Abstract
Objective:
To determine the incidence of parkinsonism in community-dwelling older adults without Parkinson disease.
Methods:
Four parkinsonian signs were assessed with a modified motor portion of the Unified Parkinson's Disease Rating Scale in 2,001 older adults without parkinsonism. We used Cox proportional hazards models to determine the associations of age and sex with incident parkinsonism (2 or more signs). We calculated the number of events per 1,000 person-years of observation in 3 age strata. Next, we investigated several potential risk factors for incident parkinsonism. Then, we examined longitudinal progression of parkinsonism using discrete-time multistate Markov models.
Results:
Average age at baseline was 76.8 years (SD 7.62 years). During an average of 5 years of follow-up, 964/2,001 (48.2%) developed parkinsonism. Age (hazard ratio [HR] 1.09, 95% confidence interval [CI] 1.08–1.10) but not male sex (HR 1.06, 95% CI 0.91–1.23) was associated with incident parkinsonism. The incidence of parkinsonism per 1,000 person-years of follow-up was 36.0 for adults <75 years of age, 94.8 for those 75–84, and 160.5 for those 85 years or older. Depressive symptoms, neuroticism, urinary incontinence, sleep complaints, and chronic health conditions were associated with incident parkinsonism. Secondary analyses suggest that risk factors are linked with incident parkinsonism via early motor signs of parkinsonism and cognitive function. Transition modeling suggests that while parkinsonism may fluctuate, it is progressive in most older adults and its risk factors increase the odds of its progression.
Conclusions:
Parkinsonism is common in older adults and increases with age. Identifying modifiable risk factors may decrease the magnitude of this growing public health problem.
Motor impairments such as parkinsonian signs are common in older adults without Parkinson disease (PD) and are associated with a wide range of adverse health outcomes. Cross-sectional studies suggest that parkinsonian signs may affect up to half of older adults.1 Since parkinsonism is strongly associated with age, its incidence is likely to increase in our aging population and could constitute a major public health problem. Nonetheless, little data are available regarding age- and sex-specific incidence rates of parkinsonism.
In this study, we employed a previously validated categorical measure of parkinsonism to determine the age- and sex-specific incidence of parkinsonism in 2,000 older community-dwelling older adults.2 Then we examined motor and cognitive measures that might capture early signs of parkinsonism and several possible risk factors to determine which were associated with incident parkinsonism.
METHODS
Participants.
Participants were from the Religious Orders Study (ROS) and Memory and Aging Project (MAP), which employ similar data collection procedures, allowing for combined analyses.3,4 Participation in the annual follow-up evaluations and autopsy rate exceeds 90%. At the time of these analyses, 3,061 participants had completed their baseline clinical assessment of parkinsonism.
To investigate incident parkinsonism, we eliminated 309 patients who did not have follow-up assessment (died before first follow-up [n = 105] or had not reached second assessment [n = 204]), and 703 individuals with parkinsonism, the condition of interest, and 48 treated with medications that could mimic or mask this condition (a history of PD treated with dopaminergic medications [n = 29] or those receiving neuroleptic medications [n = 19]).
Assessment and categorization of parkinsonism.
The annual evaluation includes a modified version of the United Parkinson's Disease Rating Scale.3,4 There were 26 items that assessed 4 parkinsonian signs and were summarized as a continuous global parkinsonian score (appendix e-1 at Neurology.org).5,6 Categories of parkinsonism, previously validated (appendix e-1), were based on the number of parkinsonian signs present.2 A sign was present if 2 or more of their respective items had at least a score of 1 indicating a mild abnormality. Parkinsonism was present if 2 or more signs were documented. Possible parkinsonism was present if there was 1 sign and no parkinsonism if there were none.2
Demographics and other predictors.
Age, sex, and years of education were recorded at baseline interview. Global motor score summarized 10 motor performances (appendix e-1).7 Physical activity was based on self-reported exercise.3 A composite measure summarized 19 cognitive tests.3 A 10-item version of the Center for Epidemiologic Studies Depression Scale assessed depressive symptoms.3 Six items from the Neuroticism-Extraversion-Openness Personality Inventory–Revised scale assessed neuroticism.3 Chronic health conditions included 7 self-reported health conditions, smoking, and measured body mass index (BMI).3 Urinary incontinence and sleep quality were self-reported.3,8
Statistical analyses.
We employed a set of discrete-time Cox proportional hazards models to examine the association of age and sex with incident parkinsonism, defined as the first instance of parkinsonism. We calculated incidence rates for 3 age strata by counting the number of incident cases in each age group and dividing by the number of person-years at risk in the age interval. Person-years from a single individual were included in more than one age stratum as appropriate. As illustrated in the figure, we employed a multistate Markov model for longitudinal data with categorical responses to assess the transitions between different categories of parkinsonism and death. This is described more fully in appendix e-1.9,10 Then, we conducted a series of discrete-time Cox proportional hazards models to examine the associations of baseline measures of motor and cognitive function and a third group of potential risk factors with incident parkinsonism. We then added a term to determine if these associations varied with age. We examined each of these predictors alone and then together as groups of covariates to identify which predictors showed independent associations with incident parkinsonism. In a final model that included all 3 groups of predictors, we examined whether motor and cognitive function attenuated the association of potential risk factors with incident parkinsonism. If motor and cognitive function mediates the association (i.e., is a critical step in the causal chain linking risk factors to incident parkinsonism), then the effect of risk factors on incident parkinsonism should be markedly reduced. Finally, we examined whether risk factors for incident parkinsonism affected the transitions between categories of parkinsonism. A priori level of statistical significance was 0.05. Deviance tests were used to compare models with different blocks of predictors. Models were examined graphically and analytically and assumptions were judged to be adequately met. Programming was done in SAS version 9.3 (SAS Institute Inc., Cary, NC).11
Figure. Possible visit-to-visit transitions between categories of parkinsonism and death.
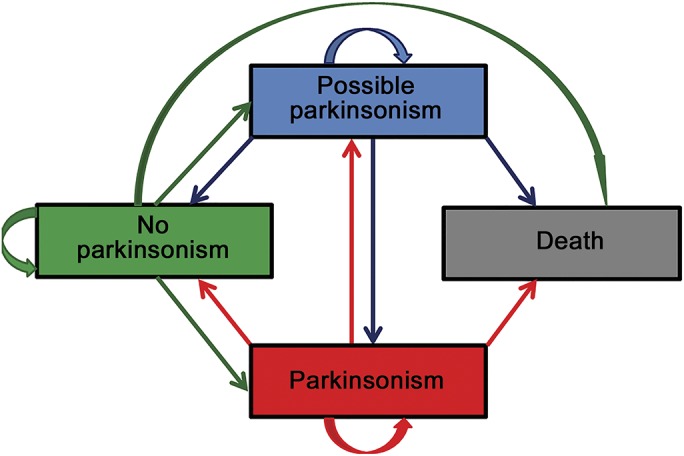
The categories of parkinsonism (no, possible, and yes) are not absorbing because individuals may (1) stay the same (category directed arrow), (2) improve, or (3) worsen from visit to visit (bidirectional arrows), or (4) they may die, an absorbing state (unidirectional arrows). A multistate model can be employed to examine the probability of visit-to-visit transitions between the categories of parkinsonism or death and the relationship of risk factors to these probabilities.
Standard protocol approvals, registrations, and patient consents.
Both studies were approved by the Institutional Review Board of Rush University Medical Center. Written informed consent and an anatomic gift act for brain donation at the time of death was obtained from all study participants.
RESULTS
Parkinsonian signs at study baseline.
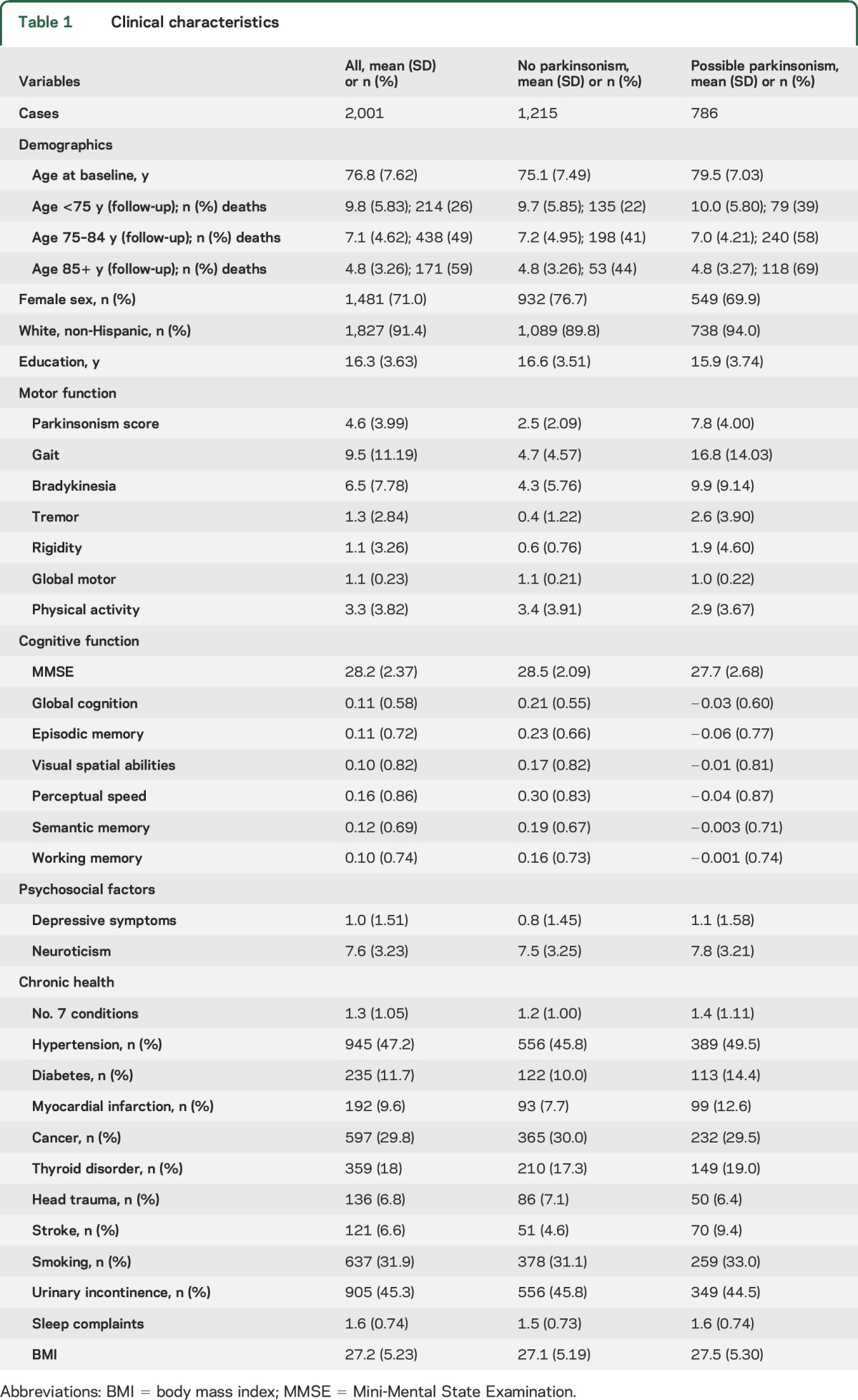
There were 2,001 individuals without a history of PD or parkinsonism at baseline included in these analyses (ROS: 895; MAP: 1,106). More than 60% (n = 1,215, 60.7%) had no parkinsonian signs. The remainder had only a single parkinsonian sign: 20% had signs of parkinsonian gait (n = 399, 19.9%) and the remaining had tremor (n = 225, 11.2%), bradykinesia (n = 101, 5.1%), or rigidity (n = 61, 3.1%). Other clinical characteristics at baseline are included in table 1.
Table 1.
Clinical characteristics
Incident parkinsonism.
During 5 years of follow-up (mean 5.4 years, SD = 4.60 years), 964 of 2,001 (48.2%) developed parkinsonism. The incidence of parkinsonism per 1,000 person-years of follow-up was 89.9/1,000 person-years (36.0 for adults <75 years of age, 94.8 for those 75–84, and 160.5 for those 85 years or older).
At the time of incident parkinsonism, most showed 2 signs (n = 800, 83.0%), but some showed 3 signs (n = 142, 14.7%) or 4 signs (n = 20, 2.1%). Parkinsonian gait (n = 843, 87.4%) was the most common sign, which occurred in combination with one or more of the other signs.
Demographic factors and incident parkinsonism.
A higher hazard for parkinsonism was strongly related to age (hazard ratio [HR] 1.09 [95% confidence interval (CI) 1.08–1.10]) but not sex (HR 1.06 [95% CI 0.91–1.23]) or education (HR 0.99 [95% CI 0.97–1.01]). The association of age did not vary by sex (age × sex HR 1.01 [95% CI 0.99–1.03]).
Transitions between categories of parkinsonism.
Possible parkinsonism may represent a less severe stage of parkinsonism that may progress.2 As illustrated in the figure, parkinsonism can improve or worsen from visit to visit. We employed a multistate model to examine the visit-to-visit transitions between the categories of parkinsonism or death (appendix e-1).9,10
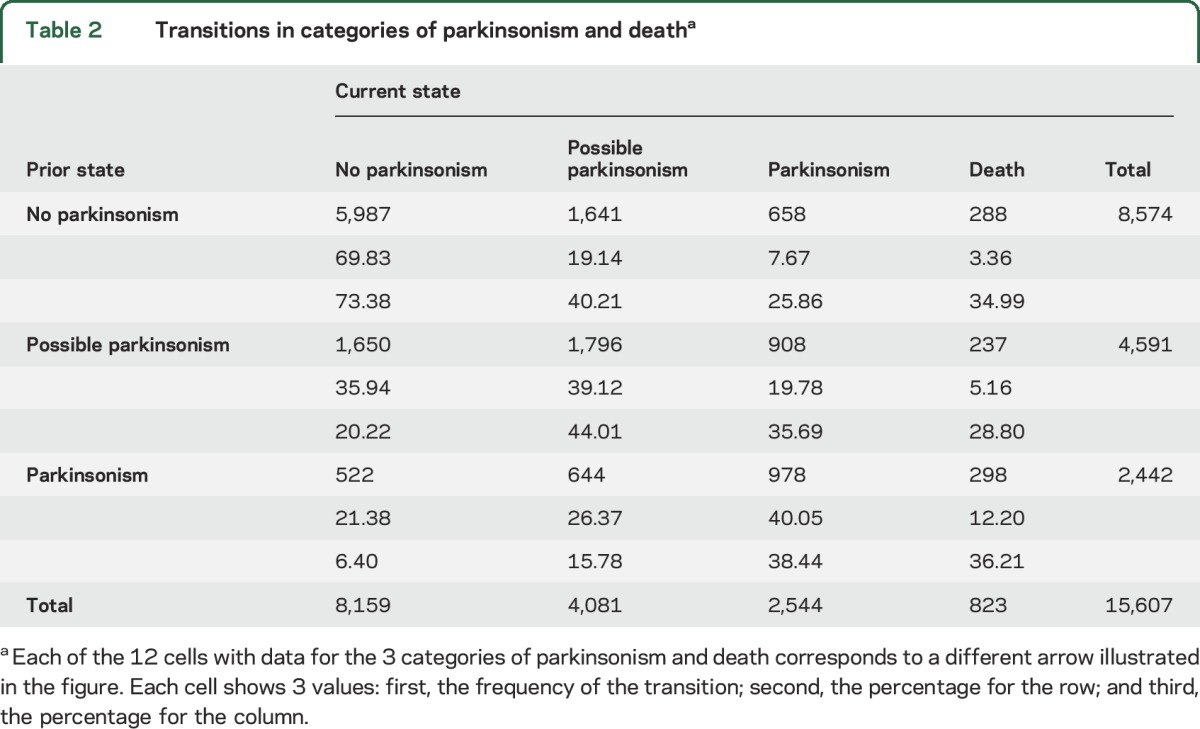
Table 2 summarizes the more than 15,000 transitions recorded for the participants included in these analyses. For each of the parkinsonism categories, participants were more likely to remain in that category at the next annual assessment than to transition to another category (e.g., 69.8% of those without parkinsonism at the previous examination remained without parkinsonism at their subsequent examination). Thus, for a total of 8,761 (56.1%) pairs of consecutive assessments, the categories remained the same. However, in the remaining 6,846 (43.9%) pairs of assessments, transition to a different category occurred, including worsening in parkinsonism (n = 4,857, 31.1%), an improvement in parkinsonism (n = 1,166, 7.5%), and death (823, 5.3%).
Table 2.
Transitions in categories of parkinsonism and deatha
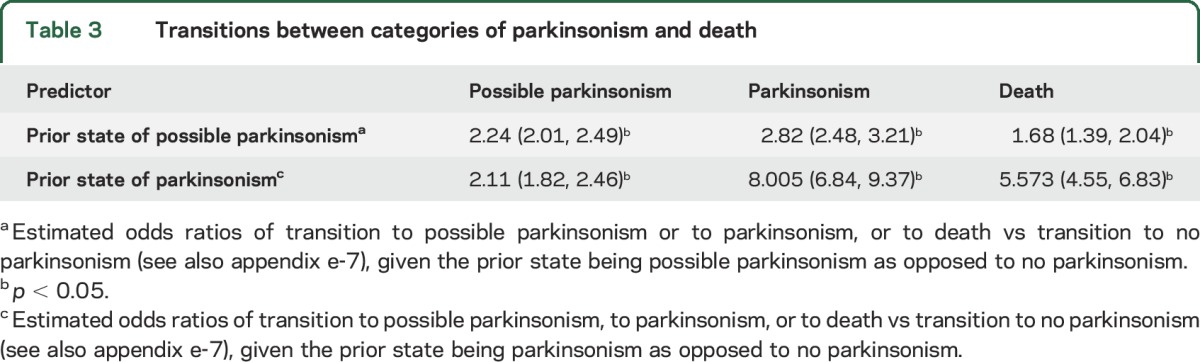
Results from the multistate model show that compared to no parkinsonism, individuals with possible parkinsonism showed a higher odds of remaining as possible parkinsonism, or progressing to parkinsonism or to death at their next assessment. Similarly, individuals with parkinsonism showed a higher odds of continued parkinsonism or death at their next assessment (table 3).
Table 3.
Transitions between categories of parkinsonism and death
Risk factors for incident parkinsonism.
First, we examined the associations of several predictors with incident parkinsonism in a series of separate Cox proportional models adjusted for age and sex. These separate analyses showed that several measures that describe early signs of parkinsonism including a higher global parkinsonian score, a lower global motor score, less physical activity, and lower cognitive function were associated with incident parkinsonism. Risk factors for parkinsonism included more depressive symptoms and neuroticism, more sleep complaints and chronic health conditions, and more frequent urinary incontinence (table 4, column B). These factors also predicted possible parkinsonism (table 4, column D).
Table 4.
Risk factors associated with incident possible parkinsonism and parkinsonism
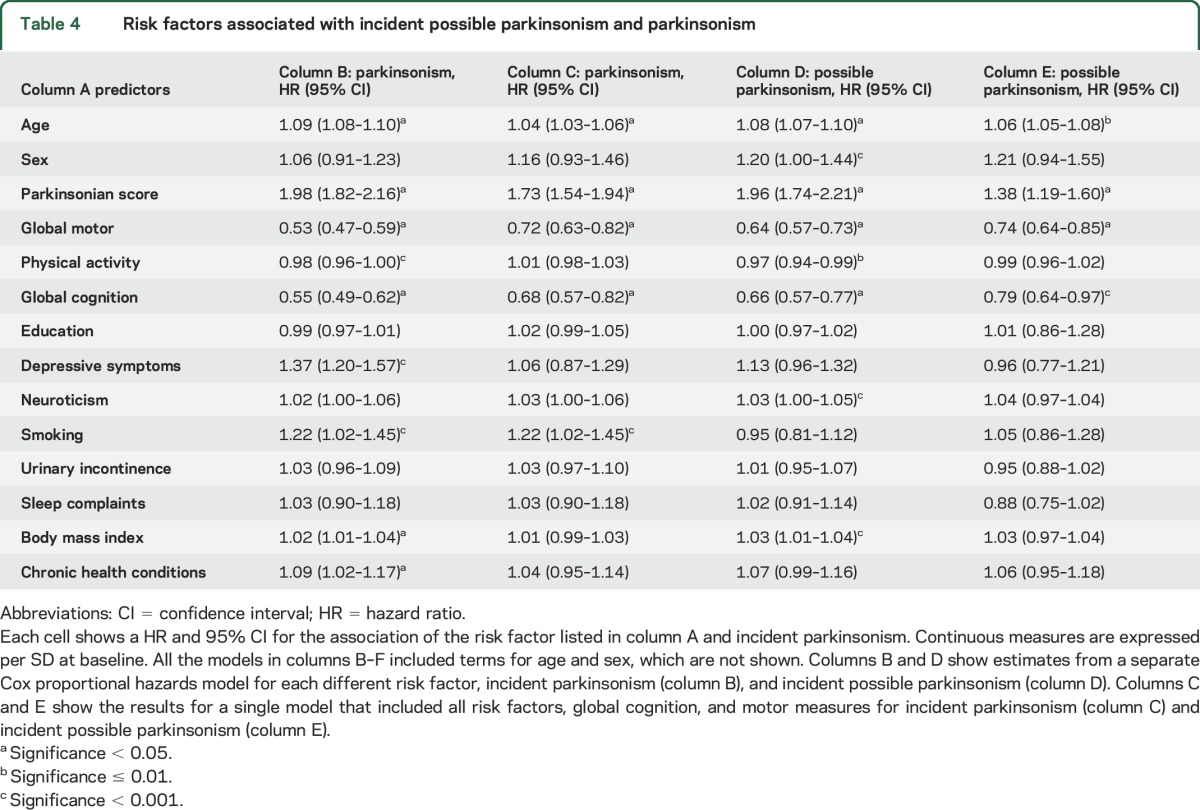
Although the incidence of parkinsonism was higher in older persons, the associations of nonmotor predictors with incident parkinsonism did not vary by age. In contrast, the association of baseline global parkinsonism and motor scores varied with age at baseline (appendix e-2).
Independent predictors of early motor signs of parkinsonism.
Several motor measures may be necessary to describe early manifestations of parkinsonism.12,13 More severe baseline global parkinsonian score, a lower global motor score, and less physical activity were associated with a higher risk of parkinsonism (table 4, column C). Next, by including all terms in a single model, we found that both global parkinsonian score and global motor score were independently associated with incident parkinsonism (appendix e-3, model 1).
Next, we examined which components of these summary motor measures predicted incident parkinsonism. All 4 parkinsonian signs were independent predictors of incident parkinsonism (appendix e-4, model 1). Dexterity and gait remained independently associated with incident parkinsonism, but not strength (appendix e-4, model 2).
Intact cognition is essential for control of volitional movement and would likely be necessary to capture early parkinsonism. Global cognition was associated with incident parkinsonism (table 4, column B). The cognitive abilities used to construct global cognition did not contribute differently to incident parkinsonism when included together in a single model (χ2 [df = 4] = 3.07, p = 0.55) (appendix e-4, model 3).
Next, we examined early motor signs of parkinsonism and cognitive function together in a single model and both were independently associated with incident parkinsonism (appendix e-3, model 2).
Independent risk factors for incident parkinsonism.
We examined potential risk factors for parkinsonism in a single model to see which showed independent associations with incident parkinsonism. Depressive symptoms, neuroticism, and BMI showed independent associations with incident parkinsonism (appendix e-3, model 3).
In a final model, we examined whether the associations of risk factors with incident parkinsonism were attenuated when motor and cognitive functions were included with risk factors in a single model. Motor signs and cognition remained associated with incident parkinsonism. By contrast, the associations of nearly all the risk factors with incident parkinsonism were attenuated and were no longer associated with incident parkinsonism (table 4, column C). This suggests that motor and cognitive function may be a step in the causal chain linking (mediating) the association of risk factors with incident parkinsonism. This pattern was similar in all 3 age strata described above (appendix e-5). This pattern was also observed for incident possible parkinsonism (table 4, column E).
Risk factors predicting incident parkinsonism also predicted incident parkinsonian gait (appendix e-6).
Risk factors and transitions between categories of parkinsonism and death.
Next we examined whether the risk factors for incident parkinsonism (age, global scores for parkinsonism, motor and cognitive performance) affected the odds of transitions among the categories of parkinsonism and death. Overall, older age and poorer cognitive and motor function were associated with a higher odds of developing possible parkinsonism and parkinsonism or death (appendix e-7).
DISCUSSION
In this study of older adults, almost half of the participants developed parkinsonism during follow-up. The incidence of parkinsonism increased with age, but the risk did not vary by sex. More depressive symptoms, neuroticism, and higher BMI showed independent associations with incident parkinsonism. These factors may be linked with incident parkinsonism via early motor signs of parkinsonism and cognitive function. Although parkinsonism may fluctuate over time, it is progressive in most older adults and risk factors for its incidence also increase the odds of its progression. Further work is needed to identify modifiable risk factors to decrease the development of parkinsonism in older adults.
There are currently about 40 million people over the age of 65 in the United States, and by 2030, this number is projected to increase to 70 million and the most rapid increase is projected to occur in adults older than 80.14 In prior cross-sectional studies, estimates of the prevalence of parkinsonism have varied from 15% to up to 50% of older adults.1,15,16 Prospective data providing estimates of its incidence and risk factors are crucial for the rational allocation of health resources and research efforts to decrease parkinsonism in older adults. The current study fills an important gap in our knowledge about parkinsonism by providing prospective data on age- and sex-specific incidence rates of parkinsonism in older adults without a clinical diagnosis of PD. Almost half of the participants in the current study, whose average age was about 75 at study baseline, developed parkinsonism during follow-up. Since parkinsonism increases with age, parkinsonism is likely to affect a large number of older adults over the coming decades.
Prior studies have focused on a subset of 8–10 nongait items from the Unified Parkinson's Disease Rating Scale (UPDRS) and have found that parkinsonism is common in older adults.15,17–19 Parkinsonism in this study was based on 26 items from the UPDRS including both gait and nongait items.6 Parkinsonian gait was the most frequent isolated sign as well as the most common sign observed in combination with the other parkinsonian signs in individuals developing parkinsonism. These observations underscore the importance of the contribution of gait to the adverse health outcomes associated with parkinsonism.20–23
The 3-level categorical measure employed in this study categorized individuals as having parkinsonism based on the presence of 2 or more parkinsonian signs, and also categorized individuals as having possible parkinsonism when only one parkinsonian sign was present. Prior work in these cohorts suggests that possible parkinsonism may be analogous to mild cognitive impairment, a less severe and an earlier stage of impaired cognition that may progress in some individuals to dementia.2 Transition modeling showed that while cases with possible parkinsonism have higher odds of progressing to more severe motor impairment, some may persist as mild motor signs.24–29 Transition modeling suggests that while parkinsonism is not an all-absorbing state, it is progressive in most older adults and its risk factors increase the odds of its progression.
The current study suggests that parkinsonism and possible parkinsonism are likely to affect large numbers of older adults. The specific motor abilities impaired in old age vary, and encompass a wide spectrum, including reduced gait speed and loss of muscle strength and bulk, balance, and dexterity as well as parkinsonism. Thus, the growing public health challenge of identifying impaired motor function in old age is complicated by the variability of its clinical expression. Moreover, currently there is no single scale that can be used to assess motor impairments. Investigators have assessed different motor abilities to document motor impairments in old age. For example, these have included sarcopenia, based on muscle strength and bulk,30 physical frailty, based on grip strength and gait speed,31 the parkinsonian signs score, based on signs of bradykinesia, tremor, rigidity, and parkinsonian gait,1 and a wide range of motor performances.32,33 The independent contributions of impaired parkinsonian signs and motor performances in predicting incident parkinsonism in the current study extend prior studies and suggest that a wider range of motor signs, i.e., several motor instruments, may be necessary to more fully describe parkinsonism and the varied manifestations of late-life motor impairment.12,13,29,34
Since parkinsonism is associated with a wide range of adverse health outcomes and increases with age, the social and personal burden of parkinsonism will pose a growing public health problem for our aging population.2 This underscores the importance of identifying modifiable risk factors. Although several chronic health conditions we examined were associated with incident parkinsonism when examined alone, only depressive symptoms, neuroticism, and BMI showed independent associations with incident parkinsonism. A secondary analysis lends support to the idea that the association of these factors with incident parkinsonism may be mediated by several motor measures and cognition, which may manifest early signs of parkinsonism. Further work is needed to determine whether these are risk factors as opposed to clinical manifestations of the accumulation of the diverse neuropathologies or other processes that cause parkinsonism in older adults (i.e., markers of parkinsonism rather than causal factors).
There are diverse causes for the clinical phenotype of parkinsonism in older adults. For example, medications, cerebrovascular disease, and neurodegenerative disorders can all cause clinical parkinsonism in older adults. Moreover, brain imaging and autopsy studies have reported that a wide range of age-related neuropathologies are related to parkinsonism in older adults without PD.2,22 Thus, the phenotypic expression of parkinsonism in an older adult is not specific for PD, but rather encompasses diverse etiologies. Moreover, the number of cases with parkinsonism in the current study is at least 2-fold to 3-fold more common than the highest reported prevalence for Lewy body pathology in older adults without a clinical diagnosis of PD.35 It is the site rather than the type of pathology that produces parkinsonian signs.36 Thus, clinical parkinsonism is more likely to be due to vascular pathologies, which are more common than Lewy body pathology.2,37 This may have important translational consequences as more aggressive and specific treatments to prevent vascular pathologies might decrease the burden of parkinsonism in older adults. Since brains from older adults commonly show several different neuropathologies, large numbers of brain samples will be needed to control for the many combinations of neuropathologies associated with parkinsonism.37 Nonetheless, while only a minority of individuals with parkinsonism may progress to manifest traditional clinical PD, the current results may inform on growing efforts to delineate the prodromal clinical PD phenotypes.38–40 The development of clinical risk profiles based on clinical functions, neuroimaging, genetic or biochemical biomarkers, and indices of neuropathologies are likely needed to disentangle the various etiologies underlying clinical parkinsonism in older adults.
The study has several strengths, including the community-based cohorts with large numbers of women and men that employed uniform clinical procedures. There are a number of limitations as well. The estimated incidence of parkinsonism in this study depends on the cutpoint employed for the positivity of parkinsonian signs, for which there is no secure agreement. The qualitative parkinsonian signs employed in this study lack specificity; quantitative motor measures may identify signature motor findings with more specificity for different causes of parkinsonism in older adults.12 It is possible that some of the patients identified with parkinsonism during this study may have had undiagnosed PD. As noted above, given the high prevalence of parkinsonism in older adults, it is more likely that neuropathologies other than PD are the underlying cause of parkinsonism.2
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants in the Religious Orders Study and the Rush Memory and Aging Project and the staff of the Rush Alzheimer's Disease Center.
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- MAP
Memory and Aging Project
- PD
Parkinson disease
- ROS
Religious Orders Study
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drafting/revising manuscript for content: A.S.B., S.E.L., L.Y., B.D.J., R.S.W., A.S.L., J.M.S., D.A.B. Study concept or design: A.S.B., S.E.L., L.Y., B.D.J., R.S.W., A.S.L., J.M.S., D.A.B. Analyses or interpretation of the data: A.S.B., S.E.L., B.D.J., R.S.W., A.S.L., J.M.S., D.A.B. Acquisition of data: A.S.B., D.A.B. Statistical analysis: A.S.B., S.E.L., L.Y. Study supervision or coordination: A.S.B., D.A.B. Obtaining funding: A.S.B., D.A.B.
STUDY FUNDING
Supported by the National Institute of Health R01AG17917, P30AG10161, and R01AG15819 to D.A.B., R01NS078009, R01AG040039, and R01AG47976 to A.S.B., K01AG050823 to B.D.J. and the Illinois Department of Public Health, and the Borwell Endowment Fund to D.A.B. J.M.S. was supported by Huffington Foundation, the Jan and Dan Duncan Neurologic Research Institute at Texas Children's Hospital, and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
DISCLOSURE
A. Buchman receives support from NIH grants (R01AG043379, R01NS078009, R01AG017917, P30AG10161, P20MD0068860, R01AG040039, and R01AG022018). S. Leurgans receives support from the NIH (P20MD006886, P20MD006886-S1, P30AG010161, RF1AG015819, U01AG046152-S1, R01AG034374, R01NS078009, R01AG047976, R01AG033570, and R01AG042210). L. Yu receives support from the NIH (R01AG038651, R01AG017917, U18NS082140, RF1AG015819, R01AG036042, U01AG046152, R01AG033678, U01AG032984, and R01DK099269) and the Shapiro Foundation. R. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition, Psychology and Aging, and Neuropsychology; has served as a consultant for Pain Therapeutics, Inc.; and receives research support from the NIH (P30AG010161, RF1AG015819, R01AG017917, R01AG034374, R01AG039478, R01AG036042, R01AG036836, R01AG041797, R01AG042210, and R01NR013151), the Alzheimer's Association (NIRGD-11-205469), and Zinfandel Pharmaceuticals. A. Lim receives support from Heart and Stroke Foundation of Ontario 7437 and Canadian Institutes of Health Research MOP125934, MMC112692, and MSH136642. B. James receives support from NIH grant K01AG050823. Dr. James consults for the Alzheimer's Association. J. Shulman was supported by grants from the NIH (K08AG034290, R21NS089854, R01NS078009, R01AG033193, U01AG046161), the Alzheimer's Association, the American Federation for Aging Research, Huffington Foundation, the Robert and Renee Belfer Family Foundation, the Jan and Dan Duncan Neurologic Research Institute at Texas Children's Hospital, and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. D. Bennett serves on the editorial board of Neurology®; has received honoraria for non-industry-sponsored lectures; has served as a consultant to Danone, Inc., Wilmar Schwabe GmbH & Co., Eli Lilly, Inc., Schlesinger Associates, and Geson Lehrman Group; and receives research support for NIH grants P30AG010161, R01AG015819, R01AG017917, R01AG036042, U01AG046152, R01AG039478, R01AG040039, R01NS084965, R01AG022018, P20MD006886, R01AG043617, R01NS078009, R01AG036836, R01NS082416, R01AG038651, R01NS086736, R01AG041797, P01AG014449, U18NS082140, U01AG032984, R01AG042210, R01AG043975, and R01AG034119, and research support from Zinfandel. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Louis ED, Bennett DA. Mild parkinsonian signs: an overview of an emerging concept. Mov Disord 2007;22:1681–1688. [DOI] [PubMed] [Google Scholar]
- 2.Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in older adults and its association with adverse health outcomes and neuropathology. J Gerontol A Biol Sci Med Sci 2016;71:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses' ratings of parkinsonian signs with a modified Unified Parkinson's Disease Rating Scale. Neurology 1997;49:1580–1587. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer's disease. J Gerontol A Biol Sci Med Sci 1999;54:M191–M196. [DOI] [PubMed] [Google Scholar]
- 7.Fleischman DA, Yang J, Arfanakis K, et al. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology 2015;84:1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park M, Buchman AS, Lim AS, Leurgans SE, Bennett DA. Sleep complaints, and incident disability in a community-based cohort study of older persons. Am J Geriatr Psychiatry 2014;22:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L, Griffith WS, Tyas SL, Snowdon DA, Kryscio RJ. A nonstationary Markov transition model for computing the relative risk of dementia before death. Stat Med 2010;29:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salazar JC, Schmitt FA, Yu L, Mendiondo MM, Kryscio RJ. Shared random effects analysis of multi-state Markov models: application to a longitudinal study of transitions to dementia. Stat Med 2007;26:568–580. [DOI] [PubMed] [Google Scholar]
- 11.SAS/STAT® User's Guide. Version 9.3 [computer program]. Cary, NC: SAS Institute Inc.; 2012. [Google Scholar]
- 12.Buchman AS, Leurgans SE, Weiss A, et al. Associations between quantitative mobility measures derived from components of conventional mobility testing and parkinsonian gait in older adults. PLoS One 2014;9:e86262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC: United States Census Bureau; 2014. [Google Scholar]
- 15.Uemura Y, Wada-Isoe K, Nakashita S, Nakashima K. Mild parkinsonian signs in a community-dwelling elderly population sample in Japan. J Neurol Sci 2011;304:61–66. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Duan L, Sun F, Yan B, Ren S. Association between mild parkinsonian signs and mortality in an elderly male cohort in China. J Clin Neurosci 2010;17:173–176. [DOI] [PubMed] [Google Scholar]
- 17.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology 1993;43:2184–2188. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol 2004;61:1273–1276. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology 2005;64:1157–1161. [DOI] [PubMed] [Google Scholar]
- 20.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke 2011;42:3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman AS, Nag S, Shulman JM, et al. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson's disease. Mov Disord 2012;27:1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol 2012;71:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology 2002;58:1815–1819. [DOI] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Albert M, Knopman DS, et al. Introduction to revised criteria for the diagnosis of Alzheimer's disease: National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement 2011;7:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siderowf A, Lang AE. Premotor Parkinson's disease: concepts and definitions. Mov Disord 2012;27:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerche S, Hobert M, Brockmann K, et al. Mild parkinsonian signs in the elderly–is there an association with PD? Cross-sectional findings in 992 individuals. PLoS One 2014;9:e92878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 32.Onder G, Penninx BWJH, Lapuerta P, et al. Change in physical performance over time in older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 2002;57:M289–M293. [DOI] [PubMed] [Google Scholar]
- 33.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc 2007;55:11–19. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol 2006;63:1763–1769. [DOI] [PubMed] [Google Scholar]
- 35.Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics, and pathogenesis. Annu Rev Pathol 2011;6:193–222. [DOI] [PubMed] [Google Scholar]
- 36.Critchley M. Arteriosclerotic parkinsonism. Brain 1929;52:23–83. [Google Scholar]
- 37.Buchman AS, Yu L, Wison RS, et al. Post-mortem brain pathology is related to declining respiratory function in community-dwelling older adults. Front Aging Neurosci 2015;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg D, Lang AE, Postuma RB, et al. Changing the research criteria for the diagnosis of Parkinson's disease: obstacles and opportunities. Lancet Neurol 2013;12:514–524. [DOI] [PubMed] [Google Scholar]
- 39.Racette BA, Willis AW. Time to change the blind men and the elephant approach to Parkinson disease? Neurology 2015;85:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Mov Disord 2014;29:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


