Abstract
Purpose of Review
The ability of macrophage lysosomes to degrade both exogenous and internally derived cargo is paramount to handling the overabundance of lipid and cytotoxic material present in the atherosclerotic plaque. We will discuss recent insights in both classical and novel functions of the lysosomal apparatus as it pertains to the pathophysiology of atherosclerosis.
Recent Findings
Lipid-mediated dysfunction in macrophage lysosomes appears to be a critical event in plaque progression. Consequences include enhanced inflammatory signaling (particularly the inflammasome/IL-1β axis) and an inability to interface with autophagy leading to a proatherogenic accumulation of dysfunctional organelles and protein aggregates. Aside from degradation, several novel functions have recently been ascribed to lysosomes including involvement in macrophage polarization, generation of lipid signaling intermediates, and serving as a nutrient depot for mTOR activation, each of which can have profound implications in atherosclerosis. Finally, the discovery of the transcription factor TFEB as a mechanism of inducing lysosomal biogenesis can have therapeutic value by reversing lysosomal dysfunction in macrophages.
Summary
Lysosomes are a central organelle in the processing of exogenous and intracellular biomolecules. Together with recent data that implicates the degradation products of lysosomes in modulation of signaling pathways, these organelles truly do lay at a nexus in nutrient sensing and processing. Dissecting the full repertoire of lysosome function and ensuing dysfunction in plaque macrophages is pivotal to our understanding of atherogenesis.
Keywords: atherosclerosis, macrophage, lysosome, heterophagy, autophagy
Introduction
Atherosclerosis denotes a condition in which lipids and other substances as well as infiltrating inflammatory cells accumulate in the walls of arterial blood vessels. This progressive and insidious disease underlies the large majority of cardiovascular complications worldwide including myocardial infarction, stroke, and heart failure. Over the years, studies have revealed the critical role of macrophages in the development of atherosclerosis. Macrophages infiltrate the arterial intima (a small region between endothelium and smooth muscle-rich media) as an early response to the buildup of lipoproteins in the vasculature. Lipids and various related species are engulfed by macrophages leading to alterations in macrophage phenotype including progression to lipid-laden foam cells and a proinflammatory state. Such early events exacerbate further immune cell infiltration and a progressive dysfunction in the plaque milieu, including the initiation of cell death pathways. Such increases in lesion size and dysfunction are a sine qua non of the so-called “complex” atherosclerotic plaque which underlies plaque rupture and the majority of acute cardiovascular events.
Since macrophages constitute the major physiological response to lipid accumulation in the early lesion and contribute to both plaque progression and complexity, dissecting the culprit cellular processes will further our understanding of the pathophysiology of atherosclerosis. Although the importance of the lysosomal machinery in degradation has been known for some time, recent data linking lysosomes to more complex functions such as nutrient sensing and prominent roles in lipid metabolism and inflammatory signaling, has led to a resurgence of interest in this organelle. Furthermore, evidence that macrophage lysosomes become dysfunctional in atherosclerosis indicates that it might serve as a promising therapeutic target [1, 2]. In this review, we summarize recent progress in our understanding of lysosomal function in the context of macrophages and atherosclerosis.
Lysosomes, a critical organelle for a professional phagocyte
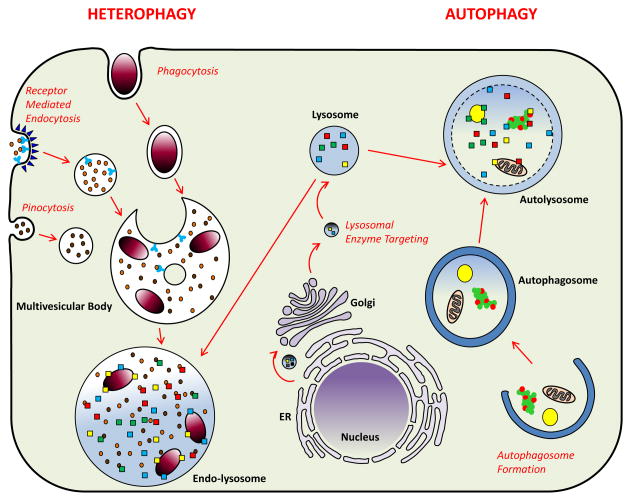
Lysosomes are highly acidic enzyme-containing organelles with the ability to denature and hydrolyze the entire repertoire of biomolecules including proteins, lipids, sugars, and nucleotides. Two primary mechanisms supply material destined for degradation to lysosomes: heterophagy (i.e. degradation of extracellular cargo) and autophagy (i.e. degradation of intracellular cargo). In heterophagy, cells uptake extracellular contents by universal processes such as receptor-mediated endocytosis and pinocytosis or by specialized processes specific to macrophages such as phagocytosis. Ingested material contained in single membrane vesicles is then trafficked to and fused with lysosomes. In contrast, autophagy involves intracellular engulfing of cytoplasmic material including aggregated proteins, lipid droplets, and organelles within double membrane vesicles, which in turn fuse with lysosomes for degradation (Figure 1). Regardless of the fueling mechanism, the products of lysosomal degradation are released to the cytoplasm for use as new substrates in anabolic processes. We will now discuss the relevance of each pathway to atherosclerosis.
Figure 1. Mechanisms of Lysosomal Degradation in Macrophages (Heterophagy versus Autophagy).
Two primary mechanisms supply lysosomes: heterophagy (left) and autophagy (right). In heterophagy, cells take up exogenous material by universal processes such as receptor-mediated endocytosis and pinocytosis or specialized processes such as phagocytosis. Endosomes, single membrane vesicles which carry exogenous material, create multivesicular bodies which are distinct subsets of endosomes containing membrane-bound intraluminal vesicles. The content of multivesicular bodies is degraded via fusion with lysosomes. In autophagy, double membrane vesicles are formed intracellularly by engulfing cytoplasmic material including protein aggregates, lipid droplets, and organelles. After formation, autophagosomes fuse with lysosomes to generate autolysosomes. The inner membrane of autophagosomes and their cytoplasmic content are degraded into basic metabolites by lysosomal hydrolysis. Formation of lysosomes requires translation of lysosomal proteins in the ER and their sorting to the correct vesicles in the Golgi. Vesicles carrying lysosomal proteins and enzymes bud from the trans-Golgi network for eventual targeting to lysosomes (middle).
Dealing with the outside: What does heterophagy bring to macrophage lysosomes?
Cellular LDL uptake is a highly regulated system with feedback mechanisms limiting excessive uptake and lipid overload [3]. In contrast, scavenger receptor-mediated uptake of modified lipids such as oxidized LDL largely bypasses this feedback leading to unfettered intracellular lipid accumulation characteristic of foam cells present in the atherosclerotic plaque. Regardless of the source, externally-derived lipid (primarily in the form of cholesteryl esters) is routed to lysosomes where several lysosomal proteins are pivotal in its hydrolysis and redistribution. Examples include Lysosomal acid lipase (LAL) and the Niemann-Pick Type C (NPC) proteins. LAL liberates free cholesterol (FC) which is shuttled sequentially from NPC2 to NPC1 and transported out of the lysosome mainly to the ER for redistribution to other cellular membranes or re-esterification by acyl-coenzyme A:cholesterol acyltransferase (ACAT) and incorporation into neutral lipid stores. Mouse models lacking NPC1 [4, 5] or LAL [6] are atherosclerosis-prone, indicating the importance of efficient lysosomal lipid handling in ameliorating plaque formation. In humans, complete absence of LAL leads to Wolman’s Disease and neonatal demise but individuals with minimal enzyme activity (dubbed Cholesteryl Ester Storage Disease) survive to early adulthood and develop premature atherosclerotic vascular disease [7]. Interestingly, two recent large-scale genome-wide association studies identified SNPs in LIPA (the gene encoding LAL) as a susceptibility locus for coronary artery disease [8, 9]. Although monocytes derived from patients with the culprit SNPs were reported to have paradoxically increased LIPA transcripts [8], concomitant assessment of LAL enzyme activity and localization in monocytes or macrophages would be necessary to develop any mechanistic links between these SNPs and atherosclerosis. Taken together, these observations support the notion that heterophagy-mediated lipid uptake and processing by macrophages requires an intact lysosomal lipid degradation and distribution machinery. More importantly, a dysfunction in this process results in lysosomal lipid accumulation and appears to contribute to atherosclerotic progression. Is there evidence beyond genetic disruptions of the lysosomal lipid-handling machinery that support such a pathogenic link?
Earlier work by Jerome and colleagues has demonstrated that incubation of macrophage cell-lines with various forms of modified lipids can disrupt lysosomal function resulting in further accumulation of intralysosomal cholesteryl esters [10–12]. Emanuel et al. recently built on this concept by demonstrating the development of lysosomal dysfunction in primary macrophages exposed to two atherogenic lipids (ox-LDL and cholesterol crystals) [13]. Morphological changes included lysosomal engorgement and less efficient detection of the lysosomotropic dye Lysotracker Red, whereas functional changes included increased lysosomal pH and decreased degradation capacity. Moreover, macrophages isolated from atherosclerotic aortas of ApoE-null mice showed parallel signs of lysosomal dysfunction that was not found in macrophages from other tissues of the same mice. Sheedy et al. provided an elegant link between ox-LDL and cholesterol crystals by demonstrating that inefficient lysosomal hydrolysis and transport of ox-LDL-derived cholesteryl esters leads to in situ cholesterol crystal formation [14]. In their model, oxidized LDL is routed to macrophage lysosomes via a CD36-dependent mechanism. Cholesterol crystals are the main product of this degradation and start to accumulate in the lysosomes. Such formation and accumulation requires both oxidized-LDL (LDL is not sufficient) and an intact lysosomal degradation machinery (blockage of degradation by bafilomycin blocks crystal formation). Cholesterol crystal formation initiates lysosomal damage by disrupting lysosomal integrity, resulting in leakage of lysosomal content into the cytoplasm.
The presence of many types of crystalline material appears to be universally damaging to lysosomal membrane integrity and ability to sequester its contents [15]. An important consequence of compromised lysosomal membrane integrity is marked activation of the inflammasome system (the rate-limiting step in production of the highly atherogenic cytokine IL-1β secretion) [14, 15]. Although still not well characterized, lysosomal leakage likely releases enzymes such as proteases that degrade endogenous inhibitors of inflammasome complex formation.
Overall, accumulating evidence suggests that heterophagic uptake of modified lipids initiates a condition very similar to lysosomal lipid storage diseases. Lipids accumulate inside lysosomes causing dysfunction and in cases where lipid content overwhelms the transport machinery, cholesterol crystals are formed leading to disruptions in membrane integrity and inflammasome activation. This model has received much recent attention since it links cholesterol derangements to inflammation (so-called “sterile inflammation”), both of which are pathognomonic features of atherosclerosis.
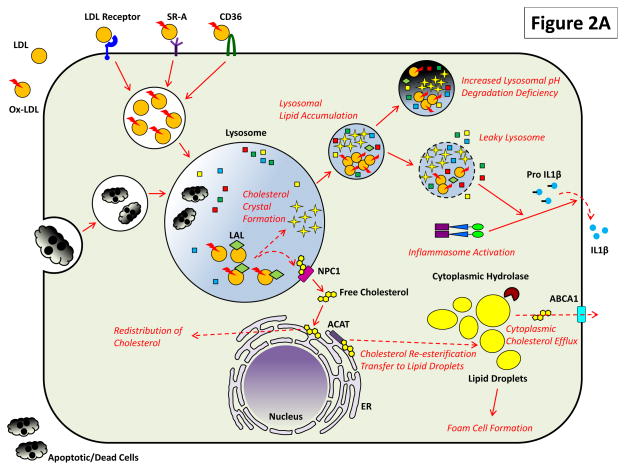
Progressive dysfunction in the lysosomal apparatus would also be predicted to adversely impact the ability of macrophages to handle other exogenous cargo. For example, compromised phagocytosis of apoptotic cells (i.e. efferocytosis) is readily seen with plaque progression and is a critical feature of increasing plaque complexity [16]. Although not directly investigated, the concomitant lysosomal dysfunction that is seen with plaques suggests that such defects are mechanistically linked. The heterophagy-lysosomal degradation pathway and its relation to atherosclerotic progression is summarized in Figure 2A.
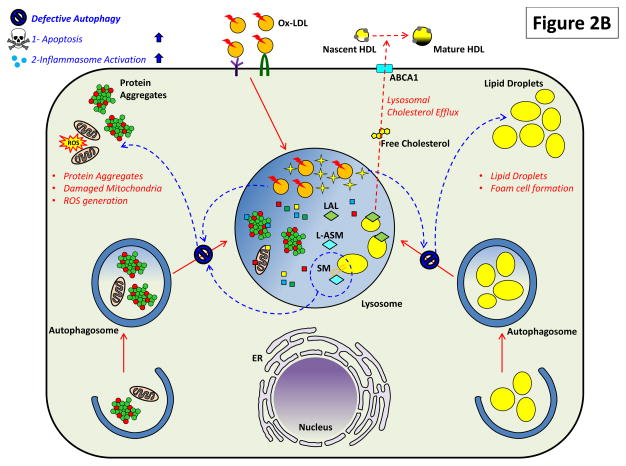
Figure 2. The Critical Roles of Macrophage Lysosomes in Atherosclerosis.
Both (A) heterophagy and (B) autophagy play important roles in the atherosclerotic macrophage and impairment of these pathways has deleterious effects.
(A) LDL, especially upon its modification/oxidation and detection by scavenger receptors such as SR-A and CD36, is endocytosed to lysosomes for hydrolysis and redistribution. Cholesteryl esters are acted on by lysosomal acid lipase (LAL) to generate free cholesterol which is transferred to ER via a mechanism requiring lysosomal proteins NPC2 and NPC1. From there, free cholesterol is either distributed to other membrane compartments or is re-esterification by ACAT on ER and transferred to lipid droplets. Excess cholesterol accumulation is balanced by the action of cytoplasmic hydrolases on lipid droplets upon which cholesterol is removed from the cell via an ABCA1-dependent cholesterol efflux. Lipid overload in atherosclerosis directly affects lysosomal degradation: modified LDL and cholesterol crystals – a byproduct of hydrolyzed but poorly handled cholesterol - accumulates in lysosomes and render them dysfunctional by preventing optimal acidification and inducing lysosomal leakage. Proatherogenic consequences of this dysfunction include activation of the inflammasome/secretion of IL-1β and an inability to clear apoptotic/dead cells by phagocytosis (efferocytosis).
(B) Lysosomal lipid accumulation/lysosomal dysfunction also prevents optimal autophagic degradation (shown by blue arrows). This could be mediated by similar mechanisms affecting heterophagic degradation as well as others such as accumulation of sphingomyelin. Reduction in autophagy also has several deleterious effects on atherosclerosis. Macrophages lose a parallel mechanism of cellular cholesterol removal mediated by autophagic engulfment of lipid droplets (lipophagy), hydrolysis by LAL, and efflux of cholesterol again by an ABCA1-dependent process. Blockage of this alternate route for cholesterol efflux induces lipid droplet accumulation and foam cell formation. Deficient autophagy also affects homeostatic turnover of organelles (like mitochondria) and the clearance of protein aggregates. Although accumulation of protein aggregates and dysfunctional organelles have not been directly studied in atherosclerosis, their ability to trigger deleterious effects such as ROS generation, apoptosis, and inflammasome activation can be surmised to exacerbate plaque progression.
Internal affairs: Linking macrophage autophagy to atherosclerosis
Macroautophagy (hitherto referred to as autophagy) is the predominant mechanism by which intracellular biomolecules are delivered to and degraded via lysosomes and an increasing body of evidence supports a protective role for macrophage autophagy in plaque progression. The most direct evaluation of this was conducted recently with the demonstration that genetic ablation of the critical autophagy gene ATG5 in macrophages accelerates atherosclerosis and lesion complexity in mouse models [17, 18]. Although several potential mechanisms have been proposed, derangements in two pathways appear to be critical since they are directly linked to lysosomal degradation: 1) decreased lipophagy (i.e. autophagic removal of lipid droplets) with effects on cholesterol efflux, and 2) the accumulation of dysfunctional/cytotoxic organelles and proteins. In the autophagy-deficient setting, inefficiencies in degradation of this cargo triggers deleterious downstream events such as foam cell formation, hyperactivation of inflammasome, and enhanced apoptosis.
As discussed above, exogenously-derived cholesteryl esters are first hydrolyzed in lysosomes before eventual distribution of free cholesterol to different membrane compartments. When membrane cholesterol content exceeds its buffering capacity, lipid droplet formation is favored and is a defining feature of the atherosclerotic foam cell. A primary response to such lipid overload is the efflux of excess cholesterol out of macrophages to nascent HDL lipoproteins. For decades, this process was thought to occur cytoplasmically where a family of cholesteryl ester hydrolases are known to liberate free cholesterol from macrophage lipid droplets followed by ABCA1-mediated efflux to HDL. The discovery of lipophagy first in the liver as a bona fide mechanism of lipid handling [19] led Ouimet et al. to evaluate this in foam cell macrophages. Indeed, autophagic uptake of lipid droplets with subsequent LAL-dependent degradation of cholesteryl esters in lysosomes was shown to be an alternative mechanism of generating free cholesterol for ABCA1-mediated efflux to HDL [20]. Thus, autophagy-deficiency increases macrophage susceptibility to foam cell formation.
Although cholesterol is the focal lipid substrate in lysosomal degradation and dysfunction in atherosclerosis, other lipid species may affect macrophage lysosomal function and cellular phenotypes through mechanisms both dependent and independent of interaction with cholesterol. Sphingomyelin (SM) is a prominent cellular phospholipid and a great deal of evidence supports its multifaceted roles in atherogenesis [21]. The lysosomal acid sphingomyelinase (L-ASM) enzyme, encoded by SMPD1, converts SM to ceramide, and mutations in this gene are the cause of the lysosomal storage disorders Niemann-Pick types A and B. In the setting of atherosclerosis, intracellular SM inhibits autophagosome-lysosome fusion and lysosomal stability [22]. Li et al. recently demonstrated greatly impaired autophagosome-lysosome fusion with SMPD1 knockout in smooth muscle cells, leading to several atherogenic cellular phenotypes [23]. Prior evidence indicates deficiency of L-ASM leads to similar dysfunction in macrophages, an effect that may partially occur through SM strongly binding and sequestering cholesterol within lysosomes [24]. Additionally, transplant of bone marrow lacking SM synthases 1 and 2, which catalyze the reverse reaction of ceramide to SM and cause SM accumulation, partially rescues western-diet induced atherosclerosis. In these models, decreased SM accumulation lead to improved cholesterol efflux, reduced inflammatory cytokine production, and reduced lesion size and complexity [25, 26]. Clearly, lipid signaling has an important role in control of autophagy-lysosomal function independent of cholesterol.
Biomolecules that are degraded through autophagy are quite diverse and not limited to lipids, importantly including organelles (e.g. mitochondria, peroxisomes) and intracellular protein aggregates. Although inefficiencies in degradation start with lysosomal lipid overload in the expanding plaque, it is the inability of macrophages to efficiently turnover organelles and protein aggregates that likely exacerbates atherosclerosis in later stages of disease. These “secondary” autophagy deficiencies have been studied more extensively in other disease models (especially neurodegenerative disorders) but not as rigorously in atherosclerosis. ATG5-null macrophages have inflammasome hyperactivation, increased IL-1β secretion, and enhanced apoptosis, all of which are recapitulated in atherosclerotic plaques of macrophage-specific ATG5-null mice [17, 18]. Although the mechanisms are unclear, intracellular accumulation of dysfunctional proteins and mitochondria is likely involved and would merit detailed investigation. Links between autophagy deficiency and atherosclerosis are summarized in Figure 2B.
Beyond degradation: new functions of lysosomes
We have thus far focused on the classical function of macrophage lysosomes as epicenters of biomolecule degradation. Recent reports however have begun to broaden the roles of lysosomes as platforms for intracellular signaling. Intralysosomal levels of degradation products such as amino acids and lipids appear to modulate downstream signaling pathways, influencing cellular growth and differentiation. Although these new concepts have not been directly examined in the setting of atherosclerosis per se, the notion that macrophage lysosomes have roles in nutrient sensing/processing and downstream signaling has tremendous implications for understanding the pathophysiology. We discuss three pertinent examples below.
First, in a recent report by Huang et al., lysosomal lipolysis was shown to be required for alternative (M2) polarization of macrophages [27]. Although macrophage phenotype is nuanced and typically on a continuum, they have traditionally been categorized as classically activated/pro-inflammatory (so-called M1) versus alternatively activated/anti-inflammatory (so-called M2) based on their cytokine profile and overall functional predilection. Determinants of macrophage polarization are pivotal given the pathogenic role of pro-inflammatory macrophages in atherosclerosis. M2-polarization of bone-marrow derived macrophages with IL-4 leads to concomitant increases in LAL expression and fatty acid oxidation. CD36-mediated lipoprotein uptake and lysosomal lipolysis are both essential as treatment of differentiating macrophages with the lipase inhibitor orlistat or deletion of LAL or CD36 abrogates alternative activation. These results indicate exogenous lipid delivery, endocytic-lysosomal lipid processing, and oxidation of liberated fatty acids in mitochondria are necessary events in macrophage differentiation toward alternative rather than classical activation. Although not yet evaluated in the atherosclerotic plaque, the importance of LAL, lysosomal lipolysis, and macrophage polarization was recently echoed in a recent study by Xu et al. focused on adipose tissue macrophages and obesity [28]. They showed that in settings of diet-induced obesity, expanding adipose tissue is inundated with macrophages that have characteristic increases in lysosomal enzymes including LAL with only secondary increases in traditional pro-inflammatory markers. Furthermore, the lysosomal inhibitor chloroquine impairs lipid metabolism, induces lipid accumulation in macrophages, and decrease overall adipose tissue lipolysis. This data challenges the notion that adipose tissue macrophages are inherently pro-inflammatory and rather suggests a compensatory role in handling the tremendous lipid excess observed in obesity.
Second, recent data by Folick et al. suggests that biomolecules generated in the lysosomes after degradation could also be used as signaling molecules [29]. Using Caenorhabditis elegans as a model organism, the lipid chaperone protein LBP8 was found to shuttle the lipid molecule oleoylethanolamide to bind and activate nuclear hormone receptors NHR-49 and NHR-80. Generation of oleoylethanolamide required an active lysosomal lipase (LIPL-4) which has functional and sequence similarities with mammalian LIPA (encoding LAL). Activation of these nuclear receptors induced transcription of acs-2, a mitochondrial acyl-CoA synthetase, and increased life-span of the animals. This study supports the idea that degradation products of lysosomes are not merely building blocks for new anabolic reactions but also serve as signaling molecules regulating cell metabolism. The presence of such a mechanism in higher organisms and specifically in macrophages and atherosclerosis is unkown but an exciting possibility.
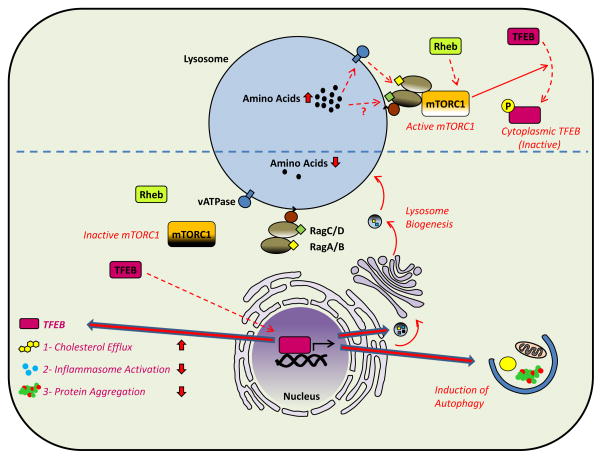
Lastly, an increasing body of work spearheaded by Sabatini and colleagues has defined a highly intriguing intralysosomal amino acid sensing mechanism that modulates mTOR complex 1 (mTORC1), a central regulator of nutrient homeostasis and cellular growth. In this mechanism, activation of mTORC1 is dependent on its localization on the outer face of the lysosomal membrane. Under conditions of nutrient deprivation, lower cellular protein uptake leads to lower lysosomal amino acid content and inactivation of a lysosome-bound vATPase-Ragulator-Rag GTPase complex. This in turn abrogates interaction with and activation of the mTORC1 complex which remains cytoplasmic [30]. In contrast, nutrient excess leads to elevations in lysosomal amino acid concentration and activation of the complex via an as yet undefined “inside-out” amino acid flux mechanism involving induction of a Rag GTPase nucleotide switch [31]. This leads to mTORC1 localization to the lysosomal membrane where its kinase function is activated by the small GTPase Rheb. Thus, amino acid availability in lysosomes is tied to mTORC1 kinase activity and its effector roles such as cellular and organismal growth. These results are important since they establish that degradation and nutrient availability are not distinct but rather strongly integrated via the lysosome. Understanding this new mechanism takes on special significance when considering the long-known atheroprotective effects of mTORC1 suppression (such as seen with rapamycin) as well as a recent study reporting the atheroprotective phenotypes of myeloid-specific mTORC1 deletion [32]. Clearly, further work in this arena would enhance our understanding of nutrient sensing by macrophages in atherosclerosis.
Lysosomal Biogenesis, a mechanism to circumvent lysosomal dysfunction in atherosclerosis
As detailed above, macrophage lysosomes are central to the pathogenesis of atherosclerosis due to their roles as degradative organelles, regulators of lipid metabolism, and modulators of signaling pathways. The progressive lysosomal dysfunction that is a characteristic feature of plaque progression raises the issue of whether lysosomal number or function can be induced as a potential atheroprotective strategy. Recent discovery of the transcription factor TFEB (and its related family member TFE3) as the only known transcriptional activators of a broad network of autophagy and lysosomal genes (i.e. master regulators of lysosomal biogenesis) has enabled serious consideration of this idea [33–35]. Using a macrophage-specific overexpressor of TFEB, Emanuel et al. showed the ability of lysosomal biogenesis to reverse the lysosomal dysfunction instigated by atherogenic lipids and to have several functional benefits such as induction of cholesterol efflux, dampening of inflammasome activation, and clearance of polyubiquitinated protein aggregates [13]. It will be informative to see whether TFEB overexpression in macrophages can also ameliorate atherosclerosis in vivo.
Interestingly, several recent papers have established TFEB as a protein which is tightly regulated by cellular nutrient status and mTORC1 signaling [36–38]. Under conditions of nutrient excess, active mTORC1 complex phosphorylates TFEB and causes its cytoplasmic retention. With nutrient deprivation, unmodified TFEB freely translocates to the nucleus for initiation of a prodegradative transcriptional program [37, 38]. Further studies elucidating the roles of mTOR and TFEB in atherogenesis in vivo should also pay particular attention to lysosomal nutrient-sensing in the context of this mTORC1-TFEB link. Regulation of cellular pathways by lysosome-dependent mechanisms are summarized in Figures 3 and 4.
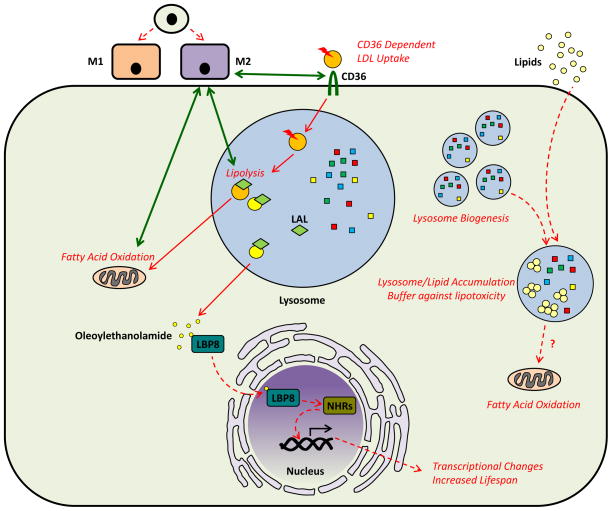
Figure 3. Lysosomes as Platforms for Intracellular Signaling (Lysosomal Acid Lipase and Lipid Signaling).
Molecules generated in lysosomes may play an important role in cellular pathways that affect the differentiation and phenotype of macrophages. A recent example is the relation between LAL-dependent lipid hydrolysis and alternative activation (M2 polarization) of macrophages. In this model, M2 polarization of macrophages induces CD36-mediated uptake of LDL, LAL-dependent degradation in lysosomes, and oxidation of the liberated free fatty acids in mitochondria. Blockage of this pathway at various levels prevents alternative activation of macrophages. Another example of the products of lysosomal degradation being able to regulate cellular pathways comes from studies in C. elegans. Oleoylethanolamide, a product of lysosomal acid lipase, binds the lipid chaperone LBP8, induces its nuclear localization and interaction with nuclear hormone receptors (NHRs). NHR-dependent transcriptional changes manifest as increased lifespan in these simple organisms. Lastly, induction of lysosomal biogenesis in macrophages appears to act as a buffer in the states of lipid overload. Adipose tissue macrophages activate lysosomal biogenesis in order to handle the increased lipid burden of the adipose tissue. Whether the increased generation of free fatty acids as a result of lipid hydrolysis in lysosomes is directly linked to increased oxidation in adjacent mitochondria is not known but is an intriguing possibility. The study of such mechanisms in atherosclerotic macrophages can be very insightful.
Figure 4. Lysosomes as Platforms for Intracellular Signaling (Amino Acid Sensing and mTOR).
Lysosomes have recently been recognized as critical mediators of amino acid sensing in cells. Under states of nutrient deprivation, amino acid concentration is low in lysosomes and mTORC1 (the predominant nutrient-sensing kinase) is inactive. Among its numerous regulatory roles in cellular signaling, mTORC1 has a particularly interesting feedback link to lysosomes. TFEB, a master transcription factor for lysosomal and autophagic genes, is an mTORC1 phosphorylation target. Unphosphorylated (active) TFEB can translocate to the nucleus and initiate autophagy and lysosomal biogenesis which has several atheroprotective features (i.e. increased cholesterol efflux, decreased inflammasome activation, and increased clearance of protein aggregates (bottom). However, under conditions of nutrient excess, high lysosomal amino acid concentration induces a nucleotide switch on Rag GTPases that is at least partially dependent on an inside-out amino acid flux mechanism requiring lysosomal vATPase. An active Rag GTPase complex leads to mTORC1 localization to lysosomal membranes where it is activated by the small GTPase Rheb. Active mTORC1 prevents TFEB nuclear localization by phosphorylation, thus blocking autophagy and lysosomal biogenesis (top).
Conclusion
The discovery of lysosomes by Christian de Duve in the 1950s heralded intense investigation of these little organelles in health and disease. There was even early suggestions by de Duve himself that lysosomal dysfunction might be an important phenomenon in atherosclerotic plaque cells [39, 40]. Unfortunately, the past few decades saw waning interest in the topic until a recent resurgence driven by expanding research in autophagy, the discovery of lysosomes as nutrient-sensing organelles, and the potential for lysosomal biogenesis. Each of these concepts has profound implications in macrophage biology, especially in the context of atherosclerosis. Our goal was to review the emerging literature in this area and lay the framework for promising new avenues of investigation.
Key Points.
Lysosomes, organelles with an ability to process both extracellular (heterophagy) and intracellular cargo (autophagy), have emerged as central mediators of macrophage function in the atherosclerotic plaque.
Inefficient handling of modified lipids in lysosomes appears to initiate a progressive lysosomal dysfunction in atherosclerotic macrophages.
Given the intricate link between autophagy and lysosomes, disruptions in lysosomal processing affect autophagic processing with deleterious consequences in macrophages.
Beyond degradation, recent data implicates lysosomes as platforms for intracellular nutrient sensing and downstream signaling with profound implications in understanding atherogenesis.
The discovery of master transcriptional regulators of lysosomal biogenesis such as TFEB open new avenues of reversing lysosomal dysfunction in atherosclerotic macrophages.
Acknowledgments
Financial support and sponsorship:
B. Razani is supported by the National Heart, Lung, and Blood Institute grants K08 HL098559 and R01 HL125838
Footnotes
Conflicts of interest:
None
References
- 1.Sergin I, Razani B. Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis. Trends Endocrinol Metab. 2014;25:225–234. doi: 10.1016/j.tem.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Settembre C, Ballabio A. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 2014;24:743–750. doi: 10.1016/j.tcb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein JL, Brown MS. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch CL, Sun Y, Arey BJ, et al. Spontaneous atherothrombosis and medial degradation in Apoe−/−, Npc1−/− mice. Circulation. 2007;116:2444–2452. doi: 10.1161/CIRCULATIONAHA.107.701276. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JR, Coleman T, Langmade SJ, et al. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J Clin Invest. 2008;118:2281–2290. [Google Scholar]
- 6.Du H, Heur M, Witte DP, et al. Lysosomal acid lipase deficiency: correction of lipid storage by adenovirus-mediated gene transfer in mice. Hum Gene Ther. 2002;13:1361–1372. doi: 10.1089/104303402760128586. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds T. Cholesteryl ester storage disease: a rare and possibly treatable cause of premature vascular disease and cirrhosis. J Clin Pathol. 2013;66:918–923. doi: 10.1136/jclinpath-2012-201302. [DOI] [PubMed] [Google Scholar]
- 8.Wild PS, Zeller T, Schillert A, et al. A genome-wide association study identifies LIPA as a susceptibility gene for coronary artery disease. Circulation Cardiovascular genetics. 2011;4:403–412. doi: 10.1161/CIRCGENETICS.110.958728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consortium CADCDG. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 10.Jerome WG. Lysosomes, cholesterol and atherosclerosis. Clinical lipidology. 2010;5:853–865. doi: 10.2217/clp.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox BE, Griffin EE, Ullery JC, Jerome WG. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J Lipid Res. 2007;48:1012–1021. doi: 10.1194/jlr.M600390-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res. 2005;46:2052–2060. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- **13.Emanuel R, Sergin I, Bhattacharya S, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34:1942–1952. doi: 10.1161/ATVBAHA.114.303342. This is the first demonstration that harnessing the lysosomal biogenesis response can have functional benefits in atherosclerotic macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. This study demonstrates in situ cholesterol crystal formation in lysosomes and spotlights lysosomal dysfunction as a nidus for inflammasome activation (i.e. sterile inflammation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouimet M, Franklin V, Mak E, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornemann T, Worgall TS. Sphingolipids and atherosclerosis. Atherosclerosis. 2013;226:16–28. doi: 10.1016/j.atherosclerosis.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Truman JP, Al Gadban MM, Smith KJ, Hammad SM. Acid sphingomyelinase in macrophage biology. Cell Mol Life Sci. 2011;68:3293–3305. doi: 10.1007/s00018-011-0686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Li X, Xu M, Pitzer AL, et al. Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med (Berl) 2014;92:473–485. doi: 10.1007/s00109-014-1120-y. This study provides a novel link between acid sphingomyelinase (a lysosomal enzyme) and autophagy and provides the impetus to evaluate such links in macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leventhal AR, Chen W, Tall AR, Tabas I. Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. J Biol Chem. 2001;276:44976–44983. doi: 10.1074/jbc.M106455200. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Fan Y, Liu J, et al. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:1577–1584. doi: 10.1161/ATVBAHA.112.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Huan C, Chakraborty M, et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. 2009;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. This is the first suggestion that lysosomal lipolysis mediated by lysosomal acid lipase can be a critical determinant of macrophage polarization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Xu X, Grijalva A, Skowronski A, et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. Together with the prior study, lysosomal lipid metabolism is again implicated as a potentially protective response in settings of lipid overload such as seen in obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Folick A, Oakley HD, Yu Y, et al. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science. 2015;347:83–86. doi: 10.1126/science.1258857. Although described in simple organisms, this study raises the intriguing prospect of lysosomes as generators of lipid signaling molecules in mammalian systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancak Y, Bar-Peled L, Zoncu R, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoncu R, Bar-Peled L, Efeyan A, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Ai D, Jiang H, Westerterp M, et al. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ Res. 2014;114:1576–1584. doi: 10.1161/CIRCRESAHA.114.302313. This study is the first to implicate macrophage mTORC1 as a proatherogenic pathway. Given the emerging roles of lysosomes in mTORC1 signaling, future work should evaluate this link in the context of atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- *35.Martina JA, Diab HI, Lishu L, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7:ra9. doi: 10.1126/scisignal.2004754. This recent study along with several in the past few years detail the role of transcription factors TFEB and TFE3 as master regulators of autophagy and lysosomal biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, et al. Regulation of TFEB and V-ATPases by mTORC1. Embo J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shio H, Farquhar MG, de Duve C. Lysosomes of the arterial wall. IV. Cytochemical localization of acid phosphatase and catalase in smooth muscle cells and foam cells from rabbit atheromatous aorta. Am J Pathol. 1974;76:1–16. [PMC free article] [PubMed] [Google Scholar]
- 40.Peters TJ, De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974;20:228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]