Abstract
Purpose
Radiotherapy remains a primary treatment modality for the majority of central nervous system tumors, but frequently leads to debilitating cognitive dysfunction. Given the absence of satisfactory solutions to this serious problem, we have used human stem cell therapies to ameliorate radiation-induced cognitive impairment. Here, past studies have been extended to determine whether engrafted cells provide even longer-term benefits to cognition.
Materials and methods
Athymic nude rats were cranially irradiated (10 Gy) and subjected to intrahippocampal transplantation surgery 2 days later. Human embryonic stem cells (hESC) or human neural stem cells (hNSC) were transplanted, and animals were subjected to cognitive testing on a novel place recognition task 8 months later.
Results
Grafting of hNSC was found to provide long lasting cognitive benefits over an 8-month post-irradiation interval. At this protracted time, hNSC grafting improved behavioral performance on a novel place recognition task compared to irradiated animals not receiving stem cells. Engrafted hESC previously shown to be beneficial following a similar task, 1 and 4 months after irradiation, were not found to provide cognitive benefits at 8 months.
Conclusions
Our findings suggest that hNSC transplantation promotes the long-term recovery of the irradiated brain, where intrahippocampal stem cell grafting helps to preserve cognitive function.
Keywords: Human stem cells, transplantation, radiation, cognition, hippocampus
Introduction
Ionizing radiation is a first-line treatment to control primary and metastatic brain tumors and can induce a progressive and long-lasting decline in cognition that can severely impact quality of life (Butler et al. 2006, Meyers and Brown 2006). Given the growing population of long-term survivors of intracranial tumors, quality of life has become an increasing concern with no satisfactory, long-term solutions. We have recently demonstrated that intrahippocampal transplantation of human stem cells prevented the development of radiation-induced cognitive impairment in rodents at 1- and 4-months post-surgery (Acharya et al. 2009, 2011, 2012, 2013). These studies provided the first evidence that engraftment of either pluripotent human embryonic stem cells (hESC) or multipotent human neural stem cells (hNSC) could protect the brain from a serious side effect of cranial irradiation (IRR).
The mechanisms underlying radiation-induced cognitive impairment are not well understood and are likely multifaceted involving microenvironmental factors such as oxidative stress and inflammation (Fike et al. 2009), which, in turn, can influence neural stem/progenitor cell (NSC) populations associated with cognitive function. Recently, we have shown that cranial IRR alters mature neuronal architecture (dendrites and spines) and modulates proteins involved in synaptic function in the hippocampus (Parihar and Limoli 2013, Parihar et al. 2014). The hippocampus is a brain region critical for the acquisition (learning), consolidation and retrieval of declarative memories (for review see: Squire 1994, Eichenbaum 2001). These processes modulate the strength and efficacy of synaptic signaling (i.e., synaptic plasticity), which impacts neurotransmission and provides a mechanism for translating synaptic plasticity into changes in synaptic strength (memory). How radiation impacts these processes was a lifelong interest of Mike Robbins, and much of his work analyzing the capability of peroxisomal proliferating-activated receptor activation (Greene-Schloesser et al. 2014) or inhibition of the renin-angiotensin system (Lee et al. 2012) demonstrated how pharmacologic strategies could be used to attenuate the adverse effects of IRR on cognition. Mike Robbins was always keen on elucidating the molecular pathways contributing to radiation-induced cognitive dysfunction (Greene-Schloesser et al. 2013) and much of this passion persuaded our efforts to use stem cell transplantation as an alternative strategy to ameliorate radiation-induced cognitive deficits. In this study we sought to determine the longer-term benefits of human stem cell grafting in the irradiated brain. The present findings suggest that grafted hNSC (but not hESC) provide cognitive benefits lasting as long as 8 months after IRR and transplantation surgery.
Materials and methods
Animals and cranial IRR procedure
All animal procedures described are in accordance with the National Institutes of Health (NIH) and approved by the Institutional Animal Care and Use Committee (IACUC). Immunodeficient athymic nude (ATN) rats (strain 0N01, Cr:NIH-rnu, X50 colony, NCI Frederick National Laboratory, Frederick, MD, USA) were maintained in sterile housing conditions (20 °C ± 1 °C; 70% ± 10% humidity; 12 h:12 h light and dark cycle), and had free access to sterilized diet and water. A total of 48 young (2 month old) ATN rats were divided in four experimental groups: 0 Gy (no IRR) receiving sham-operation surgery with vehicle (Cont-sham, n = 12), 10 Gy (head-only IRR) receiving vehicle and sham-operation surgery (IRR-sham, n = 12), 10 Gy irradiated receiving hESC grafting (IRR + hESC, n = 12) and 10 Gy irradiated receiving hNSC grafting (IRR + hNSC, n = 12). A subset of animals (n = 4) from each group was followed for 8 months post-transplantation for the long-term study. For the IRR procedures, animals were anesthetized, eyes and body were lead-shielded and were exposed to cranial γ-IRR (10 Gy) using a 137 Cs irradiator (J.L. Shepard, Mark I, San Fernando, CA, USA) at a dose rate of 2.07 Gy/min, as described in detail previously (Acharya et al. 2009, 2011). None of the animals received immune-suppression throughout the study.
Transplantation surgery
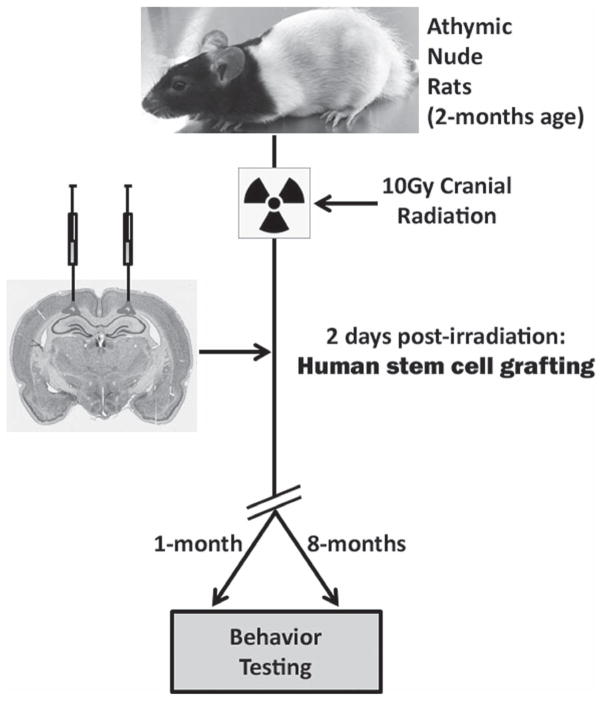
The use of hESC (H9, WiCell Research Institute, Inc., Madison, WI, USA) and hNSC (ENStem-A cell line, EMD Millipore, Billerica, MA, USA) was approved by the Institutional Human Stem Cell Research Oversight Committee (hSCRO). The hESC were cultured on a mitotically inactive mouse embryonic fibroblast (MEF) feeder layer (EMD-Millipore) while hNSC were maintained as a monolayer in T25 flasks in neural expansion media (EMD-Millipore) as described previously (Acharya et al. 2009, 2011). For transplantation studies, hESC were used at passages 42 – 49 while hNSC were used at passages 5 – 9. Two days post-IRR, rats received bilateral, intrahippocamoal transplantation of human stem cells as described in detail previously (Acharya et al. 2009, 2011). A total of 4.0 × 105 live hESC or hNSC were transplanted in 4 distinct hippocampal sites (1.0 × 105 cells per site) per hemisphere using precise streotaxic coordinates (Acharya et al. 2009, 2011). Therefore, a total of 8.0 × 105 live human stem cells were transplanted per brain. The sham surgery groups ‘Control’ (0 Gy) and ‘IRR’ (10 Gy) received sterile vehicle (neural expansion media) at the same stereotaxic coordinates. The schematic of the research design is shown in Figure 1.
Figure 1.
Study design. Athymic nude rats (2 months age) received a 10 Gy cranial dose of γ-rays and were transplanted with human stem cells 2-days post-IRR. Two groups of animals (1- and 8-month post-transplantation) were administered a novel place recognition (NPR) task.
Novel place recognition task
Groups of rats were tested on a novel place recognition (NPR) task at 1 month or 8 months post-transplantation. All 1-month NPR data was previously published (Acharya et al. 2009, 2011) and adapted here for comparative purposes. The NPR task assesses spatial recognition memory that has been shown to rely on intact hippocampal function (Save et al. 1992, Mumby et al. 2002). We employed a standard protocol (Acharya et al. 2009, Christie et al. 2012) involving video recording and live tracking of animals using Noldus Ethovision XT system (v7.0, Noldus Information Technology, Inc., Leesburg, VA, USA). Briefly, for this study, animals were habituated for two days in an open field arena with two toy objects for a 20-min session for acclimatization. On the third day, a familiarization phase using identical plastic blocks (8 × 3 × 10 cm high) was administered for 5 min and rats were allowed to explore freely in the arena. Rats were then returned to holding cages for a 5-min retention interval. Following this delay, placement of an identical copy of one block was altered, while an identical copy of the other block was placed in the same spatial location as during the familiarization phase, and the rats were again allowed to explore the novel place stimuli freely for the duration of 3 min. The time gap between familiarization and test phase was 5 min, thus, referred to as the 5 min test phase. A positive score was counted when the nose of the rat was within 1 cm and pointed in the direction of the object. Time was not scored for rats that were near but not facing the object. Data collection and analysis for behavior studies were carried out blind to the observer.
Statistical analyses
For the behavioral analyses (NPR task), exploration ratio, or the proportion of total time spent exploring the novel spatial location (tnovel / tnovel + tfamiliar), was used as the main dependent measure for statistical analyses. Exploration ratio data were analyzed using univariate ANOVAs for the 5-min test phases. In all cases, we confirmed that the data were normally distributed using the Kolmogorov-Smirnov test, and that the error variances did not differ between groups by using Levene’s test of equality of error variances. When a statistically significant overall group effect was found, multiple comparisons were made using Fisher’s protected least significant difference (FPLSD) post hoc tests to compare the individual groups. Additional analyses of recognition memory were conducted using one-sample t-tests to determine whether the mean proportion of time spent exploring the novel spatial location for each group differed significantly from chance (i.e., 0.5) and if differences existed between short- and long-term time-points. The statistical analyses were carried out by PASW statistics (v17.0, SPSS, IBM Inc., Armonk, NY, USA) and p-values less than 0.05 were considered statistically significant.
Results
Human stem cell transplantation improves cognition at 1- and 8-month post-grafting
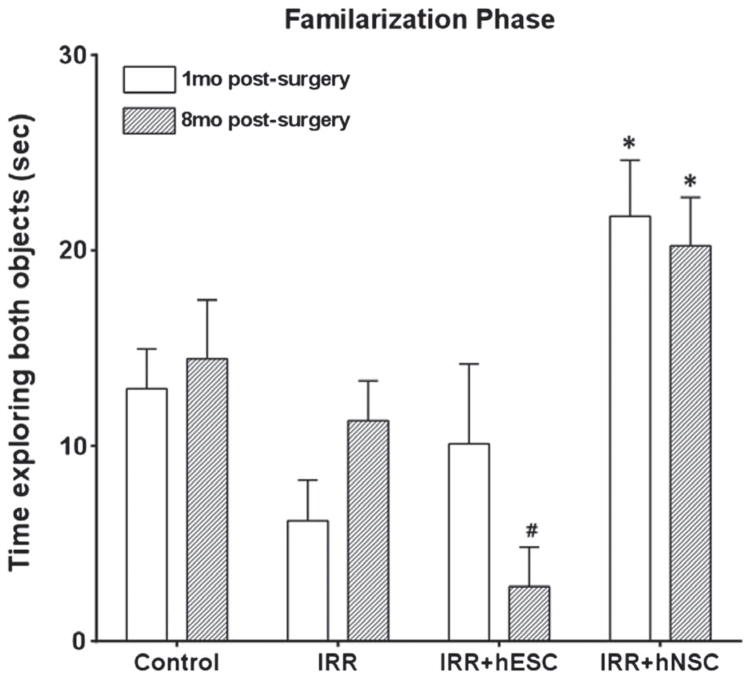
We have previously shown that transplantation of hESC or hNSC improved cognition at 1- and 4-months post-IRR (Acharya et al. 2009, 2011). In the current study, we have extended these experiments to 8-month post-IRR time-points and compared data for the restoration of cognition at 1 and 8 months following IRR. During the familiarization phase (Figure 2), IRR animals explored significantly less than hNSC transplanted animals (IRR + hNSC) at both time-points (1 month, p = 0.001, n = 12 and 8 months, p = 0.006, n = 4, Figure 2). For animals transplanted with hESC, cognitive benefits were not found, and at the 8-month time, hESC transplantation was not beneficial (Figure 2). At both 1- and 8-month times, hNSC transplanted animals explored significantly more than non-irradiated control animals (1 month, p = 0.02, n = 12, and 8 months, p = 0.044, n = 4, Figure 2).
Figure 2.
Cognitive testing (familiarization phase) conducted 1 and 8 months after human stem cell transplantation and cranial IRR. Transplantation of hNSC ameliorates IRR-induced deficits in novel place recognition (NPR) performance at 1- and 8-months post-surgery. Time spent exploring both objects during the initial familiarization phase is shown. The 1- and 8-month IRR + hNSC group explored significantly more than the corresponding IRR group (IRR + hNSC, *, p’s < 0.01, Post hoc, Fisher’s LSD). The IRR + hESC group did not show improvement in object exploration at either post-grafting time. 1-month data adapted from (Acharya et al. 2009, 2011). Error bars indicate the standard error of the mean ± SEM (n = 12, 1-month data; n = 4, 8-month data). +, #, indicates significant difference versus 8 month post-surgery Control and IRR groups.
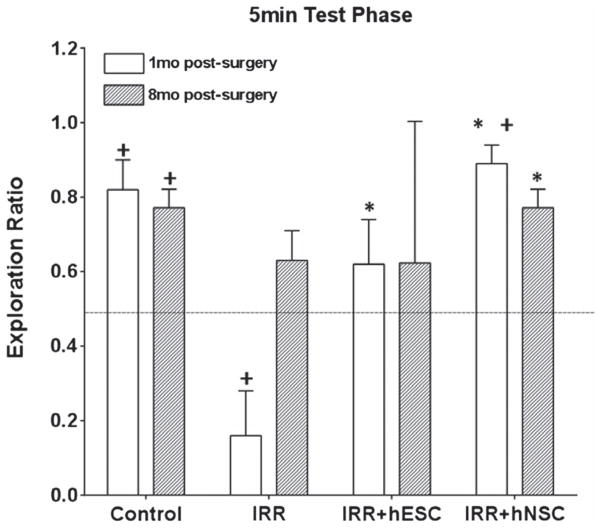
During the 5 min NPR test phase, the 1-month IRR animals spent a significantly lower proportion of time exploring the novel place compared to all other groups (p’s < 0.001, Post hoc, Fisher’s least square difference (LSD) test, Figure 3). Although not statistically different compared to Control, IRR + hESC and IRR + hNSC cohorts, the 8-month IRR group did not spend significantly more time exploring the novel place than expected by chance (dashed line, Figure 3). Transplantation of irradiated animals with hESC improved cognition significantly 1 month after IRR, such that they explored the novel place significantly more (p = 0.013, Post hoc, Fisher’s LSD) compared to IRR animals. The cognitive benefits of engrafted hESC did not persist when animals were evaluated on the NPR task 8 months after IRR, where they were not found to be significantly different from sham-irradiated controls or the 8-month IRR-sham group (Figure 3). However, for animals transplanted with hNSC preference for the novel object was improved significantly at both the 1-month and 8-month times after IRR (1 month IRR + hNSC, p = 0.015, n = 12; and 8 months IRR + hNSC, p = 0.009, n = 4, Figure 3). These data demonstrate that engrafted hNSC provide superior cognitive benefits when compared to animals grafted with hESC.
Figure 3.
Cognitive testing (5-min novel place recognition) conducted 1 and 8 months after human stem cell transplantation and cranial IRR. Exploration ratios (time novel/time novel + time familiar) for the first minute of the 5 min NPR test session are plotted. Irradiated animals (IRR) at 1 month did not explore the novel spatial location more than expected by chance (indicated by dashed line) and showed a significant decline when compared to the non-irradiated Control group (p = 0.015). While the cohort of 8-month IRR animals did exhibit a trend toward improved performance they did not spend significantly more time exploring the novel place than expected by chance. The IRR + hNSC group at 1- and 8-month post-surgery showed comparable cognitive performance with controls. At the 1-month time the IRR + hESC group showed significant improvement (p = 0.013) in novel place exploration compared to the IRR group. Error bars indicate the standard error of the mean ± SEM (n = 12, 1-month data; n = 4, 8-month data). 1-month data adapted from (Acharya et al. 2009, 2011). * indicates significant difference between 8-month and 1-month IRR groups (i.e., p < 0.05 on Post hoc, Fisher LSD group comparisons). +, indicates significant difference compared to 0.5 (i.e., more than expected by chance, p’s < 0.05, one-sample t -test comparison).
Discussion
The present results demonstrate that intrahippocampal transplantation of human stem cells were able to provide certain cognitive benefits over protracted times after IRR and transplantation surgery. Clinical management of primary and metastatic brain tumors routinely involves cranial IRR treatment plans designed to forestall the advancement of malignant growth. While such therapies remain beneficial, resultant cognitive dysfunction has the potential to severely compromise quality of life. The debilitating clinical side effects of cranial IRR have been problematic for decades, and to date, this remains an unmet medical need, with no satisfactory long-term solutions available to alleviate the persistent and progressive neurocognitive sequelae (Butler et al. 2006, Meyers and Brown 2006).
While it remains uncertain precisely how IRR impacts specific subpopulations of cells within the central nervous system (CNS) to disrupt cognition, the present results demonstrate the capability of hNSC transplantation to restore neuronal function at the behavioral level at a protracted treatment interval (i.e., 8 months). Past work from our laboratory has characterized the functional decrements in cognition caused by cranial IRR (Acharya et al. 2009, 2011, 2012) and demonstrated that intrahippocampal transplantation of human stem cells could ameliorate radiation-induced cognitive dysfunction at earlier times (i.e., ≤4 months). In the present study we analyzed the functional consequences of grafted cells in the brain at an extended time (8 months) after IRR and transplantation surgery. While irradiated animals showed some recovery in cognition over 8 months, present evidence points to the fact that grafted hNSC were able to provide beneficial effects over extended times following a single transplantation surgery (Figures 2 and 3).
Previous work from our group has defined the beneficial effects of multiple stem cell types on cognition following IRR, and defined the yields and differentiated phenotypes associated with those effects at 1 and 4 months following treatment (Acharya et al. 2009, 2011, 2012). We have recently reported that following hNSC transplantation, grafted cells expressed the behaviorally-induced plasticity-related activity-regulated cytoskeleton-associated protein (Arc) and neuron-specific nuclear antigen (NeuN) when analyzed 1 month after transplantation, suggesting that grafted cells possess the capability to functionally integrate into hippocampal circuits (Acharya et al. 2011). Arc is known to play key roles in the neuronal mechanisms underlying long-term synaptic plasticity and memory, and provides a reliable marker for detecting neurons that are actively engaged in spatial and contextual information processing associated with memory consolidation (Guzowski et al. 2005, Ramírez-Amaya et al. 2005). The long-term cognitive benefits of hNSC grafting may involve a facilitation of Arc expression in neurons of the host brain. Should the long term benefits of stem cell grafting in the irradiated brain be found more dependent on neurotrophic support from engrafted cells, rather than functional replacement and/or integration of cells into the damaged tissue bed, then this would be consistent with the preponderance of evidence using stem cell therapies in a wide range of animal-based injury models (Benderitter et al. 2014). While further experimentation will be required to address the underlying mechanisms, stem cell therapy in the irradiated brain may ultimately rely on the protection of existing host neuronal circuitry.
Acknowledgments
We are thankful to Mary Lan for excellent technical assistance and for the inspiration provided by Mike Robbins.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the NIH-NINDS R01 NS074388581 (C.L.L.) and the California Institute for Regenerative Medicine (CIRM) training grant TG-01152 (M.M.A.).
References
- Acharya MM, Christie LA, Lan ML, Limoli CL. Comparing the functional consequences of human stem cell transplantation in the irradiated rat brain. Cell Transplant. 2012;22:55–64. doi: 10.3727/096368912X640565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Christie LA, Hazel TG, Johe KK, Limoli CL. Transplantation of human fetal-derived neural stem cells improves cognitive function following cranial irradiation. Cell Transplant. 2013 doi: 10.3727/096368913X670200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, Limoli CL. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci USA. 2009;106:19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71:4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, Malard O, Stewart F, Tamarat R, Luijk PV, Limoli CL. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5652. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: Radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser D, Payne V, Peiffer AM, Hsu FC, Riddle DR, Zhao W, Chan MD, Metheny-Barlow L, Robbins ME. The peroxisomal proliferator-activated receptor (PPAR) alpha agonist, fenofibrate, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat Res. 2014;181:33–44. doi: 10.1667/RR13202.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD, Robbins ME. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci USA. 2013;110:12822–12827. doi: 10.1073/pnas.1307301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Function. 2014 doi: 10.1007/s00429-014-0709-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: Evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Buhot MC, Foreman N, Thinus-Blanc C. Exploratory activity and response to a spatial change in rats with hippocampal or posterior parietal cortical lesions. Behav Brain Res. 1992;47:113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and forgetting: Long-term and gradual changes in memory storage. Int Rev Neurobiol. 1994;37:243–269. [PubMed] [Google Scholar]