatory, immunomodulatory, antioxidant, and antithrombotic effects.[4,5] In previous studies, statins have been shown to reduce the unregulated immune response, influence the gene transcription, and reduce the expression of mRNA to sepsis. The use of statins was associated with an improved mortality and morbidity following sepsis.[6,7] In animal studies, statins reduced the severity of sepsis.[8,9] It is also thought that statins exert a protective effect through down-regulation of toll-like receptor 4, inhibition of nuclear factor-kappa-B and protection against endothelial cell apoptosis.[10,11] Despite the initial promise from animal data and early clinical trials, these benefits have not been reproduced in the large, well-designed, randomized controlled trials (RCTs) published recently.[12,13]
This paper aims to assess the impact of both high and low dose statin therapy on in-hospital and 28-day mortality in patients with sepsis.
Methods
Search strategy for published studies with aggregate data
Electronic databases, including PubMed and EMBASE, were searched using a combination of keywords: “sepsis,” “intensive care” “statin” “simvastatin,” “atorvastatin,” and “rosuvastatin” to create a list of articles published before October 2015. The search was limited to articles published in the English language.
Study selection criteria
Articles were included if the study population was adult patients with a suspected or confirmed infection and reported 28 day or in-hospital mortality reported as a primary or secondary outcome. A total of 215 articles were identified. The following were excluded; 160 were not clinical trials, 2 post hoc analyses, 2 cell-based studies, 3 were not randomized control trials, and 31 were the wrong patient population. A total of 15 studies were fully assessed for eligibility. Of these, 3 were excluded as mortality was not reported as a primary or secondary outcome, and 4 were excluded as they were the wrong patient populations: 3 were postcardiac surgery patient populations and 1 was neurosurgical patients. Eight studies were included in the meta-analysis [Figure 1].
Figure 1.
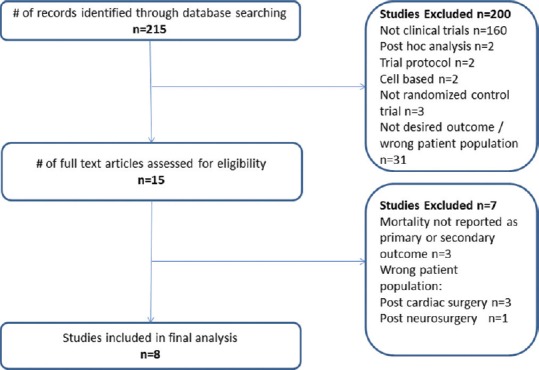
Studies included in this meta-analysis
Data extraction and statistical analysis
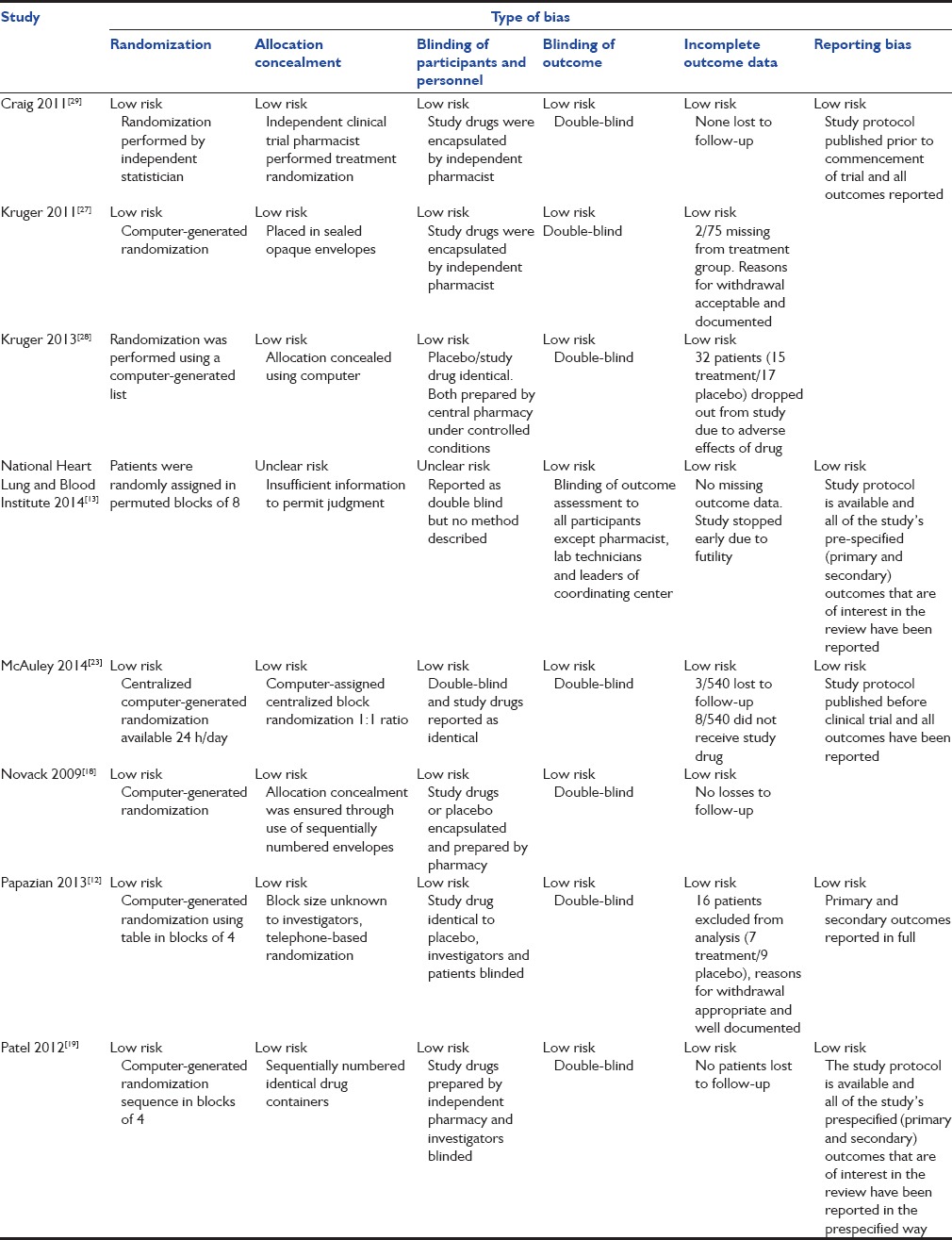
Data were extracted independently by two authors and analyzed using Review Manager (RevMan) [Computer program]. Version 5.3.[14] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. For each study, the characteristics of the study, number of participants, characteristics of included patients, selection criteria, drug and dose used, and outcomes observed in the study were extracted. Most studies reported in-hospital mortality and 28 days mortality outcomes. Some studies reported 28 days mortality only and others reporting in-hospital mortality only. To include all RCTs in this meta-analysis, the authors have performed a separate analyses on both 28 days mortality and in-hospital mortality for this reason. All studies had adequate randomization and blinding. The quality of the RCTs was evaluated using the method described in the Cochrane Handbook for Systematic Reviews of Interventions.[15] In each study, patients were given either a dose of statin (simvastatin 20/40/80 mg, rosuvastatin 40 mg or atorvastatin 20 mg) or a placebo. Low-dose statin was defined as simvastatin 20 mg or atorvastatin 20 mg. High dose was defined as simvastatin 40 mg or 80 mg or rosuvastatin 40 mg.[16,17]
Results
We examined a total of 8 RCTs with a total of 2275 patients. Figures 2 and 3 show forest plots of 28-day mortality and in-hospital mortality, respectively. Table 1 shows the 8 RCTs and the characteristics of each study. Table 2 shows assessment of bias for each study. All studies used in the meta-analysis are listed in Appendix 1.
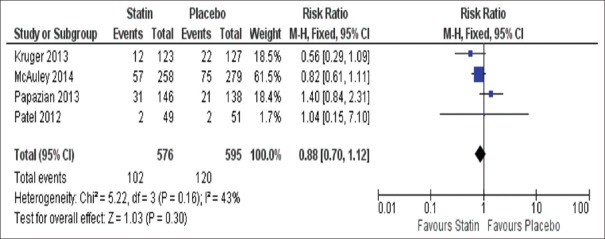
Figure 2.
Forest plot of proportional effect on 28-day mortality. Forest plot of proportional effect of all studies (low and high doses) on 28-day mortality (Diamonds = totals and subtotals [95% confidence interval]. Squares = individual studies [horizontal lines are 95% confidence intervals]. Area of square proportional to amount of statistical information in that category. Risk ratios are weighted to represent heterogeneity)
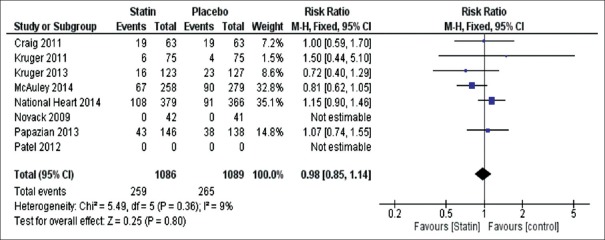
Figure 3.
Forest plot of proportional effect for in-hospital mortality of individual studies. Forest plot of proportional effect on in-hospital mortality (Diamonds = totals and subtotals [95% confidence interval]. Squares = individual studies [horizontal lines are 95% confidence intervals]. Area of square proportional to amount of statistical information in that category. Risk ratios are weighted to represent heterogeneity)
Table 1.
Characteristics of included studies
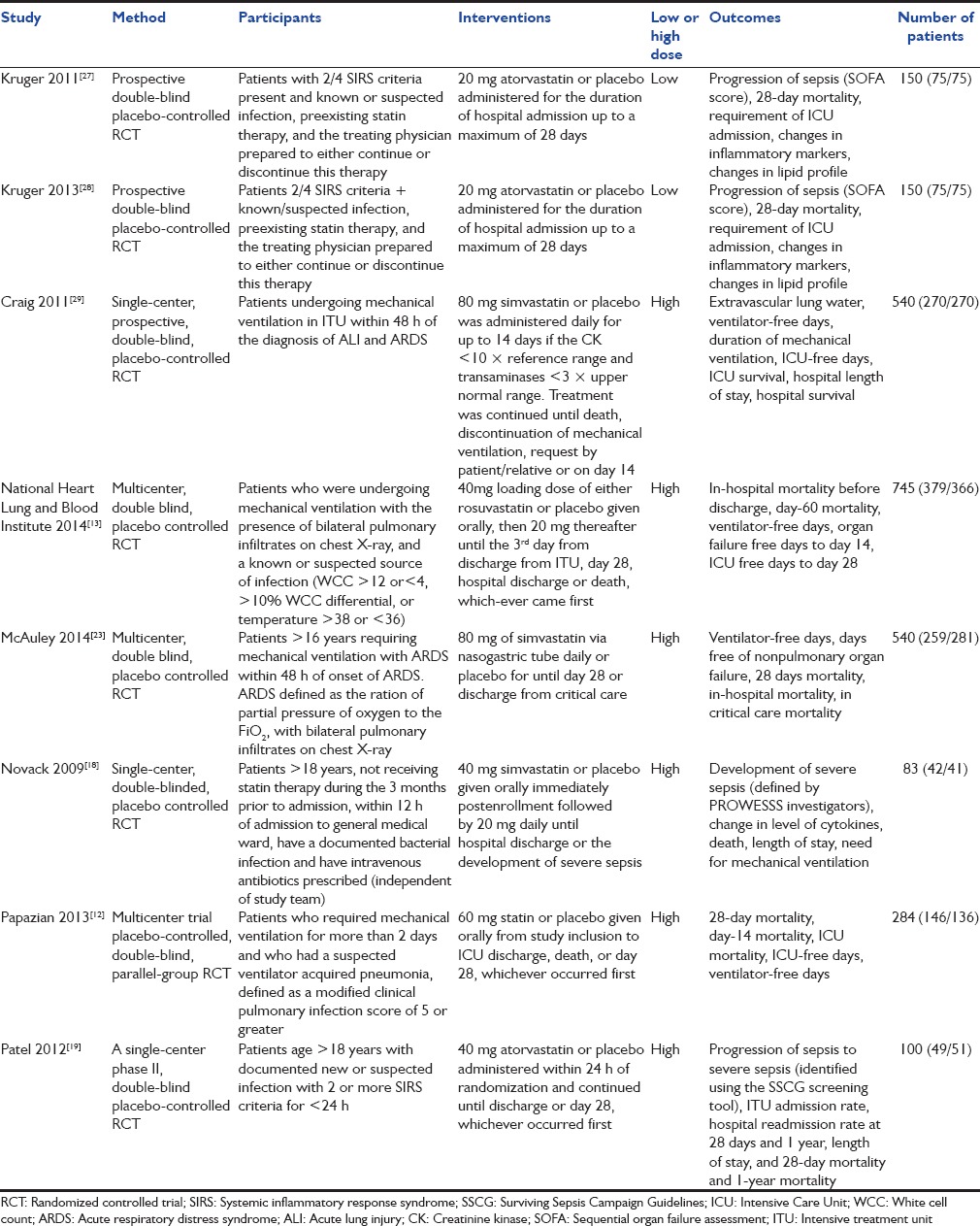
Table 2.
Assessment of risk of bias
All dose statin, 28-day mortality
Results from 1171 patients demonstrated that there was no significant difference between statin (102/576) and placebo (120/595) for 28 day mortality (relative risk [RR] 0.88 95% confidence interval [CI], 0.70–1.12 and P = 0.16), with a heterogeneity of the trials I2 = 43%, P = 0.16 [Figure 2].
All dose statin, in-hospital mortality
Results from 2175 patients demonstrated that there was no significant difference between statin (259/1086) versus placebo (265/1089) for in-hospital mortality (RR 0.98 95% CI, 0.85–1.14 P = 0.36) with low heterogeneity between the studies I2 = 9% P = 0.36. The authors excluded 83 patients from the Novack 2009 study from the analysis, as both arms of the trial had zero events [Figure 3].[18]
Low dose statin, in-hospital mortality
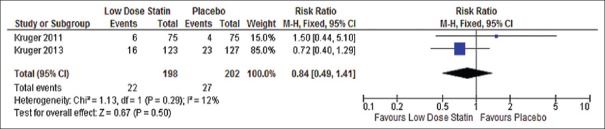
Results from 400 patients demonstrated no statistically significant difference between low-dosage statin use (22/198) versus placebo (27/202) for in-hospital mortality [RR 0.81 CI (0.44–1.49) P = 0.27, I2 = 16% Figure 4].
Figure 4.
Forest plot of proportional effect for low dose statin, in-hospital mortality. Forest plot of proportional effect on in-hospital mortality (Diamonds = totals and subtotals [95% confidence interval]. Squares = individual studies [horizontal lines are 95% confidence intervals]. Area of square proportional to amount of statistical information in that category. Risk ratios are weighted to represent heterogeneity)
High dose statin, in-hospital mortality
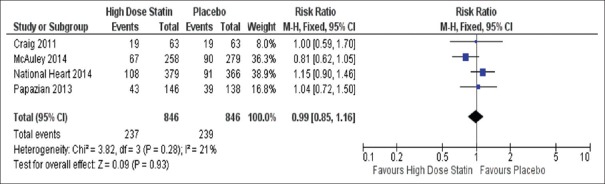
Results from 1692 patients demonstrated that there was no significance between high-dose statin usage (237/846) versus placebo (239/846) for in-hospital mortality [RR 0.99 95% CI 0.85–1.16 P = 0.28, I2 = 21%, Figure 5].
Figure 5.
Forest plot of proportional effect for high dose statin, in-hospital mortality. Forest plot of proportional effect on in-hospital mortality (Diamonds = totals and subtotals [95% confidence interval]. Squares = individual studies [horizontal lines are 95% confidence intervals]. Area of square proportional to amount of statistical information in that category. Risk ratios are weighted to represent heterogeneity)
Adverse effects in high dose and low dose groups
From all reported trials, patients with sepsis who received statins did not have a significantly higher incidence of adverse effects compared to placebo. In patients who were treated with high-dose statins, the incidence of adverse effects was higher (11.6% vs. 8.5%, P > 0.05), but this failed to reach statistical significance.
Discussion
This meta-analysis demonstrated that statin therapy does not reduce in-hospital or 28 days mortality in sepsis when compared to placebo. This meta-analysis also failed to show any significant difference in outcome of treating patients with low or high dose statins.
Statins were shown to be beneficial in animal model studies,[6] and early RCTs demonstrated benefits in critically ill patients with sepsis, however these studies were single center RCTs with a relatively small sample size.[19] In this study, we investigated whether the dose of statin was associated with adverse events. Statin therapy is associated with musculoskeletal side effects including myopathy, myositis, and rhabdomyolysis. Myalgia and arthralgia are reported in up to 5% of participants in clinical trials.[20,21,22] Reported incidence of dose dependent adverse events such as deranged hepatic transaminases was 0.5–2%.[16,20] In one study, there were a significant number of adverse events related to the intervention arm when using 80 mg of simvastatin.[23] However, across all studies in this analysis, the total incidence of adverse events in patients who were treated with high dose statins was not significantly different compared to placebo or to low dose statins.
In this up to date meta-analysis, we focused only on eligible RCTs to eliminate the risk of confounding variables often seen in observation studies. Compared to previous meta-analyses, we have not only included up to date RCTs but also tried to delineate the role of high and low-dose statins.[24] These findings are in contradiction to previous literature reviews and observational studies.[7,25] As our meta-analysis relies purely on RCTs, this limits the study to a relatively small sample size. This is particularly true for the low-dose statin subgroup of patients.
While preclinical and observational studies hypothesized the potential benefits of statin usage in sepsis, our meta-analysis contradicts this presumption. Although the beneficial effects in vitro and animal models have been well documented in literature,[6,11] these effects do not confer a detectable benefit in large human model RCTs. This could be due to the heterogeneity of the populations being studied; there is an inter subject variability in the host response due to age, existing comorbidities and genetic profile. Genes involved in sepsis have been analyzed for links between single-nucleoside polymorphisms and sepsis susceptibility, organ dysfunction and mortality.[26] Despite the heterogeneity of the populations being studied, there are no suggestions from this study that a specific population group would benefit from statin therapy.
Conclusions
This meta-analysis found no beneficial effect of statin therapy in the context of sepsis and the mortality of critically ill patients. Our findings are in contradiction to previous reviews of the literature and also to those of observational studies.[7,19,25]
Financial support and sponsorship
Dr. Veenith is supported by NIAA clinical research training fellowship and Beverly Sackler Studentship.
Conflicts of interest
There are no conflicts of interest.
Appendix
Appendix 1: Studies included in this meta-analysis
Papazian L, Roch A, Charles PE, Penot-Ragon C, Perrin G, Roulier P, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA 2013;310:1692-700.
National Heart, Lung, and Blood Institute ARDS Clinical Trials Network, Truwit JD, Bernard GR, Steingrub J, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014;370:2191-200.
Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care 2012;16:R231.
McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695-703.
Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, et al. Continuation of statin therapy in patients with presumed infection: A randomized controlled trial. Am J Respir Crit Care Med 2011;183:774-81.
Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013;187:743-50.
Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, et al. A randomized clinical trial of hydroxymethylglutaryl. coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011;183:620-6.
Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: A randomized double-blind placebo controlled clinical trial. Intensive Care Med 2009;35:1255-60.
Search criteria
((“sepsis”[MeSH Terms] OR “sepsis”[All Fields]) AND (((“atorvastatin”[Supplementary Concept] OR “atorvastatin”[All Fields]) OR (“rosuvastatin”[Supplementary Concept] OR “rosuvastatin”[All Fields])) OR (“simvastatin”[MeSH Terms] OR “simvastatin”[All Fields]))) OR ((“intensive care”[MeSH Terms] OR (“intensive”[All Fields] AND “care”[All Fields]) OR “intensive care”[All Fields]) AND (((“atorvastatin”[Supplementary Concept] OR “atorvastatin”[All Fields]) OR (“rosuvastatin”[Supplementary Concept] OR “rosuvastatin”[All Fields])) OR (“simvastatin”[MeSH Terms] OR “simvastatin”[All Fields])))
References
- 1.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS Data Brief. 2011;62:1–8. [PubMed] [Google Scholar]
- 3.Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006. [Last cited on 2016 Jul 24]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24199255 . [PubMed]
- 4.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: Panacea for sepsis? Lancet Infect Dis. 2006;6:242–8. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 5.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–24. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 7.Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32:75–9. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- 8.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: Multiple modifications at multiple levels. Lancet Infect Dis. 2007;7:358–68. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 9.Drage SM, Barber VS, Young JD. Statins and sepsis: Panacea or pandora's box? Lancet Infect Dis. 2007;7:80. doi: 10.1016/S1473-3099(07)70003-8. [DOI] [PubMed] [Google Scholar]
- 10.De Loecker I, Preiser JC. Statins in the critically ill. Ann Intensive Care. 2012;2:19. doi: 10.1186/2110-5820-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papazian L, Roch A, Charles PE, Penot-Ragon C, Perrin G, Roulier P, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA. 2013;310:1692–700. doi: 10.1001/jama.2013.280031. [DOI] [PubMed] [Google Scholar]
- 13.Heart TN. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Review Manager (RevMan) [Computer Program] Copenhagen: The Nordic Cochrane Centre; 2014. The Cochrane Collaboration. [Google Scholar]
- 15.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011. [Last accessed on 2016 Sep 07]. Available from http://handbook.cochrane.org .
- 16.Hsu I, Spinler SA, Johnson NE. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother. 1995;29:743–59. doi: 10.1177/106002809502907-818. [DOI] [PubMed] [Google Scholar]
- 17.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 18.Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: A randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–60. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 19.Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial) Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151:43–9. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 22.Farmer JA. Learning from the cerivastatin experience. Lancet. 2001;358:1383–5. doi: 10.1016/S0140-6736(01)06489-3. [DOI] [PubMed] [Google Scholar]
- 23.McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 24.Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: A meta-analysis of randomized trials. Am J Med. 2015;128:410–7.e1. doi: 10.1016/j.amjmed.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 25.Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–5. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 26.Dhas BB, Ashmi H, Bhat BV. Sepsis genomics: Stepping forward toward sepsis prevention.? Int J Adv Med Health Res. 2014;1:8–15. [Google Scholar]
- 27.Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, et al. Continuation of statin therapy in patients with presumed infection: A randomized controlled trial. Am J Respir Crit Care Med. 2011;183:774–81. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 28.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–50. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 29.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–6. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]