Abstract
Introduction:
Febrile neutropenia (FN) is an oncological emergency. The choice of empiric therapy depends on the locally prevalent pathogens and their sensitivities, the sites of infection, and cost. The Infectious Diseases Society of America guidelines are being followed for the management of FN in India.
Methods:
This is a prospective observational study conducted at a tertiary care cancer centre from September 2012 to September 2014.
Objectives:
The objectives of this study were as follows: (1) To review the pattern of microbial flora, susceptibility pattern, and important clinical variables among bloodstream infections in febrile neutropenic patients with solid tumors and hematological malignancies. (2) As per the institutional protocol to periodically review the antibiotic policy and susceptibility pattern, and compare the findings with an earlier study done in our institute in 2010. This was a prospective study conducted from September 2012 to September 2014.
Results:
About 379 episodes of FN were documented among 300 patients. About 887 blood cultures were drawn. Of these, 137 (15%) isolates were cultured. Isolates having identical antibiograms obtained from a single patient during the same hospitalization were considered as one. Hence, 128 isolates were analyzed. About 74 (58%) cultures yielded Gram-negative bacilli, 51 (40%) were positive for Gram-positive cocci, and 3 (2%) grew fungi. Among Gram-negative organisms, Escherichia coli followed by Acinetobacter baumannii and Klebsiella pneumoniae accounted for 78% of the isolates. Among Gram-positive cocci, Staphylococcus species accounted for 84% of the isolates. We have noted a changing trend in the antibiotic sensitivity pattern over the years. Following the switch in empirical antibiotics, based on the results of the study done in 2010 (when the empirical antibiotics were ceftazidime + amikacin), the sensitivity to cefoperazone-sulbactam has plunged from about 80% to 60%%. Similar reduction in susceptibility was noted for piperacillin-tazobactam, imipenem, and meropenem. On the contrary, there was a marked increase in sensitivity to ceftazidime (50–76%). Based on these results, we have reverted to ceftazidime + amikacin as the empirical antibiotics.
Conclusion:
Every institute must have a regular revision of antibiotic policy based on periodic assessment of the clinical and microbiological profile in FN. This will combat antibiotic resistance.
Key words: Blood culture and sensitivity, changing trends, febrile neutropenia, hematological malignancy, solid tumor
INTRODUCTION
Febrile neutropenia (FN) is an oncological emergency. Despite advances in the therapy, including more effective empirical broad-spectrum antibiotics, antifungals, and granulocyte colony-stimulating factors, FN remains a therapeutic challenge. It prolongs hospital stay, increases health-care costs, and compromises chemotherapy efficacy, due to delays and dose reductions.
Bacteremia is the cause of fever in 25% of neutropenic patients.[1] The reported mortality rates vary from 5% to 20%, especially in those with major infections and medical comorbidities. Mortality rates exceed 50% in patients presenting with septic shock or pneumonia, despite prompt antibiotic treatment.[2]
As per the published literature from the West, there has been a substantial change in the epidemiologic spectrum of pathogens isolated from febrile neutropenic patients over the past 40 years. Until the mid-eighties, Gram-negative bacilli (Escherichia coli, Klebsiella species, and Pseudomonas aeruginosa) were the most frequently isolated organisms. Among the Gram-positive cocci, Staphylococcus aureus predominated. Since then, Gram-positive cocci (coagulase-negative staphylococci [CoNS], viridans streptococci) have been the leading cause of infections in FN. Enterococci are in majority responsible for colonization in neutropenic patients and raise the problem of resistance to antibiotics, mainly glycopeptides.[3] However, there are a few reports on the profile of infections in FN from India.[4,5,6,7]
Microbial etiology of FN is often unknown at the time of initiation of treatment. However, choice of empiric therapy should depend on the locally prevalent pathogens and their sensitivity patterns, risk group (high or low risk for complications), potential sites of infection, and the cost of various regimens. The Infectious Diseases Society of America (IDSA) guidelines are being followed by well-recognized treatment centers in India.[3]
This study was designed to review the recent pattern of microbial flora, their susceptibility pattern, and clinical variables among bloodstream infections (BSIs) in febrile neutropenic patients with solid tumors (STs) and hematological malignancies (HM) at a regional cancer center catering to the diagnosis and treatment of cancer patients in South India. As per the institutional protocol to periodically review the antibiotic sensitivity pattern and antibiotic policy so as to curtail the development of resistance, we compared our findings with a study done in our institute in 2010 by Jacob et al.[3]
MATERIALS AND METHODS
Patients
This was a prospective observational study conducted at a tertiary care cancer center. We analyzed the clinical and microbiological profile of BSI that occurred in adult patients (>15 years of age) with FN, diagnosed and treated for HM and ST from September 2012 to September 2014.
Blood cultures and bacteremia
Blood was sampled for cultures when the patient developed fever and prior to initiation of antibiotic therapy, absolute neutrophil count (ANC) was ≤100, and the patient had severe oral mucositis, skin/soft tissue infection, hypotension, pneumonia, and evidence of catheter-related BSI. A blood culture was considered to be positive if one or more samples yielded an organism, with the exception of CoNS, where two separate positive blood cultures were required to be considered a true bacteremia.[8] Samples that represented BSI included peripheral blood, blood drawn through central venous catheters, peripherally inserted central catheters, and catheter tip cultures from patients with an appropriate clinical syndrome. Blood samples were inoculated into BacT/ALERT blood culture bottles and incubated in the BacT/ALERT system. The bacterial isolates were identified by routine biochemical reactions. Antimicrobial susceptibility testing on isolates was performed using VITEK 2C AST cards (bioMérieux Inc., Durham, NC, USA).
Antibiotic use policy
All patients received initial empirical antibiotic therapy with cefoperazone-sulbactam as per the institutional policy. If fever persisted beyond 2 days, we switched to piperacillin-tazobactam or meropenem. In case of further deterioration of clinical status, we added tigecycline or colistin. Vancomycin or teicoplanin was added in the presence of hemodynamic instability, suspected catheter-related infection, skin and soft tissue infection, pneumonia, and severe oral mucositis. Antibiotics were modified based on the culture and sensitivity reports, if clinically indicated.
Use of antifungals
By day 5, if fever persisted despite adequate systemic antibiotics, amphotericin B (AmB) was added. Early escalation of antibiotics and administration of antifungals were done in patients with rapid deterioration prior to this point. The other indications were persistent fever despite 48 h of second-line antibiotic therapy, bronchopneumonia, extensive candidiasis involving tongue, buccal mucosa and palate, suspicion of esophageal candidiasis in patients with oral candidiasis and odynophagia, sinonasal infection – nasal discharge, nose or palatal ulceration, periorbital swelling, and maxillary tenderness. High-resolution computed tomography (HRCT) of the chest was withheld as the poor general condition of our patients forbade us from shifting them for the procedure. Serum galactomannan assay is unavailable in our institution at present. Invasive fungal infections (IFIs) were diagnosed according to the EORTC/MSG 2008 guidelines.[9] Suspected IFI was defined as fever >38°C persisting for >96 h of intravenous antibiotics without positive blood culture.[10] AmB was given as a first-line drug at a dose of 1 mg/kg/day. Second-line antifungals were voriconazole (200 mg PO twice daily) and caspofungin (70 mg infused intravenously over 1 h as a loading dose on day 1 followed by maintenance dose of 50 mg infused over 1 h daily), given in case of failure to respond to AmB, suspected or proven invasive aspergillosis (IA), or renal derangement/severe hypokalemia due to AmB.
Statistical analysis
For outcome analysis, patients were stratified on the basis of age, gender, type of malignancy, severity and duration of neutropenia, presence or absence of hypotension, pneumonia, oral mucositis and gastrointestinal symptoms at presentation, and culture results. The data were expressed in percentages.
Statistical analysis was done using SPSS 13 (SPSS Inc. Released 2004. SPSS for Windows, Version 13.0. Chicago, SPSS Inc.). Data were evaluated by a 2 × 2 contingency table employing the Fisher's exact test. Results of P < 0.05 were statistically significant.
Definitions used in this study
FN: A single oral temperature of 101°F or temperature of >100.4°F for 1 h with an ANC <500/mm3 or an ANC that was expected to decrease to <500/mm3 during the next 48 h.
Profound neutropenia: ANC of <100/mm3
Prolonged neutropenia: Neutropenia lasting >10 days
Hypotension: Systolic blood pressure <100 mmHg.
Clinical sepsis was defined as per the criteria established by the American College of Physicians and Society of Critical Care Medicine, which included temperature >38°C, heart rate >90/min, and respiratory rate >20/min.
Multi-drug resistance (MDR), extremely drug resistant, and pan drug resistant were defined as per the international expert proposal for interim standard definitions for acquired resistance by the European Centre for Disease Prevention and Control and Centre for Disease Control and Prevention.[11]
RESULTS
From September 2012 to September 2014, 379 episodes of FN, among 300 patients, were documented. Out of these, 350 (92%) episodes occurred in patients ≤60 years and 29 (8%) occurred in those >60 years of age. About 223 episodes (59%) were documented in males and 156 (41%) in females.
Majority of the episodes were in patients with HM, namely 236 (62%), while 143 episodes (38%) were in those with ST. Among the HM, acute myeloid leukemia (AML) (25%), acute lymphoblastic leukemia (ALL) (19%), and non-Hodgkin's lymphoma (NHL) (14%) were the most common [Table 1]. Among the ST, head and neck cancers (19%), followed by cancers of breast (15%) and esophagus (11%) were the most common [Table 2].
Table 1.
Incidence of febrile neutropenia in hematological malignancies (n=236)
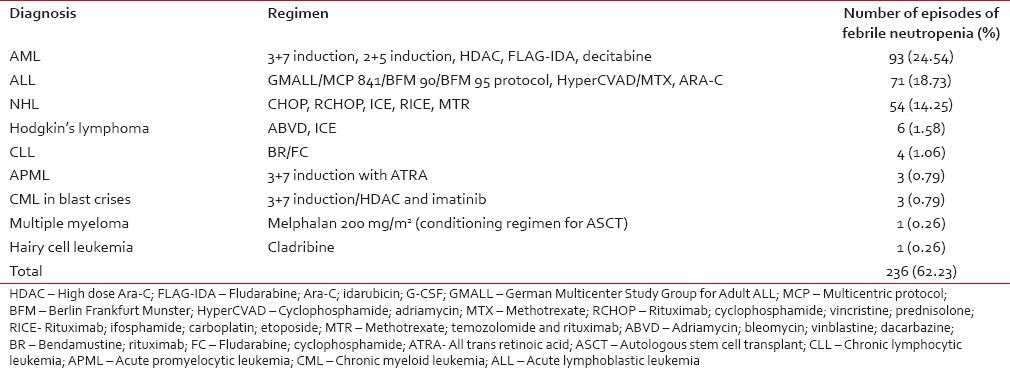
Table 2.
Incidence of febrile neutropenia in solid tumors (n=143)

We looked into the frequency of FN in acute leukemia. Out of 93 episodes in patients with AML, 56 (15%) were in those receiving induction chemotherapy. Among these, 31 were culture-positive. E. coli was the most common organism (10 isolates). About 35 (9%) patients developed FN while receiving consolidation for AML, 14 of which were culture-positive. S. aureus was the most common isolate (6 isolates). Out of 71 episodes in patients with ALL, 49 (13%) were in those receiving induction chemotherapy. Among them, 24 were culture-positive. Acinetobacter baumannii was the most common isolate (5 isolates). Seven patients (2%) developed FN while receiving consolidation for ALL, only one grew P. aeruginosa. The median time for the development of FN postchemotherapy was day 10 (range: day 6–45) for HM and day 11 for ST (range: day 5–23). The median duration of neutropenia for HM and ST was 8 days (range: 3–60 days) and 5 days (range: 3–21 days), respectively. Hypotension was documented in 45 episodes and acute kidney injury in eight. The median nadir total count was 500/mm3 (range 0–1600/mm3), median nadir ANC was 100/mm3 (range: 0–500/mm3), median nadir platelet count was 25,000/mm3 (range 1000/mm3–169000/mm3), and median nadir hemoglobin was 8.2 g/dL (range: 3.8g/dL – 13 g/dL).
Microbiological profile of bloodstream infection
A total of 887 blood cultures from febrile neutropenic patients were drawn. Of these, 137 (15%) yielded growth; 103 in HM and 34 in ST. Identical isolates obtained from a single patient during the same hospitalization were considered as one. Hence, 128 isolates were analyzed; 74 (58%) yielded Gram-negative bacilli, 51 (40%) were positive for Gram-positive cocci, and 3 (2%) grew fungi. Cultures were positive in 73 (57%), 51 (40%), and 4 (3%) of the blood samples drawn from peripheral blood, central venous catheters (subclavian and internal jugular veins), and chemoport, respectively. None of the catheter tip cultures and blood cultures drawn from PICC were positive.
The contribution of various isolates is shown in Table 3. Gram-negative organisms were isolated predominantly (58%). Overall, the most common bacterial isolates were S. aureus (25%), followed in decreasing order of frequency by E. coli (18%), A. baumannii (16%), Klebsiella pneumoniae (12%), and CoNS (9%).
Table 3.
The contribution of various isolates
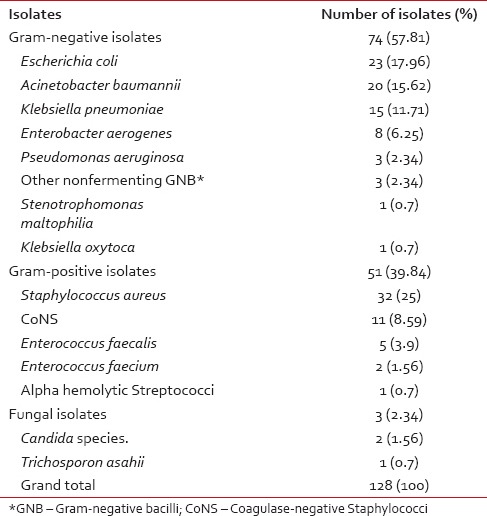
E. coli followed by A. baumannii and K. pneumoniae together accounted for 78% of the Gram-negative organisms.
Staphylococcus species accounted for 84% (43/51) of all Gram-positive isolates, with 32 S. aureus isolates and eleven CoNS.
Polymicrobial bacteremia was defined as isolation of ≥2 pathogens from the same blood sample.[5] Eight had polymicrobial etiology, namely, K. pneumoniae + E. aerogenes, E. coli + E. aerogenes + A. baumannii, E. coli + S. aureus, A. baumannii + K. pneumoniae, E. aerogenes + P. aeruginosa, E. aerogenes + methicillin resistant S. aureus (MRSA), E. coli + K. pneumoniae, and S. aureus + E. aerogenes.
Multi-drug resistant and extended spectrum beta-lactamase isolates
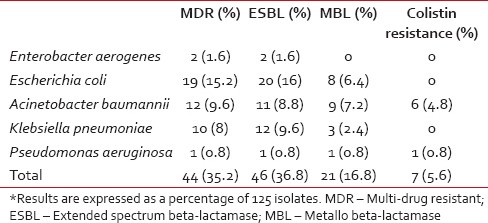
Among the MDR bacteria, 16 (13%) were Gram-positive and 44 (35%) were Gram-negative [Tables 4 and 5]. There were 46 extended spectrum beta-lactamase (ESBL) producers and 21 metallo beta-lactamase producers among the Gram-negative isolates. There were ten MRSA and MDR isolates, mainly consisting of E. coli, A. baumannii, K. pneumonia, and S. aureus. Chief among the ESBL producers were E. coli (87% of the E. coli isolates), K. pneumoniae (80% of the K. pneumoniae isolates), and A. baumannii (55% of the A. baumannii isolates).
Table 4.
Multi-drug resistant Gram-positive bacteria
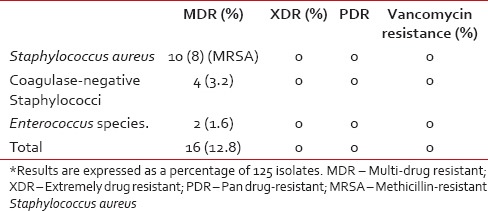
Table 5.
Multi-drug resistant Gram-negative Bacilli
Fungal Isolates
Fungal infections comprised 2% (3/128) of isolates; two of these being Candida species and one being Trichosporon asahii.
Antibiotic sensitivity patterns of Gram-negative organisms
The antibiotic sensitivity pattern of various Gram-negative isolates is shown in Table 6. A very high degree of resistance was noted to cephalosporins. Only 13% of E. coli, 33% of Klebsiella, and 45% of A. baumannii were susceptible to cephalosporins, whereas 75% of E. aerogenes were susceptible to cephalosporins. Among the third-generation cephalosporins, while only 39% of the isolates were sensitive to cefotaxime and ceftriaxone, 76% retained sensitivity to ceftazidime. On the other hand, 95% of A. baumannii, 60% of K. pneumoniae, and 67% of P. aeruginosa were susceptible to ceftazidime.
Table 6.
Antibiotic sensitivity pattern in (%) of the most prevalent Gram-negative bacteria
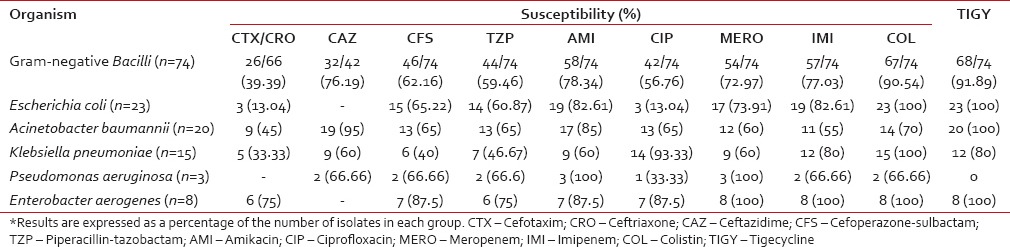
In overall activity against Gram-negative isolates, the beta-lactam/beta-lactamase inhibitor combinations fared better than most cephalosporins with the exception of ceftazidime. The sensitivity of cefoperazone-sulbactam and piperacillin-tazobactam was 62% and 60%, respectively. The highest resistance to this group of antibiotics was seen in K. pneumoniae isolates, with 60% being resistant to cefoperazone-sulbactam and 53% being resistant to piperacillin-tazobactam. On the other hand, E. coli, A. baumannii, P. aeruginosa, and E. aerogenes showed resistance ranging from 12 to 40%.
Amikacin had a higher overall activity against the Gram-negative isolates (78%) than most other antibiotics with the exception of colistin and tigecycline. More than 80% isolates of E. coli, A. baumannii, E. aerogenes, and 100% of P. aeruginosa were susceptible to Amikacin. The highest resistance to this antibiotic was seen in the K. pneumoniae group, with 40% being resistant.
Among the quinolones, ciprofloxacin showed poor efficacy against most of the Gram-negative isolates, especially the E. coli and P. aeruginosa. However, it is noteworthy that nearly 93% of the K. pneumoniae isolates were sensitive to ciprofloxacin.
Imipenem and meropenem demonstrated more than 70% efficacy toward the Gram-negative isolates. None of the isolates of P. aeruginosa and E. aerogenes were resistant to meropenem. However, resistance to carbapenems was emerging with nearly 40% of strains of A. baumannii and K. pneumoniae showing resistance.
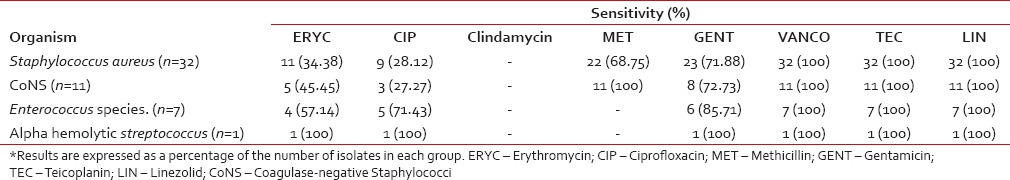
Antibiotic sensitivity patterns for Gram-positive cocci
The antibiotic sensitivity pattern for Gram-positive cocci is shown in Table 7. No resistance was noted in our study to these three antibiotics. However, high degrees of resistance (>50%) to ciprofloxacin and erythromycin were noted, especially among the Staphylococcus species. Methicillin resistance was demonstrated in 31% of S. aureus isolates. None of the CoNS isolates were resistant to methicillin.
Table 7.
Antibiotic sensitivity pattern in (%) most of the prevalent Gram-positive bacteria
Response to antibiotics
All patients received initial empirical antibiotic therapy with cefoperazone-sulbactam. In our study, meropenem was used in 77 episodes (20%), vancomycin in 62 (16%), piperacillin-tazobactam in 58 episodes (15%), and amikacin in 145 (38%). Imipenem, teicoplanin, linezolid, colistin, and tigecycline were used in 36 (10%), 14 (4%), 10 (3%), 3 (0.8%), and 2 (0.5%) of episodes, respectively. Colistin was used in three episodes in the setting of induction chemotherapy for AML. It was initiated empirically when the patients continued to be febrile despite receiving meropenem and antifungal therapy. The blood cultures were negative in all instances.
Invasive fungal infections
Antifungals were used in 126 episodes (33.2%). There were 18% possible, 14% suspected, 0.8% proven, and 0.5% probable IFI. Of the proven IFI, there were two episodes of candidemia, one in a patient receiving first cycle of MTR protocol for primary CNS lymphoma and another in a patient with relapsed ALL receiving second cycle of HyperCVAD. One patient receiving induction therapy for AML developed FN with T. asahii fungemia. There was one case of pulmonary aspergillosis (probable IFI), wherein the patient had FN during induction therapy for AML; chest X-ray revealed multiple fungal balls and sputum culture grew Aspergillus. Initial empirical antifungal therapy with AmB was used in 124 episodes. Two patients received caspofungin as first-line therapy in view of renal failure. Second-line voriconazole and caspofungin were used in 3 and 5 episodes, respectively.
Mortality
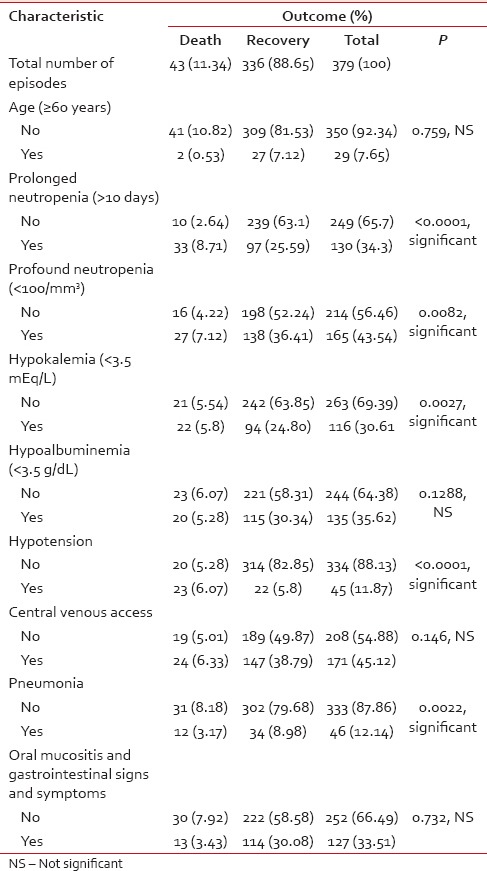
Mortality was considered attributable to the bloodstream pathogen if the patient died within 7 days of bacteremia and there was no other discernible cause. There were 43 deaths, 29 in those with HM and 14 in those with ST. Hence, the overall mortality rate was 11%, with 8% in HM and 4% in ST. Among HM, 17 deaths (45%) were during the induction phase of AML, 4 (1%) each during induction therapy for ALL and chemotherapy for NHL, 2 (0.5%) in patients receiving consolidation for AML, and 1 (0.3%) each during the first cycle of chemotherapy for Hodgkin's lymphoma and induction therapy for AML-M3. Among the ST, there were six deaths (1.6%) in patients receiving chemotherapy for HNSCC, 3 (0.8%) in patients with breast cancer, two (0.5%) in patients with gastric cancer, and 1 (0.3%) each in those with choriocarcinoma, esophageal cancer, and osteosarcoma. Fifteen (35%) had positive blood cultures, out of which 5 (12%) had Gram-positive isolates and 10 (23%) had Gram-negative isolates. Among the Gram-positive isolates, S. aureus and Enterococcus species. were isolated in 4 (9%) and 1 (2%) episodes, respectively. Among the Gram-negative isolates, E. coli and Klebsiella were isolated in 5 (12%) and 2 (5%) episodes, respectively. E. aerogenes, A. baumannii, and P. aeruginosa were isolated from one blood culture each. We analyzed the various factors associated with mortality in FN patients. Correlation between mortality due to FN and other variables is shown in Table 8. Prolonged neutropenia, profound neutropenia, hypokalemia (<3.5 mEq/L), hypotension, and presence of pneumonia were significantly associated with mortality in our study.
Table 8.
Various factors associated with mortality in febrile neutropenia
DISCUSSION
Periodic assessment of pattern of infections and antibiotic sensitivity plays a vital role in modulating anti-microbial policy so as to reduce infection-related morbidity and mortality. In a previous study from our institute by Jacob et al.,[3] in the year 2010, when empirical antibiotics used as a part of institutional protocol were a combination of ceftazidime and amikacin, it was found that the sensitivity to ceftazidime and amikacin was 50% and 83%, respectively. This prompted us to switch over to cefoperazone-sulbactam, which was more efficacious with an overall sensitivity of 83%. In the present study, we wanted to determine the predominant isolates causing BSI in patients with FN and their antibiotic sensitivity patterns. We also investigated the clinical, biochemical, and microbiological factors associated with mortality and their significance.
Indian data on FN have largely been on patients with HM.[4,5,6,7] This prospective, observational study included febrile neutropenic patients of HM and ST. In this study, we found that FN episodes were more frequent in patients with HM. Out of 164 FN episodes in patients with acute leukemia, 105 (64%) were in those receiving induction chemotherapy. This is similar to the data published by Jagarlamudi et al.[12]
Gram-negative bacilli were the predominant cause of infections in FN at our center. A rising incidence of Gram-positive bacteremia in febrile neutropenic patients has been reported over the past three decades, especially from the developed countries.[13,14,15] This has been attributed to the increasing use of indwelling venous catheters, oral fluoroquinolone prophylaxis, use of aggressive anti-neoplastic regimes causing severe oropharyngeal mucositis and bowel damage, and H2 receptor blockers.[16] In our study, 57% episodes were associated with an indwelling catheter. Predominance of Gram-negative bacilli in FN has been well established by several studies done in India and other developing countries.[5,6,7,17,18,19] Our study reinforces this clinical and microbiological data and also demonstrates that the accepted trend in the switch to Gram-positive cocci in febrile neutropenic patients may be the phenomenon noted only in the developed countries.
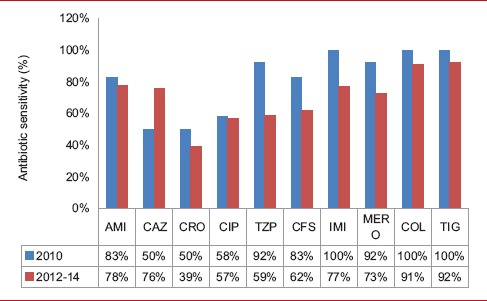
As per our institute isolates, we have noted a changing trend in the antibiotic sensitivity pattern over the years [Table 9]. It is noteworthy to observe a significant reduction in the sensitivity to several antibiotics over time. Following the switch in empirical antibiotics based on the results of the study by Jacob et al.,[3] the activity of the primary agent cefoperazone-sulbactam dropped. Similar significant reduction in susceptibility was noted for piperacillin-tazobactam, imipenem and meropenem. On the contrary, a marked increase in sensitivity to ceftazidime was noted. It was the empirical antibiotic of choice in combination with amikacin in the previous antibiotic regimen and was discontinued from use from 2012 after the results of the previous study were analyzed. The activity of amikacin remained almost the same. We found this changing trend very interesting, re-emphasizing the need for antibiotic cycling, which has been suggested as a method for decreasing antibiotic resistance.[20] Considering the results of the present study, we have reverted to ceftazidime and amikacin as the empirical antibiotics for FN. We also observed that colistin and tigecycline were not spared; with nearly 30% of isolates of A. baumannii and P. aeruginosa having shown resistance to colistin and 20% of K. pneumoniae and 100% of P. aeruginosa being resistant to tigecycline. In our study, the most active antimicrobial agent against E. coli and K. pneumoniae was colistin and that against A. baumannii was tigecycline. Amikacin and meropenem showed maximum activity against P. aeruginosa.
Table 9.
Change in antibiotic sensitivity pattern over the years
Multi-drug resistant Gram-negative bacteria accounted for 35% of the isolates with a high prevalence of ESBL producing E. coli, K. pneumoniae and A. baumannii. Similar findings have been reported by other studies from the subcontinent.[6,18,21] This highlights an increase in the resistance to third-generation cephalosporins, which are used in empirical therapy of FN.
S. aureus continued to be the predominant Gram-positive isolate at our institute. Fortunately, vancomycin, linezolid, and teicoplanin continued to be 100% effective, confirming the results of the study by Jacob et al.[3] This was attributed to the judicious use of these antibiotics. However, there was a significant resistance to erythromycin and ciprofloxacin, the most resistant isolate being S. aureus.
Although the occurrence of MRSA was lower in our study as compared to similar studies from other institutes in India,[22] it was higher than that reported by Jacob et al.[3] None of these strains were vancomycin-resistant. This reinforces the fact that strict regulation of the use of vancomycin must be practiced in the areas of low prevalence of MRSA, and its indiscriminate use must be avoided.
The incidence of fungal infections in our study is similar to that reported by Ghosh et al.[23] The rising trend has been attributed to large scale construction in the vicinity of hospitals and use of intensive regimen with prolonged and severe neutropenia.[7] Construction work was in progress in our institute at the time of this study, and we had one case of pulmonary aspergillosis (probable IFI). Extensive use of prophylactic antifungals results in the development of resistance. Hence, we have reserved prophylactic fluconazole for those receiving FLAG-IDA and HyperCVAD for relapsed acute leukemia and high-dose chemotherapy in ASCT. Definitive diagnosis was rarely achieved due thrombocytopenia and difficulty in performing invasive procedures such as FNAC and biopsy in critically ill patients. We are considering the implementation of serum galactomannan assay to aid in the diagnosis of IA and monitor the duration of antifungal therapy.
The mortality rate of 11% in our study is comparable with other published data.[23] Prolonged and profound neutropenia, hypokalemia, hypotension, and pneumonia were associated with mortality. Judicious selection of first- and second-line antibiotics, prompt initiation of antifungal therapy, aggressive correction of dyselectrolytemia, and hypotension will help improve outcomes.
CONCLUSION
This single-center experience emphasizes the fact that every institute must have a periodic assessment of the clinical variables, microbial data, and mortality in FN. This should form the basis of a regular revision of antibiotic-prescribing policy to combat the growing menace of antibiotic resistance and avoid irrational use of antibiotics. In our experience, ceftazidime + amikacin or cefoperazone-sulbactam is the reasonable option for first-line therapy. AmB is a good option for empirical antifungal therapy. Careful selection of antibiotics considering the clinical features and treatment details of each patient and early institution of antifungals, whenever there is a high index of suspicion of IFI, may reduce the morbidity and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33:947–53. doi: 10.1086/322604. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Rolston KV. How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract. 2010;6:149–52. doi: 10.1200/JOP.091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob LA, Lakshmaiah KC, Govindbabu K, Suresh TM, Lokanatha D, Sinha M, et al. Clinical and microbiological profile of febrile neutropenia in solid tumors and hematological malignancies at a tertiary cancer care center in South India. Indian J Cancer. 2014;51:464–8. doi: 10.4103/0019-509X.175330. [DOI] [PubMed] [Google Scholar]
- 4.Kumar L, Kochupillai V, Bhujwala RA. Infections in acute myeloid leukemia. Study of 184 febrile episodes. J Assoc Physicians India. 1992;40:18–20. [PubMed] [Google Scholar]
- 5.Mathur P, Chaudhry R, Kumar L, Kapil A, Dhawan B. A study of bacteremia in febrile neutropenic patients at a tertiary-care hospital with special reference to anaerobes. Med Oncol. 2002;19:267–72. doi: 10.1385/MO:19:4:267. [DOI] [PubMed] [Google Scholar]
- 6.Swati M, Gita N, Sujata B, Farah J, Preeti M. Microbial etiology of febrile neutropenia. Indian J Hematol Blood Transfus. 2010;26:49–55. doi: 10.1007/s12288-010-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Singh M, Singh H, Kumar L, Sharma A, Bakhshi S, et al. Infections in acute myeloid leukemia: An analysis of 382 febrile episodes. Med Oncol. 2010;27:1037–45. doi: 10.1007/s12032-009-9330-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosa RG, Dos Santos RP, Goldani LZ. Mortality related to coagulase-negative staphylococcal bacteremia in febrile neutropenia: A cohort study. Can J Infect Dis Med Microbiol. 2014;25:e14–7. doi: 10.1155/2014/702621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organisation for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious disease mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandhaniya S, Iqbal S, Sharawat SK, Xess I, Bakhshi S. Diagnosis of invasive fungal infections using real-time PCR assay in paediatric acute leukaemia induction. Mycoses. 2012;55:372–9. doi: 10.1111/j.1439-0507.2011.02157.x. [DOI] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Jagarlamudi R, Kumar L, Kochupillai V, Kapil A, Banerjee U, Thulkar S. Infections in acute leukemia: An analysis of 240 febrile episodes. Med Oncol. 2000;17:111–6. doi: 10.1007/BF02796205. [DOI] [PubMed] [Google Scholar]
- 13.Johansson PJ, Sternby E, Ursing B. Septicemia in granulocytopenic patients: A shift in bacterial etiology. Scand J Infect Dis. 1992;24:357–60. doi: 10.3109/00365549209061343. [DOI] [PubMed] [Google Scholar]
- 14.Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Ann Intern Med. 1993;119(7 Pt 1):584–93. doi: 10.7326/0003-4819-119-7_Part_1-199310010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Rubio M, Palau L, Vivas JR, del Potro E, Diaz-Mediavilla J, Alvarez A, et al. Predominance of gram-positive microorganisms as a cause of septicemia in patients with hematological malignancies. Infect Control Hosp Epidemiol. 1994;15:101–4. doi: 10.1086/646869. [DOI] [PubMed] [Google Scholar]
- 16.Giamarellou H, Antoniadou A. Infectious complications of febrile leukopenia. Infect Dis Clin North Am. 2001;15:457–82. doi: 10.1016/s0891-5520(05)70156-2. [DOI] [PubMed] [Google Scholar]
- 17.Zahid KF, Hafeez H, Afzal A. Bacterial spectrum and susceptibility patterns of pathogens in adult febrile neutropenic patients: A comparison between two time periods. J Ayub Med Coll Abbottabad. 2009;21:146–9. [PubMed] [Google Scholar]
- 18.Butt T, Afzal RK, Ahmad RN, Salman M, Mahmood A, Anwar M. Bloodstream infections in febrile neutropenic patients: Bacterial spectrum and antimicrobial susceptibility pattern. J Ayub Med Coll Abbottabad. 2004;16:18–22. [PubMed] [Google Scholar]
- 19.Kanafani ZA, Dakdouki GK, El-Chammas KI, Eid S, Araj GF, Kanj SS. Bloodstream infections in febrile neutropenic patients at a tertiary care center in Lebanon: A view of the past decade. Int J Infect Dis. 2007;11:450–3. doi: 10.1016/j.ijid.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Craig M, Cumpston AD, Hobbs GR, Devetten MP, Sarwari AR, Ericson SG. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a haematological malignancy and transplantation unit. Bone Marrow Transplant. 2007;39:477–82. doi: 10.1038/sj.bmt.1705591. [DOI] [PubMed] [Google Scholar]
- 21.Irfan S, Idrees F, Mehraj V, Habib F, Adil S, Hasan R. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: A descriptive study. BMC Infect Dis. 2008;8:80. doi: 10.1186/1471-2334-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhash K, Medhekar A, Ghadyalpatil N, Noronha V, Biswas S, Kurkure P, et al. Blood stream infections in cancer patients: A single center experience of isolates and sensitivity pattern. Indian J Cancer. 2010;47:184–8. doi: 10.4103/0019-509X.63019. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh I, Raina V, Kumar L, Sharma A, Bakhshi S, Thulkar S, et al. Profile of infections and outcome in high-risk febrile neutropenia: Experience from a tertiary care cancer center in India. Med Oncol. 2012;29:1354–60. doi: 10.1007/s12032-011-9858-3. [DOI] [PubMed] [Google Scholar]


