Abstract
Background
What role should previous cesarean section play in affecting clinical pregnancy outcomes and avoiding the complications of in vitro fertilization? In this article, we focus on elective single-embryo transfer (eSET) versus double-embryo transfer (DET) and assess the clinical efficacy and safety of eSET in patients who have a previous cesarean scar.
Material/Methods
The pregnancy, delivery, and neonatal outcomes of 130 patients who had a previous cesarean scar and received in vitro fertilization-embryo transfer (IVF-ET) were retrospectively analyzed. The number of transferred embryos was chosen depending on patients’ desire after acknowledging all benefits and risks, including eSET (eSET group, n=56) and DET (DET group, n=74). A total of 101 patients with previous vaginal delivery receiving IVF-ET in the same period were included as a control group.
Results
The pregnancy rates, multiple birth rates, abortion rates, ectopic pregnancy rates, gestational age at delivery, preterm birth rates, neonatal birth weight, and take-home baby rates were similar between the previous cesarean section group and the previous vaginal delivery group. A previous cesarean section scar did not affect embryo implantation and pregnancy outcomes in IVF. In the eSET and DET groups of previous cesarean section patients, the embryo implantation rates, pregnancy rates, abortion rates, and take-home baby rates were similar. However, the rate of multiple pregnancies reached 50% in the DET group, which led to more preterm births and lower birth weight.
Conclusions
Elective single-embryo transfer is a well-accepted strategy to avoid multiple pregnancies and improve the obstetric and neonatal outcomes of singleton pregnancy in IVF patients with a previous cesarean section.
MeSH Keywords: Cesarean Section, Fertilization In Vitro, Single Embryo Transfer
Background
According to World Health Statistics 2015, which was published by the World Health Organization (WHO), the use of cesarean section in clinical delivery was very high in all countries for the period 2007–2014. In China, the reported cesarean rate was 27%, which is significantly higher than the goal of less than 15% proposed by WHO during the 1980s [1,2]. An even higher percentage of patients receiving in vitro fertilization-embryo transfer (IVF-ET) accept cesarean section due to the higher cost of embryo implantation and the concerns for the babies [3]. With abolishing of the single-child policy in China, the number of patients with a history of cesarean section choosing IVF-ET as an alternative strategy for their second progeny will increase, as expected.
On the one hand, it has been reported in the literature that a cesarean section scar can reduce the chance of embryo implantation and lead to spontaneous abortion [4,5]. DET increases the embryo implantation and pregnancy rate in an IVF transfer cycle. On the other hand, DET will significantly increase the incidence of iatrogenic multiple pregnancies (IMPs) as well. Excessive uterine distension increases the risk of bleeding [6]. Most uterine rupture cases occur in women with a uterine scar, and multiple pregnancies are a risk factor for uterine rupture for patients who have had a previous cesarean section [7–9]. When a pregnancy implants on a cesarean fibrous tissue scar, a cesarean scar pregnancy (CSP) occurs [10,11]. Multi-embryo transfer is also a risk factor for CSP. As a rare and dangerous type of ectopic pregnancy in IVF-ET, the severe complications of CSP include placenta previa/accreta, uterine rupture, and life-threatening hemorrhage [12–15].
What role should previous cesarean section play in affecting clinical pregnancy outcomes and avoiding the complications of IVF? We conducted a retrospective study to investigate if previous cesarean section affects embryo implantation rate and pregnancy outcomes in patients with previous cesarean section compared to patients with a history of vaginal delivery. Moreover, in this article, we focus on the number of transferred embryos and assess the clinical efficacy and safety of eSET in patients who have a previous cesarean scar.
Material and Methods
Ethics statement
This is a retrospective study. Institutional Ethics Board approval was obtained from the Reproductive Medical Ethics Committee of Nanjing Drum Tower Hospital (Protocol number: 2014002). All participating patients were formally informed of the potential academic use of their clinical data in the future when admitted in the hospital, and the written informed consents were obtained from all participants as well.
Patients, inclusion, and exclusion criteria
A total of 231 patients with history of delivery who received IVF-ET technology in the reproductive medical center of our hospital from January 2012 to September 2014 were enrolled in this study. Patients who did not receive embryo transfer during the oocyte retrieval cycle were excluded. According to the previous ways of delivery, patients were divided into two groups: previous cesarean section group (n=130) and vaginal delivery group (n=101). After the patients were informed of all benefits and risks of single-embryo or double-embryo transfer, they were permitted to choose one-embryo transfer (eSET) or double-embryo transfer (DET) depending on their own desire. Therefore, patients in the cesarean section scar group were further divided into a, eSET group (n=56) and a DET group (n=74). One patient experienced two previous cesarean deliveries; however, the others experienced just one. Ultrasound measurement of scar thickness on the uterus of those who previously gave birth by caesarean section was done before IVF.
Mid-luteal Lupron and ovulation induction
The mid-luteal Lupron (luteal phase down-regulation) protocol was applied for all participants. For the patients with ovulation, administration of gonadotrophin-releasing hormone agonist (GnRH-a) was initiated in mid-luteal phase. Oral contraceptives were first given to the patients without ovulation for 15–17 consecutive days starting from the fourth day of menses. Then, GnRH-a was administered to them for 14–15 consecutive days as well. Ovarian stimulation was carried out with either recombinant follicle-stimulating hormone (FSH) or human menopausal gonadotropin (hMG). When follicles reached pre-ovulatory size (18–22 mm), 5,000 IU of human chorionic gonadotropin (hCG) was administered.
In vitro embryo culture and transfer
Fertilization and embryo culture manipulation were conducted according to the general protocol of our center. Briefly, short-term fertilization or intracytoplasmic sperm injection (ICSI) was performed 3 or 4 hours later after oocyte retrieval. Formation of pro-nucleus was monitored 17–19 hours after insemination. At 66–68 hours after insemination, embryos without multi-nucleus displaying more than 6 blastomeres and fewer than 20% fragments were selected for B-ultrasound-guided transfer. Moreover, luteal support was applied after embryo transfer.
Pregnancy follow-up
Urine pregnancy tests or blood hCG tests were initially conducted 14–16 days after embryo transfer. Transvaginal ultrasound was performed 30–35 days after embryo transfer to confirm the progeny by observation of a gestational sac or fetal heartbeat. Delivery complications and neonatal conditions were recorded after birth.
Observation indicators
Basic information about the patients, such as age, years after cesarean section, body mass index (BMI), gonadotropin (Gn) dose, causes of infertility, fertilization methods, and endometrial thickness for embryo transfer, was recorded. For the comparison of fertilization and embryo development between groups of patients, the fertilization rate, cleavage rate, number of cells in the transferred embryo, and embryo cryopreservation cycle rate were recorded and compared. Moreover, pregnancy rate, embryo implantation rate, rate of multiple pregnancies, early and mid-term abortion rate, ectopic pregnancy rate, take-home baby rate, gestational age of delivery, birth weight, and the rate of occurrence of birth defects were compared between groups as well for understanding the pregnancy, delivery outcomes, and neonatal conditions.
Statistical analysis
Statistical analysis of the data was conducted by using SPSS 17.0 software. Comparison of mean values between two groups was assessed by the Student’s t test. Analysis of the differences in percentages was performed using the χ2 test. P<0.05 was considered to indicate statistical significance.
Results
General information on patients receiving IVF-ET after cesarean delivery and vaginal delivery
The baseline characteristics of the participants were comparable between groups (Table 1). Generally, age was considered as the key factor affecting the clinical outcome of IVF. Patients in different age stages have different pregnancy rates. In our study, the mean age of the group with cesarean section scar (33.61±4.21 years) was lower than that of the vaginal delivery group (34.85±4.03 years). To illustrate the influence of age, we divided the patients into groups according to age (Table 1). Patients were further divided into sub-groups based on their age (<35 years, 35–37 years, and >37 years), and no significant difference was observed between the two groups. As demonstrated in Table 1, other factors such as BMI, dosage of Gn, causes of infertility, fertilization methods, and endometrial thickness were also similar between the two groups. Statistically significant differences were not observed for embryo development-related factors such as the number of retrieved oocytes (9.95±4.60 vs. 9.39±4.20), fertilization rate (87.79% vs. 89.35%), cleavage rate (97.80% vs. 97.99%), cell number of transferred embryo (8.15±0.97 vs. 7.97±0.90), and embryo cryopreservation cycle rate (74.62% vs. 72.28%). The presence of a previous cesarean section scar did not affect fertilization and embryo development after IVF.
Table 1.
General information of patients receiving IVF-ET.
| Previous cesarean section group | Previous vaginal delivery group | P value | |
|---|---|---|---|
| Number of cycles (cycle) | 130 | 101 | |
| Age (years) | 33.61±4.21 | 34.85±4.03 | 0.024* |
| Age | 0.350 | ||
| <35 years (%) | 55.38 (72/130) | 47.52 (48/101) | |
| 35–37 years (%) | 25.38 (33/130) | 25.74 (26/101) | |
| >37 years (%) | 19.23 (25/130) | 26.73 (27/101) | |
| Infertility duration (years) | 3.96±3.00 | 6.95±4.54 | 0.000** |
| BMI | 23.59±3.28 | 22.99±3.28 | 0.175 |
| Gn dosage (IU) | 2622.40±918.76 | 2606.81±741.51 | 0.887 |
| Composition of infertility factors | 0.432 | ||
| Pelvic and oviduct factors (%) | 65.38 (85/130) | 69.31 (70/101) | |
| Male factors (%) | 10.77 (14/130) | 5.94 (6/101) | |
| Other factors (%) | 23.85 (31/130) | 24.75 (25/101) | |
| Composition of fertilization methods | 0.089 | ||
| IVF (%) | 76.15 (99/130) | 85.15 (86/101) | |
| ICSI (%) | 23.85 (31/130) | 14.85 (15/101) | |
| Implant endometrial thickness (mm) | 10.62±1.95 | 11.08±1.92 | 0.072 |
| Mean number of retrieved oocytes | 9.95±4.60 | 9.39±4.20 | 0.335 |
| Fertilization rate (%) | 87.79 (1136/1294) | 89.35 (847/948) | 0.255 |
| Cleavage rate (%) | 97.80 (1111/1136) | 97.99 (830/847) | 0.767 |
| Cell number in implanted embryos (cells) | 8.15±0.97 | 7.97±0.90 | 0.059 |
| Embryo cryopreservation cycle rate (%) | 74.62 (97/130) | 72.28 (73/101) | 0.689 |
Pregnancy and delivery outcomes in patients receiving IVF-ET after cesarean delivery and vaginal delivery
Pregnancy and delivery outcomes of the participants receiving IVF-ET are shown for the previous cesarean section group and the previous vaginal delivery group (Figure 1). Despite the fact that the percentage of patients receiving single-embryo transfer in the previous cesarean section group was significantly higher than that in the previous vaginal delivery group (43.08% vs. 6.93%; P=0.000), the clinical pregnancy rates (58.46% vs. 54.46%) and take-home baby rates (45.38% vs. 40.59%) between the two groups were similar. The implantation rate in the previous cesarean section group was higher than that in the previous vaginal delivery group (49.02% vs. 38.46%; P=0.034). The occurrence of abnormal pregnancy such as multiple pregnancies, abortion, and ectopic pregnancy also was similar between the two groups (32.89% vs. 38.18%, 21.05% vs. 23.64%, and 1.32% vs. 0) (Table 2). The presence of a cesarean section scar did not affect pregnancy outcomes after IVF.
Figure 1.
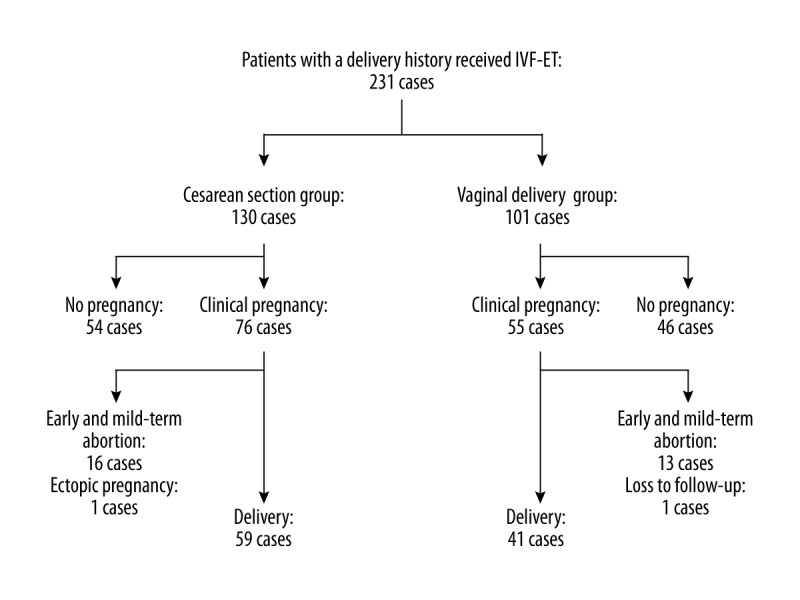
Pregnancy and delivery outcomes of IVF-ET in patients with previous cesarean delivery and patients with vaginal delivery.
Table 2.
Pregnancy and delivery outcomes after IVF-ET.
| Previous cesarean section group | Previous vaginal delivery group | P value | |
|---|---|---|---|
| Number of cycles (cycle) | 130 | 101 | |
| Mean number of implanted embryos (embryos) | 1.57±0.50 | 1.93±0.26 | 0.000** |
| Composition of number of transplanted embryos | 0.000** | ||
| Single-embryo transfer | 43.08 (56/130) | 6.93 (7/101) | |
| Double-embryo transfer | 56.92 (74/130) | 93.07 (94/101) | |
| Clinical pregnancy rate (%) | 58.46 (76/130) | 54.46 (55/101) | 0.542 |
| Embryo implantation rate (%) | 49.02 (100/204) | 38.46 (75/195) | 0.034* |
| Multiple pregnancy rate (%) | 32.89 (25/76) | 38.18 (21/55) | 0.532 |
| Abortion rate (%) | 21.05 (16/76) | 23.64 (13/55) | 0.725 |
| Ectopic pregnancy rate (%) | 1.32 (1/76) | 0 (0/55) | 1.000 |
| Take-home baby rate (%) | 45.38 (59/130) | 40.59 (41/101) | 0.466 |
There were no statistically significant differences between the previous cesarean section group (22.02%,13/59) and the previous vaginal delivery group (12.20%, 5/41) regarding preterm birth related to delivery history. In singleton deliveries among the two groups, preterm birth rates were similar (7.14% vs. 7.41%). However, a significantly lower gestational age of delivery (35.24±2.46 vs. 37.00±1.24; P=0.022) and higher preterm birth rates (58.82% vs. 21.43%; P=0.036) were observed in patients in the previous cesarean section group who experienced multiple delivery (Table 3).
Table 3.
Delivery outcome and neonatal conditions after IVF-ET.
| Previous cesarean section group | Previous vaginal delivery group | |||
|---|---|---|---|---|
| Singleton delivery1 | Multiple delivery2 | Singleton delivery3 | Multiple delivery4 | |
| Number of cases | 42 | 17 | 27 | 14 |
| Live births(cases) | 42 | 35 | 27 | 28 |
| Gestational age of delivery (weeks) | 38.31±1.24 | 35.24±2.46* | 38.44±1.85 | 37.00±1.24* |
| Gestational age of delivery (cases) | ||||
| 37–40 weeks | 39 | 7 | 25 | 11 |
| 34–36 weeks | 3 | 6 | 1 | 3 |
| <34 weeks | 0 | 4 | 1 | 0 |
| Preterm birth rate(%) | 7.14 (3/42) | 58.82 (10/17)* | 7.41 (2/27) | 21.43 (3/14)* |
| Birth weight (g) | 3449.52±486.46 | 2428.57±492.49 | 3379.63±518.92 | 2637.31±388.78 |
| Birth defects (cases) | 0 (0/42) | 3 (3/35) | 0 (0/27) | 0 (0/28) |
Gestational age of delivery P1, 3=0.533; P2, 4=0.022. Preterm birth rate P1, 3=0.967; P2, 4=0.036. Birth weight P1, 3=0.397; P2, 4=0.271. Birth defects P2, 4=0.258.
General information on patients receiving eSET or DET after cesarean delivery
We further divided patients who had previous cesarean section into groups receiving eSET or DET. For general information such as age, years after cesarean section, BMI, dosage of Gn, causes of infertility, fertilization methods, and endometrial thickness, no significant difference was observed between the two groups (Table 4). The embryo development-related indicators were similar as well (Table 4). Although the fertilization rate in the eSET group was lower than that in DET group (84.14% vs. 90.37%; P=0.024), the fertilization rates in both groups were higher than the 65% industry standard (Table 4).
Table 4.
General information of patients receiving eSET or DET after cesarean delivery.
| eSET | DET | P value | |
|---|---|---|---|
| Number of cycles | 56 | 74 | |
| Age (years) | 34.13±3.89 | 33.22±4.41 | 0.224 |
| Infertility duration (years) | 4.16±3.31 | 3.81±2.75 | 0.527 |
| Years after cesarian section | 7.75±4.09 | 7.53±4.00 | 0.756 |
| BMI | 23.95±3.53 | 23.31±3.08 | 0.271 |
| Gn dosage (IU) | 2525.89±935.83 | 2695.44±905.14 | 0.299 |
| Composition of infertility factors | 0.619 | ||
| Pelvic and oviduct factors (%) | 60.71 (34/56) | 68.92 (51/74) | |
| Male factors (%) | 12.50 (7/56) | 9.46 (7/74) | |
| Others (%) | 26.79 (15/56) | 21.62 (16/74) | |
| Composition of fertilization methods | 0.053 | ||
| IVF (%) | 67.86 (38/56) | 82.43 (61/74) | |
| ICSI (%) | 32.14 (18/56) | 17.57 (13/74) | |
| Implant endometrial thickness (mm) | 10.52±2.04 | 10.70±1.89 | 0.608 |
| Mean number of retrieved oocytes | 9.57±4.51 | 10.24±4.67 | 0.412 |
| Fertilization rate (%) | 84.14 (451/536) | 90.37 (685/758) | 0.024* |
| Cleavage rate (%) | 97.56 (440/451) | 97.96 (671/685) | 0.683 |
| Cell number in implanted embryo (cells) | 8.14±0.82 | 8.15±1.03 | 0.970 |
| Embryo cryopreservation cycle rate | 76.69 (43/56) | 72.97 (54/74) | 0.621 |
Pregnancy and delivery outcomes for eSET and DET after cesarean delivery
The implantation outcomes (50.00% vs. 48.65%) for the eSET and DET groups were similar; the clinical pregnancy rate (50.00% vs. 64.86%), abortion rate (25.00% vs. 18.75%), and take-home baby rate (37.50% vs. 51.35%) in these two groups also did not display statistically significant differences (Table 5). However, 50% of IVF pregnancies were multiples in the DET group and carried higher risk to the neonates compared with singleton pregnancy (Table 5). At delivery, multiple DET babies had significantly lower gestational age and birth weight than singleton eSET babies. There was one case of CSP and three cases of birth defects (one case of congenital pyloric obstruction and two cases of congenital heart disease) in the DET group (Table 6).
Table 5.
Pregnancy outcomes between eSET or DET after cesarean delivery.
| eSET | DET | P value | |
|---|---|---|---|
| Number of cycles | 56 | 74 | |
| Implantation rate (%) | 50.00 (28/56) | 48.65 (72/148) | 0.863 |
| Clinical pregnancy rate (%) | 50.00 (28/56) | 64.86 (48/74) | 0.089 |
| Multiple pregnancy rate (%) | 0 (0/28) | 50.00 (24/48) | 0.000** |
| Abortion rate (%) | 25.00 (7/28) | 18.75 (9/48) | 0.519 |
| Ectopic pregnancy rate (%) | 0 (0/28) | 2.08 (1/48) | 1.000 |
| Take-home baby rate (%) | 37.5 (21/56) | 51.35 (38/74) | 0.116 |
Table 6.
The delivery outcome and neonatal conditions between eSET or DET after cesarean delivery.
| eSET | DET | ||
|---|---|---|---|
| Singleton delivery1 | Singleton delivery2 | Multiple delivery3 | |
| Number of cases | 21 | 21 | 17 |
| Live births (cases) | 21 | 21 | 35 |
| Gestational age of delivery (weeks) | 38.24±1.58 | 38.38±0.80 | 35.24±2.46** |
| Gestational age of delivery (cases) | |||
| 37–40 weeks | 18 | 21 | 7 |
| 34–36 weeks | 3 | 0 | 6 |
| <34 weeks | 0 | 0 | 4 |
| Preterm birth rate(%) | 14.29 (3/21) | 0 (0/21) | 58.82 (10/17)** |
| Birth weight (g) | 3252.38±344.77 | 3647.67±533.51** | 2428.57±492.49** |
| Birth defects (cases) | 0 (0/21) | 0 (0/21) | 3 (3/35) |
Gestational age P1, 2=0.785; P1, 3=0.000; P2, 3=0.000. Preterm birth rate P=0.000. Birth weight P1, 2=0.008; P1, 3=0.000; P2, 3=0.000. Birth defects P=0.154.
Discussion
Cesarean section is applied in high-risk pregnancies by using a surgical method [16]. With advances in cesarean section technology and improvements of anesthesia as well as perioperative monitoring, the safety of cesarean section is guaranteed. However, abuse of cesarean section without medical needs resulted in rapid rising of the cesarean section rate throughout the twentieth century with a substantial increase in the last 30 years all over the world [17,18]. The Caesarean section rates of 18–20% in developed countries and 27% in China far exceed the 15% that is recommended by the WHO. The percentage of cesarean sections is even higher in IVF patients. In China, with the single-child policy recently abolished, there will be an increased need for patients who have previously given birth by caesarean section to choose IVF technology as an alternative strategy for their second pregnancy.
Cesarean section does not affect function of the ovary or quality of the ovum unless the hemorrhage caused by previous surgery affects the blood supplies for the uterus or the ovary [19]. In our study, patients with a uterine scar had a 58.46% clinical pregnancy rate, which was similar to that in vaginal delivery group; this suggests that the existence of a uterine scar does not affect embryo implantation. However, the location of embryo implantation is the major factor affecting the pregnancy condition in patients with a cesarean scar. If the location of embryo implantation is outside the scar region, prior cesarean section has little impact on the outcome in the early stage of pregnancy. However, it is more difficult to conduct surgical abortion if the embryo is implanted on the cesarean scar. Moreover, due to the weak myometrial fibers at the scar and the increased volume of gestational sac wrapping in scar tissue, there will be increased risk for massive hemorrhage or even uterine rupture for CSP [20,21]. Furthermore, the risk of spontaneous uterine rupture is also increased in patients experiencing multiple cesarean sections [22].
According to the regulation in China, the number of transferred embryos is limited to fewer than two during the application of assisted reproductive technology unless the recipient’s age is more than 35 years or there was a previous failure of IVF-EF. With double-embryo IVF-ET, the risk of iatrogenic multiple births is significantly increased [23]. In this study, no significant difference was observed for embryo implantation rates, pregnancy rates, abortion rates, and take-home baby rates between eSET or DET in patients receiving previous cesarean section. However, 50% of IVF pregnancies were multiples in the DET group, which may lead to a higher incidence of complications. Twin pregnancy in previous cesarean scar patients was associated with increased risk of placenta previa and uterine scar rupture, preterm birth, and reduced birth weights of twin infants [24,25].
In a previous report to assess the benefits of single-embryo transfer, a significantly increased incidence of preterm births (including the rate of very preterm births) and low neonatal birth weights, respiratory complications, sepsis, and jaundice in neonates were observed for twin pregnancies [26]. Therefore, there are more benefits if patients with a previous cesarean scar prefer eSET. In our study, only 43.08% patients with previous cesarean section and 6.93% patients with previous vaginal delivery accepted eSET. Patients are more willing to choose DET to achieve a better pregnancy rate, and the urging for progeny causes patients to ignore the risk related to twin pregnancy. Moreover, concerns about the low pregnancy rate with eSET and the cost for another embryo transfer cycle also affect patients’ decisions regarding the number of transferred embryos [27].
The primary goal of assisted reproductive technology is to have a healthy baby. Although prior cesarean delivery is not shown to be associated with an increased risk of stillbirth in a subsequent pregnancy [28], increased uterine volume caused by multiple pregnancies may increase intrauterine pressure and lead to ruptures at late stage of pregnancy or delivery, especially for the cases with prior cesarean deliveries [22]. In our study, pregnancy outcomes were similar for eSET and DET patients with a previous cesarean section scar. Reducing the number of embryos transferred to patients is the most effective method to prevent the multiple pregnancies. Therefore, there is no need to transfer more embryos to improve the implantation outcome, while iatrogenic multiple pregnancies result both maternal and fetal risk. Physicians should guide patients properly for accepting high-quality single-embryo IVF-ET to ensure a safer labor and delivery, as well as better neonatal outcome [29].
Conclusions
Due to the similar pregnancy efficiency and clinical outcome of eSET and DET, our data provide valuable information for guiding future patients who previously gave birth by cesarean section to accept eSET in order to ensure the safety of delivery. Since the patients enrolled in our study were limited, a large-scale investigation is need to validate our conclusion.
Footnotes
Disclosure of conflict of interest
None.
Source of support: This work was supported by the Fundamental Research Funds for the Central Universities (021414380035) and the Special Grant for Clinical Medicine science of Jiangsu Province of PR China (BL2014003)
References
- 1.World Health Organization. World Health Statistics. 2015. [Google Scholar]
- 2.Lumbiganon P, Laopaiboon M, Gulmezoglu AM, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007–08. Lancet. 2010;375:490–99. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- 3.Tomic V, Tomic J. Neonatal outcome of IVF singletons versus naturally conceived in women aged 35 years and over. Arch Gynecol Obstet. 2011;284:1411–16. doi: 10.1007/s00404-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki E. Effects of cesarean section on fertility and abortions. J Reprod Med. 1986;31:620–24. [PubMed] [Google Scholar]
- 5.Naji O, Wynants L, Smith A, et al. Does the presence of a Caesarean section scar affect implantation site and early pregnancy outcome in women attending an early pregnancy assessment unit? Hum Reprod. 2013;28:1489–96. doi: 10.1093/humrep/det110. [DOI] [PubMed] [Google Scholar]
- 6.Ismail L, Mittal M, Kalu E. IVF twins: buy one get one free? J Fam Plann Reprod Health Care. 2012;38:252–57. doi: 10.1136/jfprhc-2011-100263. [DOI] [PubMed] [Google Scholar]
- 7.Gardeil F, Daly S, Turner MJ. Uterine rupture in pregnancy reviewed. Eur J Obstet Gynecol Reprod Biol. 1994;56:107–10. doi: 10.1016/0028-2243(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 8.Yap OW, Kim ES, Laros RK., Jr Maternal and neonatal outcomes after uterine rupture in labor. Am J Obstet Gynecol. 2001;184:1576–81. doi: 10.1067/mob.2001.114855. [DOI] [PubMed] [Google Scholar]
- 9.Saciragic L, Mehdizadeh S, Amankwah Y, Singh S. Spontaneous uterine rupture of an unscarred uterus in a twin pregnancy. J Obstet Gynaecol Can. 2015;37:391–92. doi: 10.1016/S1701-2163(15)30249-8. [DOI] [PubMed] [Google Scholar]
- 10.Osborn DA, Williams TR, Craig BM. Cesarean scar pregnancy: Sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med. 2012;31:1449–56. doi: 10.7863/jum.2012.31.9.1449. [DOI] [PubMed] [Google Scholar]
- 11.Maymon R, Halperin R, Mendlovic S, et al. Ectopic pregnancies in Caesarean section scars: The 8 year experience of one medical centre. Hum Reprod. 2004;19:278–84. doi: 10.1093/humrep/deh060. [DOI] [PubMed] [Google Scholar]
- 12.Chiang AJ, La V, Chou CP, et al. Ectopic pregnancy in a cesarean section scar. Fertil Steril. 2011;95:2388–89. doi: 10.1016/j.fertnstert.2011.03.104. [DOI] [PubMed] [Google Scholar]
- 13.Wang CB, Tseng CJ. Primary evacuation therapy for Cesarean scar pregnancy: Three new cases and review. Ultrasound Obstet Gynecol. 2006;27:222–26. doi: 10.1002/uog.2644. [DOI] [PubMed] [Google Scholar]
- 14.Maymon R, Halperin R, Mendlovic S, et al. Ectopic pregnancies in a Caesarean scar: Review of the medical approach to an iatrogenic complication. Hum Reprod Update. 2004;10:515–23. doi: 10.1093/humupd/dmh042. [DOI] [PubMed] [Google Scholar]
- 15.Seow KM, Huang LW, Lin YH, et al. Cesarean scar pregnancy: Issues in management. Ultrasound Obstet Gynecol. 2004;23:247–53. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 16.Lurie S, Glezerman M. The history of cesarean technique. Am J Obstet Gynecol. 2003;189:1803–6. doi: 10.1016/s0002-9378(03)00856-1. [DOI] [PubMed] [Google Scholar]
- 17.Declercq E, Young R, Cabral H, Ecker J. Is a rising cesarean delivery rate inevitable? Trends in industrialized countries, 1987 to 2007. Birth. 2011;38:99–104. doi: 10.1111/j.1523-536X.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- 18.Witt WP, Wisk LE, Cheng ER, et al. Determinants of cesarean delivery in the US: A lifecourse approach. Matern Child Health J. 2015;19:84–93. doi: 10.1007/s10995-014-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eijsink JJ, van der Leeuw-Harmsen L, van der Linden PJ. Pregnancy after Caesarean section: Fewer or later? Hum Reprod. 2008;23:543–47. doi: 10.1093/humrep/dem428. [DOI] [PubMed] [Google Scholar]
- 20.Rheinboldt M, Osborn D, Delproposto Z. Cesarean section scar ectopic pregnancy: A clinical case series. J Ultrasound. 2015;18:191–95. doi: 10.1007/s40477-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riaz RM, Williams TR, Craig BM, Myers DT. Cesarean scar ectopic pregnancy: Imaging features, current treatment options, and clinical outcomes. Abdom Imaging. 2015;40:2589–99. doi: 10.1007/s00261-015-0472-2. [DOI] [PubMed] [Google Scholar]
- 22.Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med. 2001;345:3–8. doi: 10.1056/NEJM200107053450101. [DOI] [PubMed] [Google Scholar]
- 23.Boyle B, McConkey R, Garne E, et al. Trends in the prevalence, risk and pregnancy outcome of multiple births with congenital anomaly: A registry-based study in 14 European countries 1984–2007. BJOG. 2013;120:707–16. doi: 10.1111/1471-0528.12146. [DOI] [PubMed] [Google Scholar]
- 24.Takemura Y, Osuga Y, Fujimoto A, et al. Increased risk of placenta previa is associated with endometriosis and tubal factor infertility in assisted reproductive technology pregnancy. Gynecol Endocrinol. 2013;29:113–15. doi: 10.3109/09513590.2012.706669. [DOI] [PubMed] [Google Scholar]
- 25.Conley D, Strully KW. Birth weight, infant mortality, and race: Twin comparisons and genetic/environmental inputs. Soc Sci Med. 2012;75:2446–54. doi: 10.1016/j.socscimed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Sazonova A, Kallen K, Thurin-Kjellberg A, et al. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99:731–37. doi: 10.1016/j.fertnstert.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Scotland GS, McLernon D, Kurinczuk JJ, et al. Minimising twins in in vitro fertilisation: a modelling study assessing the costs, consequences and cost-utility of elective single versus double embryo transfer over a 20-year time horizon. BJOG. 2011;118:1073–83. doi: 10.1111/j.1471-0528.2011.02966.x. [DOI] [PubMed] [Google Scholar]
- 28.Bahtiyar MO, Julien S, Robinson JN, et al. Prior cesarean delivery is not associated with an increased risk of stillbirth in a subsequent pregnancy: Analysis of U.S. perinatal mortality data, 1995–1997. Am J Obstet Gynecol. 2006;195:1373–78. doi: 10.1016/j.ajog.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 29.Roberts SA, McGowan L, Vail A, Brison DR. The use of single embryo transfer to reduce the incidence of twins: Implications and questions for practice from the ‘towardSET?’ project. Hum Fertil (Camb) 2011;14:89–96. doi: 10.3109/14647273.2011.568037. [DOI] [PubMed] [Google Scholar]


