Abstract
Introduction
Failure to normalize lactate is associated with poor outcomes in septic shock. It has been suggested that persistently elevated lactate may result from regional ischemia due to disturbed and/or heterogenous microcirculatory blood flow.
Objectives
The goal of this study was to determine if lactate clearance may serve as a surrogate marker for changes in microcirculatory blood flow in patients with septic shock.
Methods
This was a prospective observational study performed within a previously published clinical trial of L-carnitine for the treatment of vasopressor-dependent septic shock. Intravital video microscopy was performed at enrollment and 12 hours later, and microcirculatory flow index (MFI) was assessed. Associations between enrollment MFI, lactate, and SOFA score were determined, in addition to associations between ΔMFI, lactate clearance, and ΔSOFA. A preplanned subgroup analysis of only patients with an elevated initial lactate was performed.
Results
We enrolled a total of 31 patients, 23 with survival to and sufficient quality videos both at enrollment and 12 hours. ΔMFI, lactate clearance, and ΔSOFA were 0.1 (IQR 0, 0.3), 18% (IQR −10%, 46%), and −2 (IQR −4, 0). Both ΔMFI and lactate clearance were associated with ΔSOFA (β = −5.3, p = 0.01 and β = −3.5, 0.047), but not with each other, even in the subgroup of patients with an initially elevated lactate.
Conclusion
We observed no association between degree of lactate clearance and change in microcirculatory blood flow in patients with septic shock. These data suggest against the hypothesis that lactate clearance may be used as a surrogate marker of microcirculatory blood flow.
Introduction
Elevated lactate is associated with adverse outcomes in sepsis and often attributed to tissue hypoperfusion with resultant anaerobic metabolism. Early lactate normalization is associated with decreased mortality1 and recommended as an early resuscitation target.2 However, many patients continue to demonstrate hyperlactatemia despite adequate resuscitation.1 One hypothesized reason for persistent lactate elevations is ongoing microvascular malperfusion and previous investigations have noted an association between microcirculatory blood flow and lactate.3 Impaired microcirculatory blood flow in patients with sepsis is associated with death while early improvements are associated with decreased organ failure.4 Furthermore, impaired microcirculatory flow may serve as an indicator of such ongoing hypoperfusion.
Measurement of microcirculatory blood flow is limited to a research setting as expense, lack of automation, and a steep learning curve related to performing the measurement currently limit generalizability. Furthermore, it remains unclear if persistent elevations in lactate are truly due to ongoing microvascular malperfusion as opposed to non-anaerobic sources, including activation of Na/K ATPase5, inhibition of pyruvate dehydrogenase,6 or inhibition of the electron transport chain.7 Given this background, we wished to test the hypothesis that persistent microvascular flow impairment was associated with failure of lactate clearance in patients with septic shock. If true, failure of lactate clearance might prove a clinically useful surrogate for ongoing microvascular malperfusion. A lack of association, however, may suggest alternative sources of persistently elevated lactate, with resultant implications for therapeutic interventions.
Methods
Study Overview
This was a secondary aim of a randomized control trial to determine the safety and preliminary efficacy of L-carnitine for the treatment of septic shock.8 Microcirculatory blood flow and venous lactate were measured at enrollment and at 12 hours, and Sequential Organ Failure Assessment (SOFA)9 score at enrollment and 24 hours. The hypotheses and data analysis plan were determined prior to conducting the analysis. The study was approved by the local institutional review board, conducted under the authority of the Food and Drug Administration (Initial New Drug #107,086) and registered on clinicaltrials.gov (NCT01193777). Abbreviated inclusion criteria8 included consensus criteria for septic shock with a SOFA score ≥5 and a cumulative vasopressor index10 of ≥3 for at least 4 hours, with enrollment within 16 hours of sepsis recognition, with recognition defined as initiation of a quantitative resuscitation protocol or first antibiotic administration. Patients were excluded if given any primary diagnosis other than sepsis.
Study Measurements
Lactate clearance (LC) and normalization 1 and side-stream dark-field video microscopy (Microvision Medical BV, Amsterdam, Netherlands) were measured and calculated as previously described.4 Lactate elevation was defined as >2.0 mmol/L, while normalization was defined as an elevated value followed by a subsequent normal value. Lactate clearance was calculated as: (initial lactate − subsequent lactate)/initial lactate. Video clips were not analyzed if determined to be of insufficient quality, due to either excessive pressure as evidenced by impaired venous blood flow in vessels with diameter >50 um, poor focus, or insufficient or excessive contrast. 11 SOFA score12 was calculated using laboratory values and bedside evaluations at enrollment and 24 (±4 hours), and ΔSOFA was defined as the 24 hour – initial value.
Outcomes
The primary outcome was the association between change in microcirculatory flow index (ΔMFI)13 and lactate clearance. Secondary outcomes included the association of these measures with ΔSOFA, associations between initial MFI, lactate, and SOFA score, and the difference in ΔMFI in patients with or without LC of 10% or normalization. An a priori subgroup analysis of only patients with an initially elevated lactate was performed.
Data analysis
Associations between initial MFI, lactate, and SOFA score, and the difference in ΔMFI were determined using simple linear regression. Comparisons of ΔMFI between patients with and without LC of 10% or normalization were performed using Wilcoxon ranksum. All analyses were repeated in the predefined subgroup. Potential L-carnitine effects were evaluated by comparing LC and ΔMFI between patients in each intervention arm, through the addition of the arm to regression models, and a subgroup analysis of only patients treated with placebo. All analyses were conducted using STATA 10.0 (College Station, TX). Tests were 2-sided, and p values of 0.05 were considered significant. A post-hoc power analysis was conducted using a freely available online tool.14
Power analysis
Given our fixed sample size of 23, and observed population standard deviations of 0.37 and 0.51 for ΔMFI and lactate clearance; assuming an alpha of 0.05, our study had 80% power to detect a true association of 0.04 MFI per 10% lactate clearance. Given minimal clinical significance of ΔMFI and lactate clearance smaller than these values, we submit our study is sufficiently powered to detect clinically meaningful associations.
Results
There were 31 patients who were enrolled, with 4 having insufficient quality videos available for analysis at enrollment and an additional 4 either with insufficient quality video at 12 hours or early death, leaving 23 patients analyzed for the primary outcome. Complete baseline demographic and clinical characteristics have been previously published.8 Of the patients included in this report, the median age was 65 (IQR 56, 73), 78% of patients were white and 65% male. All patients received vasopressors, 65% were intubated, and median intravenous fluid volume in the 1st 6 hours was 4.0 L (IQR 1.7, 5.4L). Enrollment and ΔSOFA scores were 11 (IQR 8, 14) and −2 (IQR −4, 0). Median lactate at enrollment was 2.5 mmol/L (IQR 1.7, 3.1), with 18 patients forming the subgroup with an elevated lactate (range: 2.1–10.1). Relative lactate clearance was 18% (IQR −10%, 46%), 9% (IQR −13%, 33%) in the subgroup. Of these 18, 10 (56%) patients attained a lactate clearance of 10%, while 8 (44%) achieved lactate normalization. Median enrollment MFI was 2.75 (IQR 2.4, 3.0) and ΔMFI was 0.1 (IQR 0, 0.3). Patients excluded due to death or insufficient quality videos exhibited non-significantly higher SOFA scores [13.5 (IQR 10, 16) vs 11 (IQR 8, 14); p = 0.28] and lactate at enrollment [4.5 (IQR 2.2, 7.1) vs 2.5 (IQR 1.7, 3.1); p = 0.13], respectively.
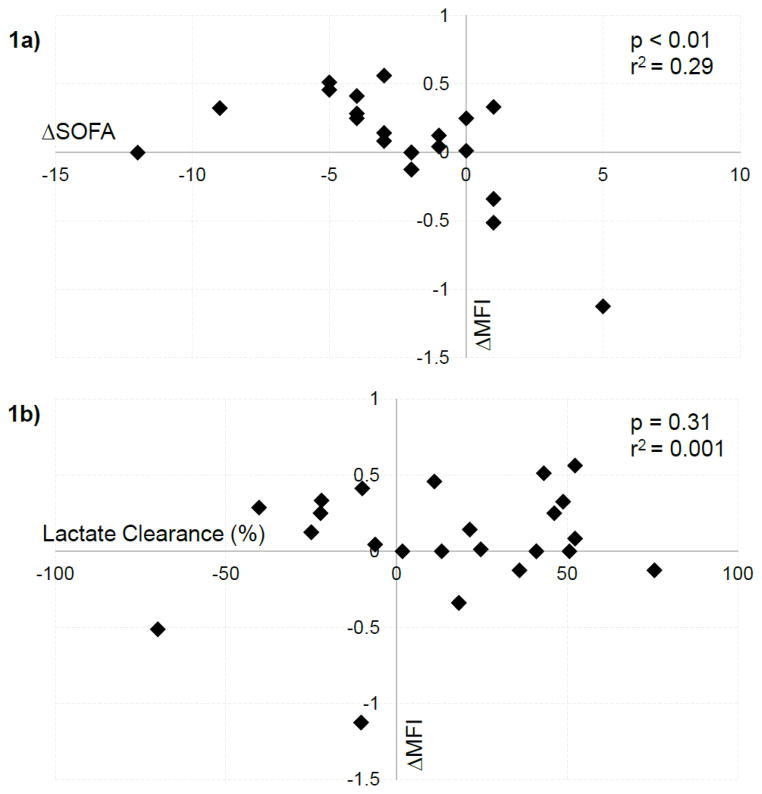
Similar to previous work,13 we found a significant linear association between early improvements in MFI and SOFA score (Figure 1a; β = −5.3; p < 0.01; r2 = 0.29). Lactate clearance was similarly associated with ΔSOFA (β = −3.5; p = 0.047; r2 = 0.11), including in the subgroup with an elevated lactate (β = −4.6; p = 0.03; r2 = 0.20). In terms of our primary analysis, we found no significant association between lactate clearance and ΔMFI either in the entire cohort (Figure 1b; β = 0.23; p = 0.31; r2 = 0.001) or in the subgroup with an elevated lactate (β = 0.44; p = 0.17; r2 = 0.08). ΔMFI was similar between groups with or without 10% clearance (0.125 vs 0.05; p = 0.90) or normalization (0.125 vs 0.08, p = 0.67), including our subgroup analysis (p = 0.55, 0.60).
Figure 1.
Relationship between a) change in microcirculatory blood flow (ΔMFI) and change in SOFA score (ΔSOFA) and b) ΔMFI and lactate clearance. Note, Figure 1a has only 20 data points due to multiple data points having the same values.
There were no significant differences in lactate clearance, normalization, and ΔMFI in patients treated with L-carnitine versus placebo. Addition of intervention to the linear regression models had no effect on the results. Confining our analysis to only patients treated with placebo yielded similar results.
Discussion
In this study, we investigated the association between microcirculatory blood flow and lactate clearance to test the hypothesis that lactate clearance may be a useful surrogate marker for changes in microcirculatory blood flow. We found no significant link between ΔMFI and LC, despite both variables being related to ΔSOFA, suggesting these two variables reflect different processes related to organ failure. We conclude lactate clearance is a poor surrogate marker of ΔMFI and that persistently elevated lactate is unlikely solely due to ongoing microvascular malperfusion. While lactate non-clearance is a robust prognostic marker associated with poor outcomes and has been suggested to reflect ongoing regional tissue hypoperfusion,3 our study suggests lactate clearance and change in MFI are poorly correlated and are not clinically interchangeable, which has implications for the development of surrogate clinical markers of impaired microcirculatory blood flow.
As a purely prognostic maker, it could be argued that the source of lactate is irrelevant. However, two clinical trials illustrate the utility of lactate clearance as a therapeutic target,15, 16 increasing the clinical relevance of this study.2 While these trials utilized goals of 10 and 20%, the Surviving Sepsis Campaign recommends targeting lactate normalization (Grade 2C),2 despite a lack of data regarding this value as an interventional target. In a more nuanced understanding, a lactate clearance driven resuscitation strategy may be effective only for a subset of patients with hypoperfusion-driven hyperlactatemia, while the same strategy may be deleterious for patients with non-ischemic sources of lactate. Further investigations to elucidate the source of lactate in sepsis and the effect of resuscitation strategies on these subgroups may help clarify this issue.
This study has several limitations that deserve consideration. This was a relatively small single center study, particularly related to subgroup analyses, and a larger study might detect smaller associations. Regarding our choice of outcome, MFI may provide a poor estimate of microvascular hypoxia; and other estimates, such as percentage of perfused vessels,17 may yield alternative results. However, MFI is the most extensively studied to date and had adequate power to demonstrate an association with SOFA score. Regarding methods, patients were enrolled relatively early, but all underwent quantitative resuscitation prior to enrollment. Several patients were necessarily excluded due to early death or poor quality video. Either of these exclusions could have biased our results, and it is possible more severely ill patients exhibit a relationship between lactate clearance and ΔMFI that we were unable to observe. Regarding lactate, we cannot rule out the possibility that arterial samples would have yielded different results or that the observed lactate clearance reflects hemodilution rather than reversal of tissue ischemia. Finally, it is possible that treatment with L-carnitine affected our outcomes though multiple subgroup analyses found no evidence of such an effect.
Conclusion
We observed no association between degree of lactate clearance and change in microcirculatory blood flow in patients with septic shock. Further research should focus on alternative surrogate markers of microcirculatory blood flow as well as further defining metabolic sources of lactate in well-resuscitated patients.
Acknowledgments
This project was supported by a grant from the American Heart Association to Dr. Puskarich (AHA 10POST356001), as well as a foundation grant from the Cannon Research Center (SRG10-004). Dr. Puskarich has received salary support through K23GM113041-01 from the National Institute of General Medical Sciences/National Institutes of Health. Drs. Shapiro and Massey received support through the National Institutes of Health grants: HL091757 and GM076659. Dr. Jones is supported by 1R01GM103799-01 from the National Institute of General Medical Sciences/National Institutes of Health.
Footnotes
The authors have no conflicts of interest to report.
This data was presented at the Society for Academic Emergency Medicine Annual Meeting, 2015 in San Diego, CA
Reference List
- 1.Puskarich M, Trzeciak S, Shapiro N, et al. Whole Blood Lactate Kinetics in Patients Undergoing Quantitative Resuscitation for Severe Sepsis and Septic Shock. Chest. 2013;143(6):1548–1553. doi: 10.1378/chest.12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger R, Levy M, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for the Management of Severe Sepsis and Septic Shock 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Filbin MR, Hou PC, Massey MJ, et al. The microcirculation is preserved in emergency department low-acuity sepsis patients without hypotension. Acad Emerg Med. 2014;21(2):154–162. doi: 10.1111/acem.12314. [DOI] [PubMed] [Google Scholar]
- 4.Trzeciak S, McCoy JV, Dellinger RP, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced milti-organ failure at 24 hours in patients with sepsis. Intensive Care Med. 2008;34(12):2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. 2005;365:871–875. doi: 10.1016/S0140-6736(05)71045-X. [DOI] [PubMed] [Google Scholar]
- 6.Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock. 1996;6(2):89–94. doi: 10.1097/00024382-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brearley D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 8.Puskarich M, Kline JA, Krabill V, Claremont H, Jones AE. Preliminary Safety and Efficacy of L-carnitine Infusion for the Treatment of Vasopressor-Dependent Septic Shock - A Randomized Control Trial. JPEN J Parenter Enteral Nutr. 2013 Jul 12; doi: 10.1177/0148607113495414. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui FS, Kumar A, Woodward B, Wang Y. Cumulative vasopressor index (CVI) as an assessment of cardiovascular organ dysfunction and indicator of outcome in patients with septic shock. Crit Care Med. 2007;35(12):A7. [Google Scholar]
- 11.Massey MJ, Larochelle E, Najarro G, et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care. 2013;28(6):913–917. doi: 10.1016/j.jcrc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande PP, Sanders NStJP, Ely EW, Shintaini A. Calculating SOFA scores when arterial blood gasses are not available: Validating SpO2/FiO2 ratios for imputing PaO2/FiO2 ratios in the SOFA scores. Crit Care Med. 2006;34(12):A1. [Google Scholar]
- 13.Trzeciak S, Rivers EP. Clinical manifestations of disordered microcirculatory perfusion in severe sepsis. Crit Care (London) 2005;9(Suppl 4):S20–S26. doi: 10.1186/cc3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed 5/21/2015 9:48:02 AM 2015]; http://hedwig.mgh.harvard.edu/sample_size/js/js_associative_quant.html.
- 15.Jones AE, Shapiro N, Trzeciak S, Arnold H, Claremont H, Kline JA. Lactate Clearance vs Central Venous Oxygen Saturation as Goals of Early Sepsis Therapy: A Randomized Clinical Trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early Lactate-Guided Therapy in Intensive Care Unit Patients: A Multicenter, Open-Label, Randomized Controlled Trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 17.DeBacker D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care (London) 2007;11(5):R101–R110. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]