Abstract
Phyllodes tumours constitute an uncommon but complex group of mammary fibroepithelial lesions. Accurate and reproducible grading of these tumours has long been challenging, owing to the need to assess multiple stratified histological parameters, which may be weighted differently by individual pathologists. Distinction of benign phyllodes tumours from cellular fibroadenomas is fraught with difficulty, due to overlapping microscopic features. Similarly, separation of the malignant phyllodes tumour from spindle cell metaplastic carcinoma and primary breast sarcoma can be problematic. Phyllodes tumours are treated by surgical excision. However, there is no consensus on the definition of an appropriate surgical margin to ensure completeness of excision and reduction of recurrence risk. Interpretive subjectivity, overlapping histological diagnostic criteria, suboptimal correlation between histological classification and clinical behaviour and the lack of robust molecular predictors of outcome make further investigation of the pathogenesis of these fascinating tumours a matter of active research. This review consolidates the current understanding of their pathobiology and clinical behaviour, and includes proposals for a rational approach to the classification and management of phyllodes tumours.
Keywords: classification, fibroadenoma, malignant, metastasis, phyllodes
Introduction
Phyllodes tumours of the breast constitute an uncommon but fascinating group of fibroepithelial neoplasms that have a morphological resemblance to the intracanalicular fibroadenoma at the benign end of the spectrum, but with increased stromal cellularity and leaf-like architecture. Phyllodes tumours are classified into benign, borderline and malignant grade categories on the basis of a constellation of histological parameters, i.e. the degree of stromal cellularity and atypia, mitotic count, stromal overgrowth, and the nature of their tumour borders.1 As each microscopic parameter has two to three tiers of stratification, there are significant challenges in accurate and reproducible categorization.
Apart from grading difficulties, the benign phyllodes tumour shows overlapping features with cellular fibroadenoma, whereas, at the other end of the spectrum, the malignant phyllodes tumour may be mistaken for primary breast sarcoma or spindle cell metaplastic carcinoma. The distinction between benign phyllodes tumour and cellular fibroadenoma is especially problematic on core biopsies. Currently, cellular fibroepithelial lesions diagnosed on core biopsy may be subjected to complete removal through either vacuum-assisted or open excision. Surgical excision is usually the preferred procedure, as it allows negative margins to be obtained in the event that the final diagnosis is a phyllodes tumour. What constitutes a sufficient margin for phyllodes tumours is yet another unresolved dilemma.
Debate regarding the relationship between fibroadenoma, a common benign neoplasm, and phyllodes tumour, a rare tumour with uncertain behaviour, continues. Fibroadenoma-like areas are not infrequently encountered in phyllodes tumours, although the frequency of such an observation is not known.
In this review, we provide a collective stance on these issues that can serve as a practical guide for pathological reporting and understanding of phyllodes tumours.
Grading of phyllodes tumours
The criteria for diagnosis and grading of phyllodes tumours are summarized in the recommendations of the World Health Organization (WHO) classification of tumours of the breast.1 Briefly, phyllodes tumours are diagnosed when the fibroepithelial architecture shows an exaggerated intracanalicular pattern with leaf-like fronds protruding into cystically dilated spaces accompanied by stromal hypercellularity. A benign phyllodes tumour shows mildly increased stromal cellularity as compared with a fibroadenoma, and has minimal nuclear atypia, pushing borders, and mitoses of ≤4/10 high-power fields (HPFs). Stromal overgrowth (defined as the presence of stroma without epithelium in at least one low-power field as observed with a × 4 microscope objective) is not present. The key feature distinguishing a benign phyllodes tumour from a fibroadenoma with an exaggerated intracanalicular growth pattern is the presence of increased stromal cellularity. In the absence of well-developed stromal fronds, the presence of elongated, branching and cleft-like ducts meandering through the cellular stroma, giving a staghorn appearance, may be a histological clue to the diagnosis of a phyllodes tumour.
At the other end of the spectrum, a malignant phyllodes tumour shows marked stromal cellularity and atypia, has permeative margins, and has mitotic activity of at least 10/10 HPFs. Stromal overgrowth is usually easily identified. Phyllodes tumours with intermediate features are assigned to the borderline category. Previous grading schemes have assessed similar histological parameters, including that described by Azzopardi in 1979, which incorporated the nature of the tumour edge, stromal overgrowth, mitotic activity, and cellular atypia.2
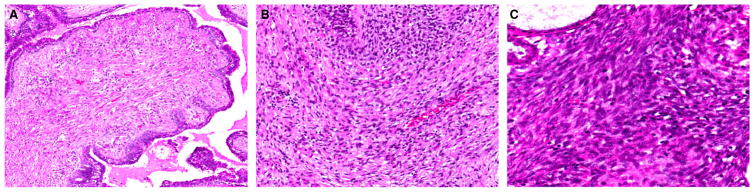
It is important to acknowledge that there are no objective criteria for separating minimal/mild from moderate and marked degrees of stromal hypercellularity and atypia, and this may confound grading attempts. A practical guide for assessing stromal cellularity is to centre on the most cellular zones of the lesion, with mild hypercellularity characterized by a slight increase in stromal cells as compared with normal perilobular stroma, with evenly spaced nuclei that are not touching or overlapping. Marked stromal cellularity shows confluent areas of densely overlapping nuclei, whereas moderate stromal cellularity has findings that are intermediate, with some overlapping stromal nuclei (Figure 1). Mild stromal atypia shows nuclei with little variation in size, with smooth nuclear contours. Moderate atypia shows some variation in nuclear size, with wrinkled nuclear membranes, to an extent exceeding that in mild atypia but less than that in marked atypia. Marked atypia shows marked variation in nuclear size, coarse chromatin, and irregular nuclear membranes with discernible nucleoli (Figure 2).3
Figure 1.
Assessment of stromal cellularity in phyllodes tumours. (A) Mild hypercellularity with slightly increased cellularity, where stromal nuclei are non-overlapping. (B) Moderate hypercellularity with some overlapping stromal nuclei. (C) Marked hypercellularity with many overlapping stromal nuclei.
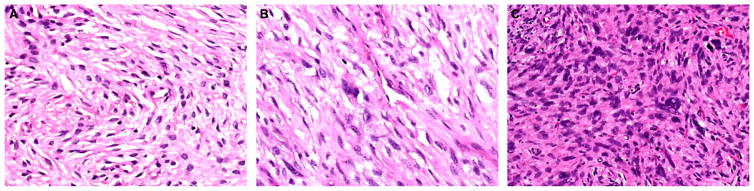
Figure 2.
Assessment of stromal atypia in phyllodes tumours. (A) Mild nuclear atypia shows minimal variation in nuclear size with even chromatin and smooth nuclear contours. (B) Moderate nuclear atypia with more variation in nuclear size and irregular nuclear membranes. (C) Marked nuclear atypia with marked nuclear pleomorphism, hyperchromasia, and irregular nuclear contours.
The perceived clinical relevance of grading phyllodes tumours is to predict clinical behaviour: benign tumours have the potential to locally recur; borderline tumours have the potential to recur locally, and have a very low risk of metastasis; and malignant tumours have the highest risk of metastatic behaviour, which may eventually prove fatal. However, it is accepted that adverse events are, in general, rare for all forms of phyllodes tumours when they are subjected to complete local excision.
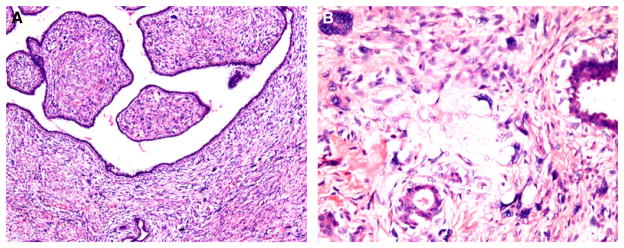
Although the guidelines may appear straightforward, their application can be fraught with ambiguity. Furthermore, how the subdivisions for each microscopic parameter interact to constitute the final grade is subjective. It is also not uncommon for phyllodes tumours to show intratumoral heterogeneity, and harbour features that typify benign lesions in some areas, and characteristics of borderline and malignant lesions in other foci. For instance, a phyllodes tumour with marked stromal atypia and brisk mitotic activity, but without permeative margins or stromal overgrowth, may be considered by some pathologists to be borderline, whereas others may regard the tumour as malignant, owing to different weighting of the relevance of each feature, with prioritization of stromal atypia (Figure 3). A practical approach is to grade a phyllodes tumour as malignant when it shows all of the histological changes of malignancy, and as borderline when not all malignant characteristics are present. The presence of a malignant heterologous element such as liposarcoma, chondrosarcoma or osteosarcoma relegates the tumour into the malignant category regardless of whether other histological parameters (stromal hypercellularity, atypia, mitotic rate, overgrowth, and nature of tumour borders) show changes characteristic of malignant phyllodes tumours. In an effort to comprehend which microscopic parameters are more influential in determining the clinical behaviour of phyllodes tumours, a study of 605 cases concluded that stromal atypia, mitoses, overgrowth and surgical margins (AMOS criteria)4 were of independent significance in predicting behaviour, with surgical margin status being the most important. A nomogram was developed by the use of a mathematical formula that could be applied to counsel patients about their individual risk for recurrence.4
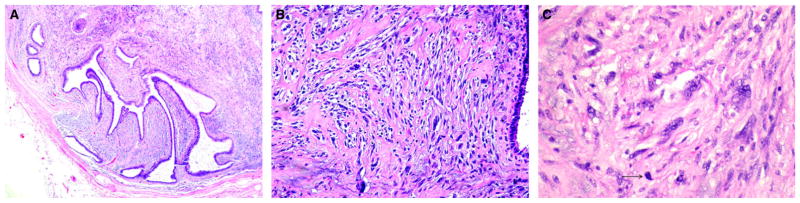
Figure 3.
Phyllodes tumour graded as borderline, as it did not fulfil all criteria of malignancy. (A) Rounded pushing contour of the tumour. (B) Stromal hypercellularity was of moderate degree, accompanied by focally marked nuclear atypia. (C) Higher magnification of atypical stromal cells showed hyperchromatic nuclei, prominent nucleoli, and occasional mitoses (arrow).
Despite the host of biological markers studied in phyllodes tumours, many with an association with grade,5–13 their use in defining grade and potential clinical behaviour in specific cases remains limited.
Biological behaviour and metastatic potential of phyllodes tumours
Recurrence rates in a large Asian series of phyllodes tumours were 10.9%, 14.4% and 29.6% for benign, borderline and malignant phyllodes tumours, respectively. 4 In another review of 33 cases from Germany, recurrence rates were reported to be 8%, 20% and 50% for benign, borderline and malignant tumours, respectively,1 with distant metastases being encountered in 9% of patients with malignant tumours. Overall, recurrence rates in the literature are 10–17%, 14–25% and 23–30% for benign, borderline and malignant phyllodes tumours, respectively.1 Interestingly, in a clinicopathological analysis by Karim et al., there was a suggestion that Asian patients experienced a higher recurrence rate than those of non-Asian ethnicity.14
Grade progression during local recurrence of phyllodes tumours can occur. There have been several suggestions regarding why this happens, including a lack of representative sampling of the initial tumour, tumour heterogeneity with the presence of stromal subclones,15 and loss of stromal–epithelial interdependency. 16
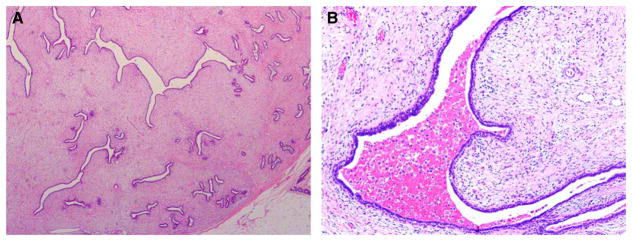
In a study of 335 phyllodes tumours, it was noted that metastases and death from phyllodes tumours were consistently preceded by a primary malignant diagnosis,17 suggesting that a key aim should be to recognize the malignant category, in order for effective therapy to be given at the outset. Metastases in phyllodes tumours invariably indicate a dismal prognosis, with ensuing death.4,18 Distant metastases occur mostly to the lung and skeleton, but almost all organ sites have been affected (Figure 4). Histologically, metastases comprise malignant stromal elements without accompanying epithelium.17,19,20 Two exceptional cases of metastatic phyllodes tumours harbouring an epithelial component have been described as case reports. One represented inclusion of lung alveolar tissue within the metastatic tumour stroma, as confirmed by immunoreactivity of the epithelial component for antisurfactant apoproteins.21 The other case showed floridly hyperplastic, adenosis-like epithelium, rimmed by actin and calponin-positive myoepithelial cells, within both the primary and metastatic tumours, which showed liposarcomatous differentiation.22 The metastatic epithelial component duplicated the immunoreactivity for oestrogen receptor (ER), progesterone receptor and gross cystic disease fluid protein 15 seen in the epithelium of the primary tumour.
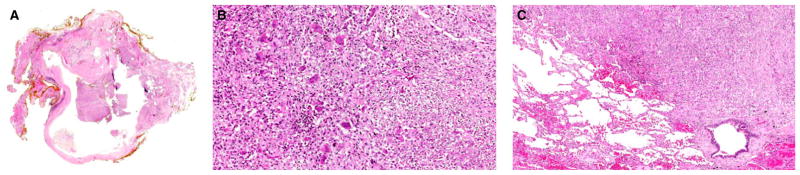
Figure 4.
Malignant phyllodes tumour with metastasis to the lung. (A) Low magnification of the primary breast phyllodes tumour with a cystic space into which stromal fronds projected. Part of the tumour showed a fibroadenoma-like appearance, whereas the remaining parts were more cellular. (B) Higher magnification of the cellular stromal areas showed sheets of plump spindled cells with enlarged vesicular nuclei with distinct nucleoli and scattered mitoses. Several osteoclastic giant cells were dispersed among the spindled cells. (C) Metastasis to the lung 1 year later showed a similar abnormal spindled population with scattered osteoclastic giant cells. No epithelial component was present in the metastasis.
How often do phyllodes tumours metastasize, and do benign tumours ever do so? Table 1 shows metastatic rates according to phyllodes tumour grades that have been described by various authors.4,23–35 The singular documentations of metastatic disease following a diagnosis of benign phyllodes tumour were by Abdalla et al. and Chaney et al., where distant metastases were reported to occur in 3.2%, 11.1% and 28.6% of benign (1/31), borderline (3/27) and malignant (6/21) tumours,26 and in 1.7%, 0% and 26.7% of benign (1/59), borderline (0/12) and malignant (8/30) tumours,23 respectively. However, pathological details of these unusual cases of metastatic benign phyllodes tumour were not provided.
Table 1.
Metastatic rates in phyllodes tumours according to grade
| Authors (case number), publication year | Tumour grade, % (no.) | ||
|---|---|---|---|
| Benign | Borderline | Malignant | |
| Chaney et al.23 (n = 101), 2000 | 1.7 (1/59) | 0 (0/12) | 26.7 (8/30) |
| Chen et al.24 (n = 172), 2005 | 0 (0/131) | 0 (0/12) | 10.3 (3/29) |
| Sotheran et al.25 (n = 50), 2005 | 0 (0/29) | 0 (0/12) | 11.1 (1/9) |
| Abdalla et al.26 (n = 79), 2006 | 3.2 (1/31) | 11.1 (3/27) | 28.6 (6/21) |
| Tan et al.27 (n = 37), 2006 | 0 (0/22) | 0 (0/9) | 50 (3/6) |
| Cheng et al.28 (n = 182), 2006 | 0 (0/138) | 7.7 (1/13) | 9.7 (3/31) |
| Belkacémi et al.29 (n = 443), 2008 | 0 (0/284) | 2.5 (2/80) | 16.5 (13/79) |
| Lenhard et al.30 (n = 33), 2008 | 0 (0/13) | 0 (0/9) | 27.3 (3/11) |
| Guillot et al.31 (n = 165), 2010 | 0 (0/114) | 0 (0/37) | 14.3 (2/14) |
| Tan et al.4 (n = 605), 2012 | 0 (0/440) | 0 (0/111) | 13 (7/54) |
| Jang et al.32 (n = 164), 2012 | 0 (0/82) | 0 (0/42) | 10 (4/40) |
| Sawalhi et al.33 (n = 42), 2013 | 0 (0/16) | 0 (0/9) | 35.3 (6/17) |
| Wang et al.34 (n = 227), 2014 | 0 (0/125) | 1.8 (1/55) | 10.6 (5/47) |
| Bumpers et al.35 (n = 50), 2015 | 0 (0/40) | 0 (0/3) | 28.5 (2/7) |
| Total | 0.13 (2/1524) | 1.62 (7/431) | 16.71 (66/395) |
It may be reasonably inferred that metastatic disease is a vanishingly rare occurrence in benign phyllodes tumours, with the qualification that all tumours should be adequately sampled to account for intratumoral heterogeneity. Conversely, metastatic behaviour is an established risk for malignant phyllodes tumours, albeit still uncommon, and pathological diagnosis should focus on accurately identifying this group of tumours.
Relationship between fibroadenoma and phyllodes tumour
Phyllodes tumours are generally regarded as de-novo lesions derived from periductal and specialized lobular stroma. The initiation of tumorigenesis may hinge on epithelial–stromal interactions. However, the histological overlap between fibroadenoma and phyllodes tumour has long raised the question of pathogenetic kinship. Table 2 shows the studies that have explored this relationship and their salient findings.36–50
Table 2.
Summary of studies evaluating the relationship between fibroadenomas and phyllodes tumours
| Study, year | Summary of findings |
|---|---|
| Noguchi et al.,36 1993 | Epithelial and stromal cells were polyclonal in all of 10 fibroadenomas, whereas stromal cells were monoclonal in all of five phyllodes tumours |
| Noguchi et al.,37 1995 | The same allele of the androgen receptor gene was inactivated in fibroadenomas and phyllodes tumours in each of three patients with both tumours |
| Kasami et al.,38 1998 | 5% (1/20) of ‘complex’ fibroadenomas and 1% (1/25) of ‘simple’ fibroadenomas showed stromal monoclonality. The one ‘simple’ fibroadenoma coexisted with a phyllodes tumour component, which showed similar stromal monoclonality |
| Kuijper et al.,39 2002 | Areas of ‘stromal expansion’ in three of 25 fibroadenomas were monoclonal. In addition, nine of 12 phyllodes tumours showed stromal monoclonality |
| Wang et al.,40 2006 | Phyllodes tumours harboured a subset of LOH loci, which were absent in fibroadenomas. Primary and recurrent phyllodes tumours shared common regions of LOH |
| Hodges et al.,41 2009 | A single, laser-microdissected fibroadenoma and phyllodes tumour (synchronous) showed similar allelic loss (D7S522) in both components, wheras the phyllodes component showed additional losses at TP53 and D22S264 |
| Abe et al.,42 2011 | Eleven of 36 cases of malignant phyllodes tumours were associated with prior diagnoses of fibroadenomas |
| Foucar et al.,43 2012 | A recent report of benign phyllodes tumours that developed in a mother and daughter pair raised the possibility of a genetic predisposition for phyllodes tumour development |
| Lim et al.,44 2014 | Recurrent somatic mutations in exon 2 of MED12 were discovered in 59% of 98 fibroadenomas on exome sequencing, with 71% of mutations occurring in codon 44 |
| Cani et al.,45 2015 | MED12 mutations were found in phyllodes tumours of all histological grades on next-generation sequencing. Additional mutations in p53, RB1 and NF1, as well as high-level copy number alterations, such as amplifications in EGFR and IGF1R, were features of malignant tumours |
| Yoshida et al.,46 2015 | All grades of phyllodes tumours showed MED12 mutations. Microdissection analysis confirmed MED12 mutations to be stroma-confined in fibroadenomas and phyllodes tumours |
| Piscuoglio et al.,47 2015 | Malignant phyllodes tumours were significantly less likely to harbour MED12 mutations than fibroadenomas, and benign and borderline phyllodes tumours |
| Nagasawa et al.,48 2015 | MED12 mutations were found in 67% of fibroadenomas (6/9) and in 45% of phyllodes tumours (5/11) |
| Pfarr et al.,49 2015 | 60% of all fibroepithelial breast lesions (fibroadenomas and phyllodes tumours) showed MED12 mutations. Intracanalicular fibroadenomas showed the highest frequency of mutations (82%), whereas malignant phyllodes tumours were least likely to contain the mutations (20%) |
| Ng et al.,50 2015 | 62.5% (70/112) of phyllodes tumours showed MED12 mutations. Tumours with MED12 mutations were associated with longer disease-free survival, whereas absence of MED12 mutations was correlated with a higher likelihood of recurrence |
LOH, loss of heterozygosity.
Interestingly, a mother and daughter pair with benign phyllodes tumours was also described, raising the possibility of hereditary linkage,43 and a TP53 founder mutation was discovered in phyllodes tumours from Brazil.51 A study from France discovered chromosome imbalances in 55%, 91% and 100% of benign, borderline and malignant phyllodes tumours, respectively, with 1q gains being associated with borderline and malignant grades. It was suggested that phyllodes tumours could be divided into two genetically distinct classes, with benign tumours in one group and borderline/malignant phyllodes in the other.52
More recently, highly recurrent mediator complex subunit 12 (MED12) somatic mutations in exon 2 were discovered in 59% of 98 fibroadenomas studied, 44 with most mutations occurring in codon 44. The same mutation in MED12 is a common genetic anomaly in uterine smooth muscle tumours.53,54 Laser capture microdissection established that MED12 mutations were present in stromal but not in epithelial cells of fibroadenomas. A subsequent study by Ng et al. found that MED12 mutations were also prevalent in phyllodes tumours, with 65.1% of benign, 65.6% of borderline and 42.8% of malignant phyllodes tumours, respectively, showing mutations. 50 The overall rate of MED12 mutations was strikingly similar in phyllodes tumours (62.5%) and fibroadenomas (59%), with a comparable rate of mutations in codon 44 of MED12 supporting a close molecular relationship.44,50 Other studies have confirmed the high prevalence of MED12 mutations in fibroadenomas and phyllodes tumours.45–49 Using targeted next-generation sequencing, Cani et al. found that malignant phyllodes tumours harboured additional genetic aberrations in tumour suppressor genes, consistent with their aggressive biological behaviour.45 Of particular prognostic import is the finding by Ng et al. that tumours with MED12 mutations were significantly associated with longer disease-free survival, possibly related to hormonal dependence.50
Although evidence for the direct evolution of phyllodes tumours from fibroadenomas remains limited, with very recent confirmation of linear progression in some cases,55 it is clear that these fibroepithelial lesions possess molecular similarities, in addition to their striking morphological resemblance in some cases.
Distinguishing cellular fibroadenoma from benign phyllodes tumour
In a study of 21 cellular fibroepithelial lesions evaluated by 10 specialist breast pathologists using the WHO criteria, only for two cases was uniform agreement achieved with regard to whether the lesion represented a cellular fibroadenoma or phyllodes tumour. It is noted, however, that these cases were highly selected from a consultation series, and problematic lesions were therefore over-represented.56 When cellular fibroadenomas and benign phyllodes tumours were combined and compared with borderline and malignant phyllodes tumours as another group, the level of agreement improved considerably, with complete concordance in 53% of cases. These findings testify to the challenges of separating cellular fibroadenomas from benign phyllodes at one end of the spectrum, and also highlight the difficulty of achieving consensus in grading phyllodes tumours (Figure 5). It is important to note that fibroadenomas in the paediatric age group tend to have increased stromal cellularity, which should not be overinterpreted. 57 Of 68 paediatric breast fibroepithelial lesions analysed in a recent study, 16 cases showed mitotic activity. These included 15 fibroadenomas of simple, cellular and juvenile types, 11 of which showed 1–2 mitoses/10 HPF, and the remaining five fibroepithelial lesions showed mitotic activity ranging from 3 to 5/10 HPF.57 In another study, by Ross et al., of breast fibroepithelial tumours in adolescent females aged up to 18 years, the mean stromal mitotic counts/10 HPF for 11 benign phyllodes tumours, five ‘usual’ fibroadenomas, 12 juvenile fibroadenomas and three ‘variant’ juvenile fibroadenomas (with pericanalicular stromal expansion) were 3 (range: 1–7), 1 (range: 0–2), 2 (range: 0–4) and 3 (range: 0–7), respectively.58 Faiz et al. also found stromal mitoses in all fibroadenoma subtypes (cellular, classic, and juvenile) among 119 paediatric cases with up to 5 mitoses/10 HPF being observed in two cases.59 Therefore, apart from potentially increased stromal cellularity in paediatric fibroepithelial tumours, mitotic activity may also be encountered, up to 7 mitoses/10 HPF.57–59 A cautious and measured approach is therefore needed when cellular and mitotically active paediatric fibroepithelial lesions are evaluated. A diagnosis of phyllodes tumour should be based on the finding of well-developed stromal fronds accompanied by increased stromal hypercellularity.
Figure 5.
Cellular fibroepithelial neoplasm that raised the differential diagnosis of a cellular fibroadenoma versus a benign phyllodes tumour. (A) Low magnification showed a few elongated epithelium-lined clefts with stromal mounds. Mild stromal hypercellularity was observed. (B) Higher magnification of a stromal frond pushing into the clefted space that contained blood and haemosiderophages, with accentuation of stromal nuclei in the peri-epithelial zone.
Numerous studies have attempted to analyse the histology of phyllodes tumours.60–64 A study by Choi et al. found a concordance rate of only 60% between core needle biopsies and excision specimens, with larger tumour size being significantly correlated with discordant biopsy results.65 Assessment of clinicoradiological tumour attributes such as size and radiographic density may contribute to clinical decision-making. 66,67 Notwithstanding that, histomorphological assessment of the excised lesion remains the practical gold standard in diagnosis and grading,60,68 with the presence of leafy architecture and increased stromal cellularity typically being used as the discriminants between cellular fibroadenoma and benign phyllodes tumour. Fibroadenomas that contain stromal multinucleated giant cells can also be mistaken for phyllodes tumours,69 and there may be a potential role for Ki67 proliferation activity as an adjunctive aid.3
The question of whether there is always a need to precisely delineate a benign phyllodes tumour from a cellular fibroadenoma arises. The answer is important, as many surgeons would perform a second surgical procedure to achieve negative margins for a benign phyllodes tumour initially enucleated without margin clearance. The WHO Working Group has proposed that the term ‘benign fibroepithelial neoplasm’ be employed in equivocal cases,70 in order to avoid overtreatment. This term, however, should be used sparingly, as it does not represent a new diagnostic category.
There has been both direct and indirect evidence that benign phyllodes tumours may be treated less aggressively. In a study of 37 women with locally recurrent phyllodes tumours, it was concluded that an expectant approach may be acceptable for initially diagnosed benign and borderline tumours, with complete resection being achieved during any subsequent recurrent episode.27 Although most surgeons would be uncomfortable with not re-excising a borderline phyllodes tumour with positive margins, it would be reasonable to assume a ‘watchful waiting’ strategy for benign lesions. The rate of recurrence for fibroadenomas after ultrasound-guided vacuum assisted percutaneous excision is listed as 15%,71 whereas Organ et al. described a recurrence rate of 17% for surgically excised fibroadenomas.72 However, as acknowledged by the authors, determining whether the recurrence was a ‘true recurrence’ of the same tumour or another primary tumour was difficult, if not impossible, owing to the retrospective nature of the study, particularly if the same breast was involved. In a previous article, also by Organ,73 it was stated that ‘recurrences’ of fibroadenomas were undoubtedly serial presentations of multicentric lesions. This contrasts with a 10.9% recurrence rate of benign phyllodes tumours in one series,4 occurring typically at the site of previous surgery. A very low recurrence rate of 3.4% was reported in benign phyllodes tumours in a study by Korean investigators, with all recurrent cases remaining benign,74 without any association with surgical margin status. Teo et al., in a retrospective review of 44 Asian cases, found no cases of local recurrence in benign tumours treated with simple excision (enucleation), regardless of margin status, after a mean follow-up of 47.6 months.75 Hence, a benign phyllodes tumour diagnosed after representative sampling of an excision specimen may be conservatively handled even when positive margins are encountered.
Conversely, malignant phyllodes tumours are associated with a recurrence rate of 29.6%4, with metastases and death being observed in 22%,1 underscoring the need to recognize this subset of aggressive phyllodes tumours for complete surgical eradication.
Distinguishing malignant phyllodes tumour from primary breast sarcoma and spindle cell metaplastic breast carcinoma
At the other end of the histological spectrum, a high-grade spindle cell neoplasm of the breast invokes different diagnostic considerations, namely malignant phyllodes tumour with sarcomatous overgrowth, spindle cell metaplastic breast carcinoma, and primary or secondary breast sarcoma.
The architectural hallmark of leaf-like fronds surmounted by benign glandular epithelium serves to delineate phyllodes tumour from its mimics. In some malignant phyllodes tumours, however, the stromal overgrowth may be so prominent that epithelial elements can be difficult to find, requiring extensive sampling with many sections for their identification. The stroma of a malignant phyllodes tumour may, on occasion, show heterologous sarcomatous differentiation, most frequently liposarcoma, but also including myosarcoma, angiosarcoma, chondrosarcoma, and osteosarcoma (Figure 6). A spindle cell metaplastic breast carcinoma contains varying proportions of a malignant epithelial component, which may be of squamous, glandular or adenosquamous type. Metaplastic carcinomas can also be entirely devoid of frank epithelial elements, or additionally show heterologous mesenchymal differentiation, although liposarcomatous elements are hardly ever seen. The presence of ductal carcinoma in situ adjacent to a malignant mammary spindle cell tumour greatly favours a diagnosis of metaplastic carcinoma. Primary breast sarcomas, which are distinctly uncommon,76 and sarcomas metastatic to the breast, which are exceptionally rare, have no distinguishing histological features of either phyllodes tumour or metaplastic breast carcinoma, and can have histological attributes common to sarcomas at any site. A history of previous or metastatic sarcoma, imaging and clinical correlation may be helpful. Table 3 summarises the features of these three entities.
Figure 6.
Malignant phyllodes tumour with liposarcoma. (A) Stromal fronds contained cells with marked nuclear pleomorphism with a few bizarre cells. (B) Among the abnormal stromal cells were scattered lipoblasts featuring hyperchromatic scalloped nuclei with vacuolated cytoplasm, indicating a liposarcomatous component.
Table 3.
Distinguishing histological features of malignant spindle cell breast lesions
| Tumour | Malignant phyllodes tumour | Spindle cell metaplastic breast carcinoma | Breast sarcoma |
|---|---|---|---|
| Epithelial component | Benign; distinct leaf-like pattern | Malignant | Absent |
| Ductal carcinoma in situ | Usually absent | May be present | Usually absent |
| Squamous differentiation | Usually absent | May be present | Absent |
| Heterologous differentiation | May be present | May be present | Tumour-specific differentiation |
| Broad-spectrum cytokeratins | Usually negative (−/+) in spindle cells | Invariably positive (+/−) in spindle cells | Usually negative (−/+) in spindle cells |
| p63 | Usually negative (−/+) in spindle cells | Usually positive (+/−) in spindle cells | Usually negative (−/+) in spindle cells |
On limited samples such as needle core biopsies, accurate diagnosis of high-grade malignant mammary spindle cell lesions can prove exceedingly challenging, especially when an epithelial element is elusive. The demonstration of diffuse cytokeratin or p63 immunoreactivity in the malignant spindle cells supports a diagnosis of metaplastic carcinoma,77,78 although interpretation must be tempered in cases of focal keratin or p63 expression, as such reactivities have been described in stromal cells of phyllodes tumours.79,80 The utility of p40 in a similar diagnostic setting remains under investigation; so far, it has been found to be more specific but less sensitive than p63.80–82 However, like p63, p40 may stain stromal cells of phyllodes tumours in some cases. CD34 reactivity, which is well described in the stromal cells of phyllodes tumours, has been reported to be inversely related to adverse histological features;6,8,13,83–86 this assumes importance if CD34 were to be considered for diagnostic utility in differentiating high-grade spindle cell lesions of the breast, as malignant phyllodes tumours are less likely to express CD34. Other markers, including bcl-286,87 which is more frequently expressed in phyllodes tumours, CD117,83,88,89 which shows increased expression in higher-grade phyllodes tumours, and sarcoma-specific molecular cytogenetic alterations are possible diagnostic adjuncts. Although routinely employed in the diagnosis of fibromatosis that may occur in the breast, aberrant nuclear expression of β-catenin has been reported in the stroma of phyllodes tumours, as well as in metaplastic carcinomas.90–94 The use of β-catenin as a solitary marker in the assessment of mammary spindle cell lesions must be cautioned against.
Adequate sampling, entailing at least one section per centimetre of maximal tumour size, with additional sampling of grossly heterogeneous areas and meticulous morphological assessment, remains the keystone of diagnosis, buttressed by clinical, radiological and immunohistochemical correlation.
Epithelial–stromal interactions in phyllodes tumours
In 1992, Sawhney et al. observed that stromal mitotic activity tended to occur close to the epithelial compartment in fibroepithelial lesions, and hypothesized that stromal growth in these tumours depended, in part, on the epithelial component. It was suggested that increasing malignancy correlated with loss of stromal dependency on the epithelium.95
Since then, there have been a number of studies supporting this view. Sawyer et al. demonstrated allelic imbalances in chromosomes 3p and 1q in both epithelial and stromal elements of phyllodes tumours.96 These authors also noted that stromal proliferation in benign phyllodes tumours was dependent on abnormalities in the Wnt pathway consequent to Wnt5a expression in the epithelial component, with malignant progression being linked to independence from the latter.91 E-cadherin is a known epithelial differentiation marker that is affected by the Wnt signalling pathway, and its expression in the epithelium of phyllodes tumours was correlated with recurrence and shorter tumour-specific survival.97 Feakins et al. found epithelial platelet-derived growth factor (PDGF)/stromal PDGF receptor-β copositivity that correlated with disease-related death in 43% of phyllodes tumours.98 Clonal abnormalities have been detected in both epithelial and stromal components of phyllodes tumours in studies by Dietrich et al.99 and Kuijper et al.39 Additional work on biomarker expression in epithelial and stromal elements of phyllodes tumours lends further credence to their interactions.16,97,100–102 For example, the level of CXCR4, an epithelial–stromal interaction-related molecule, was found to be increased in the stromal component of higher-grade phyllodes tumours.10
Hormone receptors
Table 4 summarizes findings from studies that have evaluated the expression of hormone receptors in phyllodes tumours,103–109 with one report documenting HER2/c-erbB2 reactivity as well.106 ERα expression was confined to the epithelial compartment, without any stromal positivity, with one study demonstrating an inverse correlation with grade.106 ERβ expression, on the other hand, has been observed in stromal cells of phyllodes tumours.108,109 The practical significance of these observations is unclear. Although the current data indicate a limited role for hormones in phyllodes tumours, the recently discovered MED12 mutations in these tumours may lead to a resurgence of interest, as the MED12 abnormality is linked to aberrantly activated oestrogen signalling.44,50
Table 4.
Summary of studies evaluating hormone receptor expression in phyllodes tumours
| Study, year | Summary of findings |
|---|---|
| Rao et al.,103 1981 | With the dextran charcoal method, tissue samples from five phyllodes tumours and 13 fibroadenomas were analysed for hormone receptors. PR was expressed in five of five phyllodes tumours and in 11 of 13 fibroadenomas. ER was expressed in one of five phyllodes tumours and in two of 13 fibroadenomas. The volumes occupied by epithelium and stroma in each tumour were in keeping with stromal expression of PR and epithelial expression of ER |
| Mechtersheimer et al.,104 1990 | Three phyllodes tumours and 13 fibroadenomas studied showed PR and ER expression confined to ductal epithelial cells |
| Singh et al.,105 1996 | Sixteen phyllodes tumours (nine benign; seven malignant) studied showed PR expression in all cases (16/16) and ER expression in most cases (12/16). Hormone receptor expression was limited to luminal epithelial nuclei |
| Shpitz et al.,106 2002 | Twenty-three phyllodes tumour studied showed epithelial c-erb-B2 (HER2) reactivity in 61% of cases; however, no correlation with histological features was found |
| Tse et al.,107 2002 | One hundred and forty-three phyllodes tumours studied showed an inverse relationship between epithelial hormonal (ER and PR) expression and tumour grade. Hormonal expression was largely confined to the epithelial component, with ER positivity in 58% of the epithelium and 2.8% of stromal cells, and PR positivity in 74.8% of the epithelium and 1.4% of stromal cells. In the same study, AR expression in both the stromal and epithelial components of phyllodes tumours was low (<5%) across all three tumour grades. Epithelial ER expression showed correlation with stromal mitotic activity, and was predictive of tumour grade |
| Sapino et al.,108 2006 | Thirty-three fibroadenomas and 40 phyllodes tumours studied showed ERα expression confined to epithelial cells, whereas this was undetectable in tumour stroma. Conversely, ERβ was expressed by both epithelial and stromal components of these tumours, which may be related to differentiation of stromal fibroblasts towards a myofibroblastic phenotype, as implied by correlation of ERβ positivity with stromal smooth muscle actin and calponin expression |
| Kim et al.,109 2012 | Eighty-two phyllodes tumours studied showed stromal ERβ expression in 24 (29.3%) cases, whereas ERα and PR were expressed only in the epithelial component. Stromal Ki67 expression correlated with epithelial ERβ, epithelial AR and stromal ERβ expression. However, no significant association was found between hormonal receptor expression and phyllodes tumour grade |
AR, androgen receptor; ER, oestrogen receptor; PR, progesterone receptor.
Note: apart from the first study, the remaining referenced studies utilized immunohistochemical analysis.
Surgical margins
The mainstay of phyllodes tumour management has traditionally consisted of surgical excision with wide tumour-free margins, generally defined by some authors as at least 10 mm.31 However, more recent data suggest that not all phyllodes tumours require excision with such wide margins.32,110,111 In cases of large tumours, this may render breast conservation impracticable.112,113 Table 5 summarizes pertinent findings from studies that have addressed surgical margins in phyllodes tumours.23–25,28,32,110,111,114–120
Table 5.
Surgical margins in phyllodes tumours
| Study, year | Pertinent findings | Definition of a wide margin |
|---|---|---|
| Ciatto et al.,114 1992 | A multicentre study of 59 cases found enucleation and wide excision to be associated with a greater incidence of local recurrence (three of five cases and 12 of 30 cases respectively) than mastectomy (two of 24 cases) | Not specified |
| Reinfuss et al.,115 1996 | A study of 170 cases showed wide local excision to achieve 5-year disease-free survival rates of 98.7%, 80% and 75% for patients with benign, borderline and malignant tumours, respectively | 10–20 mm |
| Barth,116 1999 | A MEDLINE review of 944 cases showed differences in local recurrence rates of benign, borderline and malignant tumours to be 21% (111/540), 46% (18/39) and 65% (26/40) after local excision, and 8% (17/212), 29% (20/68) and 36% (16/45) after wide excision, respectively | 10–20 mm |
| Mangi et al.,117 1999 | A study of 40 cases showed post-excision recurrence to be confined to cases with positive margins, or margins <10 mm | 10 mm |
| Chaney et al.,23 2000 | A study of 101 patients showed low recurrence rates (actuarial 10-year local failure rates of 7% for benign and borderline tumours, and 9% for malignant tumours, respectively) when local excision with appropriate surgical margins was used as primary treatment of phyllodes tumours, provided that the tumour-to-breast ratio was amenable to good cosmesis | ≥10 mm |
| Chen et al.,24 2005 | A study of 172 cases showed local excision to be associated with a high percentage (18.3%) of positive margins | >10 mm |
| Sotheran et al.,25 2005 | A retrospective analysis of 50 phyllodes tumours found breast conservation surgery to be as successful as mastectomy, provided that margins were sufficiently wide | >1 mm |
| Macdonald et al.,118 2006 | A SEER review of 821 malignant phyllodes tumours from 1983 to 2002 found no benefit conferred by mastectomy over wide excision with regard to disease-specific mortality | Not specified |
| Cheng et al.,28 2006 | A study of 182 phyllodes tumours showed positive surgical margins to be the only independent predictor of recurrence. Tumour grade progression was found in 16% of recurrent cases | 10–20 mm |
| Jang et al.,32 2012 | A study of 164 cases revealed no significant local control advantage conferred by wide margins over narrower margins, provided that the narrower margins were tumour-negative | 10 mm |
| Lin et al.,111 2013 | A study of 33 cases showed no relationship between width of surgical margin and disease recurrence | >10 mm |
| Mitus et al.,119 2014 | A study of 70 cases showed no significant difference in 5-year disease-free survival between patients treated with mastectomy (n = 34, 82.4%) and those treated with breast conservation surgery with clear microscopic margins (n = 36, 83.3%) | ≥10 mm |
| Onkendi et al.,110 2014 | A study of 67 cases of borderline and malignant phyllodes tumours showed the extent of surgical excision to have no impact on disease-free survival | ≥10 mm |
| Yom et al.,120 2015 | A study of 285 cases investigated the benefit of a second excision following initial inadequate (<10 mm) clearance. Tumour size and mitotic activity were found to be independently prognostic of local recurrence, whereas margin status and surgical procedure were not. It was proposed that wide margins, if necessary via re-excision, should be the goal in treating small (<50 mm) tumours with high mitotic activity (>10 mitoses per 10 high-power fields), as these tumours constituted a distinct group associated with a significant (55.6%) local recurrence rate | 10 mm |
Drawing a parallel from an opinion advanced by Wood regarding the issue of surgical margins in invasive breast cancer, there appears to be a dearth of data supporting a precise width of tumour-free tissue that is significantly associated with reduced tumour recurrence.121 Although an increasing amount of normal tissue confers greater confidence in the adequacy of excision (with ensuing diminishing cosmetic results), a single layer of cells between the tumour and the surgical plane constitutes, in theory, a clear margin.121 This does not take into account myriad factors that may undermine the accuracy of representation in any slide, including tumour irregularity, multifocality, ink seepage, sampling and technical sectioning issues, among others. Onkendi et al., in a study of 67 borderline and malignant phyllodes tumours from the Mayo Clinic, found that the extent of surgical excision had no impact on disease-free survival.110 An analysis of 164 cases by Jang et al. revealed no significant local control advantage conferred by wide (at least 10 mm) margins over narrower margins.32 Lin et al., in a single-institution series of 33 cases, found no relationship between width of surgical margin and disease recurrence.111
Notwithstanding the above, many institutions elect to offer additional surgical treatment for close margins. Mangi et al., in a study of 40 cases from the Massachusetts General Hospital, found that post-excision recurrences were confined to cases with positive margins, or margins of <10 mm. Following re-excision with a 10-mm clearance, patients remained recurrence-free.117 Yom et al., in a recent Korean study of 285 cases, investigated the benefit of a second excision following initial ‘inadequate’ (<10 mm) clearance. Tumour size and mitotic activity were found to be independently prognostic of local recurrence, whereas margin status and surgical procedure were not. On the basis of these findings, the group proposed that wide margins, if necessary via re-excision, should be the goal in treating small (<50 mm) tumours with high mitotic activity (>10 mitoses/10 HPFs), as these tumours constituted a distinct group associated with a significant (55.6%) local recurrence rate.120
As convincing evidence for an appropriate margin width in surgically excised phyllodes tumours remains elusive, it may be pragmatic to consider tumour on ink, or <1 mm, as a positive margin. A conservative approach can be accorded to benign phyllodes tumours that have been initially enucleated without margins.27,74,75 Excision with negative margins should be achieved for recurrent and malignant phyllodes tumours.
Because of the infrequency of nodal disease in phyllodes tumours, most investigators do not recommend routine axillary dissection.29,115,117,122–124
Adjuvant therapy
Adjuvant radiation therapy has been offered to patients with malignant phyllodes tumours on an individualized basis, although its precise role is controversial.23,118,125–128 An analysis of 3120 malignant cases from the US National Cancer Data Base by Gnerlich et al. showed a pronounced increase in the use of radiotherapy (9.5% in 1998–1999 versus 19.5% in 2008–2009), which, although being associated with reduced local recurrence, had no impact on disease-free or overall survival.129 In a study of malignant phyllodes tumours by Mitus et al., conservatively treated cases were subjected to radiation if tumour-free margins were <10 mm, whereas no adjuvant therapy was administered if margins were wide (≥10 mm). The two conservatively treated groups showed identical 5-year disease-free survival rates.119 Belkacémi et al., in an analysis of cases collected from the Rare Cancer Network between 1971 and 2003, found that adjuvant radiotherapy for borderline and malignant tumours yielded superior 10-year local control rates (86% with radiation versus 59% without radiation), but no survival benefit.29
There are no randomized clinical trials assessing the role of adjuvant chemotherapy in malignant phyllodes tumours. The merits of systemic therapy are therefore considered on a case-by-case basis.
Summary and practical recommendations
Phyllodes tumours present distinct challenges relating to their diagnosis, classification, predicted behaviour, and clinical management. On the basis of the currently available knowledge, a few practical recommendations may be useful:
Grading of phyllodes tumours should aim to achieve accuracy and consistency at the benign and malignant ends of the spectrum.
Definitive distinction between cellular fibroadenomas and benign phyllodes tumours may not be crucial, in light of similar reported recurrence rates. The term benign fibroepithelial lesion/neoplasm may be recommended for cases where clear diagnostic distinction cannot be made, although this should be used sparingly.
Malignant phyllodes tumours are diagnosed when there are marked stromal hypercellularity, atypia, increased mitoses of ≥10/10 HPFs, permeative tumour borders, and stromal overgrowth. The presence of a malignant heterologous component places the tumour into the malignant category regardless of other histological features.
A conservative approach can be accorded to benign phyllodes tumours that have been initially enucleated without margins.
Excision with negative margins should be achieved for recurrent and malignant phyllodes tumours. Most would recommend that borderline tumours should also be completely excised. Although the literature often refers to a margin width of at least 10 mm, a robust evidence base to support this approach is lacking. Therefore, an ideal margin width remains to be determined, and may need to be considered in relation to factors such as tumour size and cosmesis.
From a diagnostic and management perspective, it is important to accurately recognize malignant phyllodes tumours, which should be surgically eradicated and effectively treated at diagnosis, as these tumours have a well-established but relatively infrequent risk of metastasis and death.
The role of adjuvant radiation therapy in borderline and malignant tumours remains to be defined. Routine axillary dissection is not recommended.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest with respect to the authorship, research and/or publication of this article
References
- 1.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. World Health Organization Classification of Tumours of the Breast. Lyon: IARC Press; 2012. [Google Scholar]
- 2.Azzopardi J. Problems in Breast Pathology. London: WB Saunders; 1979. pp. 346–365. [PubMed] [Google Scholar]
- 3.Jara-Lazaro AR, Akhilesh M, Thike AA, Lui PC-W, Tse GM-K, Tan PH. Predictors of phyllodes tumours on core biopsy specimens of fibroepithelial neoplasms. Histopathology. 2010;57:220–232. doi: 10.1111/j.1365-2559.2010.03607.x. [DOI] [PubMed] [Google Scholar]
- 4.Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65:69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 5.Tsang JYS, Ni Y-B, Ng EK, et al. MicroRNAs are differentially deregulated in mammary malignant phyllodes tumour. Histopathology. 2015;67:294–305. doi: 10.1111/his.12648. [DOI] [PubMed] [Google Scholar]
- 6.Vilela MHT, de Almeida FM, de Paula GM, et al. Utility of Ki- 67, CD10, CD34, p53, CD117, and mast cell content in the differential diagnosis of cellular fibroadenomas and in the classification of phyllodes tumors of the breast. Int J Surg Pathol. 2014;22:485–491. doi: 10.1177/1066896914521290. [DOI] [PubMed] [Google Scholar]
- 7.Tan WJ, Thike AA, Bay BH, Tan PH. Immunohistochemical expression of homeoproteins Six1 and Pax3 in breast phyllodes tumours correlates with histological grade and clinical outcome. Histopathology. 2014;64:807–817. doi: 10.1111/his.12329. [DOI] [PubMed] [Google Scholar]
- 8.Ho SK, Thike AA, Cheok PY, Tse GM-K, Tan PH. Phyllodes tumours of the breast: the role of CD34, vascular endothelial growth factor and β-catenin in histological grading and clinical outcome. Histopathology. 2013;63:393–406. doi: 10.1111/his.12177. [DOI] [PubMed] [Google Scholar]
- 9.Kim G-E, Kim J-H, Lee KH, et al. Stromal matrix metalloproteinase-14 expression correlates with the grade and biological behavior of mammary phyllodes tumors. Appl Immunohistochem Mol Morphol. 2012;20:298–303. doi: 10.1097/PAI.0b013e318235a132. [DOI] [PubMed] [Google Scholar]
- 10.Kwon JE, Jung W-H, Koo JS. Molecules involved in epithelial– mesenchymal transition and epithelial–stromal interaction in phyllodes tumors: implications for histologic grade and prognosis. Tumour Biol. 2012;33:787–798. doi: 10.1007/s13277-011-0296-9. [DOI] [PubMed] [Google Scholar]
- 11.Ang MK, Ooi AS, Thike AA, et al. Molecular classification of breast phyllodes tumors: validation of the histologic grading scheme and insights into malignant progression. Breast Cancer Res Treat. 2011;129:319–329. doi: 10.1007/s10549-010-1204-5. [DOI] [PubMed] [Google Scholar]
- 12.Tsai W-C, Jin J-S, Yu J-C, Sheu L-F. CD10, actin, and vimentin expression in breast phyllodes tumors correlates with tumor grades of the WHO grading system. Int J Surg Pathol. 2006;14:127–131. doi: 10.1177/106689690601400204. [DOI] [PubMed] [Google Scholar]
- 13.Chen CM, Chen CJ, Chang CL, Shyu JS, Hsieh HF, Harn HJ. CD34, CD117, and actin expression in phyllodes tumor of the breast. J Surg Res. 2000;94:84–91. doi: 10.1006/jsre.2000.6001. [DOI] [PubMed] [Google Scholar]
- 14.Karim RZ, Gerega SK, Yang YH, et al. Phyllodes tumours of the breast: a clinicopathological analysis of 65 cases from a single institution. Breast. 2009;18:165–170. doi: 10.1016/j.breast.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Karim RZ, O’Toole SA, Scolyer RA, et al. Recent insights into the molecular pathogenesis of mammary phyllodes tumours. J Clin Pathol. 2013;66:496–505. doi: 10.1136/jclinpath-2012-201082. [DOI] [PubMed] [Google Scholar]
- 16.Karim RZ, Scolyer RA, Tse GM, Tan P-H, Putti TC, Lee CS. Pathogenic mechanisms in the initiation and progression of mammary phyllodes tumours. Pathology. 2009;41:105–117. doi: 10.1080/00313020802579342. [DOI] [PubMed] [Google Scholar]
- 17.Tan P-H, Jayabaskar T, Chuah K-L, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol. 2005;123:529–540. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 18.Goh CHR, Lim YP, Su JW, et al. Cardiopulmonary thromboembolism of epithelioid angiosarcoma arising from malignant phyllodes tumour of the breast. J Clin Pathol. 2014;67:450–454. doi: 10.1136/jclinpath-2013-202118. [DOI] [PubMed] [Google Scholar]
- 19.Tsubochi H, Sato N, Kaimori M, Imai T. Osteosarcomatous differentiation in lung metastases from a malignant phyllodes tumour of the breast. J Clin Pathol. 2004;57:432–434. doi: 10.1136/jcp.2003.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez BB, Hernanzez FJ, Spindler W. Metastatic cystosarcoma phyllodes: a light and electron microscopic study. Cancer. 1976;37:1737–1746. doi: 10.1002/1097-0142(197604)37:4<1737::aid-cncr2820370419>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.West TL, Weiland LH, Clagett OT. Cystosarcoma phyllodes. Ann Surg. 1971;173:8. doi: 10.1097/00000658-197104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kracht J, Sapino A, Bussolati G. Malignant phyllodes tumor of breast with lung metastases mimicking the primary. Am J Surg Pathol. 1998;22:1284–1290. doi: 10.1097/00000478-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Chaney AW, Pollack A, McNeese MD, et al. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89:1502–1511. doi: 10.1002/1097-0142(20001001)89:7<1502::aid-cncr13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Chen W-H, Cheng S-P, Tzen C-Y, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol. 2005;91:185–194. doi: 10.1002/jso.20334. [DOI] [PubMed] [Google Scholar]
- 25.Sotheran W, Domjan J, Jeffrey M, Wise MH, Perry PM. Phyllodes tumours of the breast—a retrospective study from 1982–2000 of 50 cases in Portsmouth. Ann R Coll Surg Engl. 2005;87:339–344. doi: 10.1308/003588405X51128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdalla HM, Sakr MA. Predictive factors of local recurrence and survival following primary surgical treatment of phyllodes tumors of the breast. J Egypt Natl Cancer Inst. 2006;18:125–133. [PubMed] [Google Scholar]
- 27.Tan EY, Tan PH, Hoon TP, et al. Recurrent phyllodes tumours of the breast: pathological features and clinical implications. ANZ J Surg. 2006;76:476–480. doi: 10.1111/j.1445-2197.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S-P, Chang Y-C, Liu T-P, Lee J-J, Tzen C-Y, Liu C-L. Phyllodes tumor of the breast: the challenge persists. World J Surg. 2006;30:1414–1421. doi: 10.1007/s00268-005-0786-2. [DOI] [PubMed] [Google Scholar]
- 29.Belkacémi Y, Bousquet G, Marsiglia H, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492– 500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 30.Lenhard MS, Kahlert S, Himsl I, Ditsch N, Untch M, Bauerfeind I. Phyllodes tumour of the breast: clinical follow-up of 33 cases of this rare disease. Eur J Obstet Gynecol Reprod Biol. 2008;138:217–221. doi: 10.1016/j.ejogrb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Guillot E, Couturaud B, Reyal F, et al. Management of phyllodes breast tumors. Breast J. 2011;17:129–137. doi: 10.1111/j.1524-4741.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 32.Jang JH, Choi M-Y, Lee SK, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol. 2012;19:2612–2617. doi: 10.1245/s10434-012-2307-5. [DOI] [PubMed] [Google Scholar]
- 33.Sawalhi S, Al-Shatti M. Phyllodes tumor of the breast: a retrospective study of the impact of histopathological factors in local recurrence and distant metastasis. Ann Saudi Med. 2013;33:162–168. doi: 10.5144/0256-4947.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Wang X, Wang C-F. Comparison of clinical characteristics between benign borderline and malignant phyllodes tumors of the breast. Asian Pac J Cancer Prev. 2014;15:10791–10795. doi: 10.7314/apjcp.2014.15.24.10791. [DOI] [PubMed] [Google Scholar]
- 35.Bumpers HL, Tadros T, Gabram-Mendola S, et al. Phyllodes tumors in African American women. Am J Surg. 2015;210:74–79. doi: 10.1016/j.amjsurg.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi S, Motomura K, Inaji H, Imaoka S, Koyama H. Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. 1993;53:4071–4074. [PubMed] [Google Scholar]
- 37.Noguchi S, Yokouchi H, Aihara T, et al. Progression of fibroadenoma to phyllodes tumor demonstrated by clonal analysis. Cancer. 1995;76:1779–1785. doi: 10.1002/1097-0142(19951115)76:10<1779::aid-cncr2820761015>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Kasami M, Vnencak-Jones CL, Manning S, Dupont WD, Jensen RA, Page DL. Monoclonality in fibroadenomas with complex histology and phyllodal features. Breast Cancer Res Treat. 1998;50:185–191. doi: 10.1023/a:1006050208157. [DOI] [PubMed] [Google Scholar]
- 39.Kuijper A, Buerger H, Simon R, et al. Analysis of the progression of fibroepithelial tumours of the breast by PCR-based clonality assay. J Pathol. 2002;197:575–581. doi: 10.1002/path.1161. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZC, Buraimoh A, Iglehart JD, Richardson AL. Genomewide analysis for loss of heterozygosity in primary and recurrent phyllodes tumor and fibroadenoma of breast using single nucleotide polymorphism arrays. Breast Cancer Res Treat. 2006;97:301–309. doi: 10.1007/s10549-005-9124-5. [DOI] [PubMed] [Google Scholar]
- 41.Hodges KB, Abdul-Karim FW, Wang M, et al. Evidence for transformation of fibroadenoma of the breast to malignant phyllodes tumor. Appl Immunohistochem Mol Morphol. 2009;17:345–350. doi: 10.1097/PAI.0b013e318194d992. [DOI] [PubMed] [Google Scholar]
- 42.Abe M, Miyata S, Nishimura S, et al. Malignant transformation of breast fibroadenoma to malignant phyllodes tumor: long-term outcome of 36 malignant phyllodes tumors. Breast Cancer. 2011;18:268–272. doi: 10.1007/s12282-009-0185-x. [DOI] [PubMed] [Google Scholar]
- 43.Foucar CE, Hardy A, Siziopikou KP, et al. A mother and daughter with phyllodes tumors of the breast. Clin Breast Cancer. 2012;12:373–377. doi: 10.1016/j.clbc.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Lim WK, Ong CK, Tan J, et al. Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet. 2014;46:877–880. doi: 10.1038/ng.3037. [DOI] [PubMed] [Google Scholar]
- 45.Cani AK, Hovelson DH, McDaniel AS, et al. Next-gen sequencing exposes frequent MED12 mutations and actionable therapeutic targets in phyllodes tumors. Mol Cancer Res. 2015;13:613–619. doi: 10.1158/1541-7786.MCR-14-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M, Sekine S, Ogawa R, et al. Frequent MED12 mutations in phyllodes tumours of the breast. Br J Cancer. 2015;112:1703–1708. doi: 10.1038/bjc.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piscuoglio S, Murray M, Fusco N, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast. Histopathology. 2015;67:529–537. doi: 10.1111/his.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagasawa S, Maeda I, Fukuda T, et al. MED12 exon 2 mutations in phyllodes tumors of the breast. Cancer Med. 2015;7:1117–1121. doi: 10.1002/cam4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfarr N, Kriegsmann M, Sinn P, et al. Distribution of MED12 mutations in fibroadenomas and phyllodes tumors of the breast—implications for tumor biology and pathological diagnosis. Genes Chromosom Cancer. 2015;54:444–452. doi: 10.1002/gcc.22256. [DOI] [PubMed] [Google Scholar]
- 50.Ng CCY, Tan J, Ong CK, et al. MED12 is frequently mutated in breast phyllodes tumours: a study of 112 cases. J Clin Pathol. 2015;68:685–691. doi: 10.1136/jclinpath-2015-202896. [DOI] [PubMed] [Google Scholar]
- 51.Giacomazzi J, Koehler-Santos P, Palmero EI, et al. A TP53 founder mutation, p. R337H, is associated with phyllodes breast tumors in Brazil. Virchows Arch. 2013;463:17–22. doi: 10.1007/s00428-013-1439-8. [DOI] [PubMed] [Google Scholar]
- 52.Laé M, Vincent-Salomon A, Savignoni A, et al. Phyllodes tumors of the breast segregate in two groups according to genetic criteria. Mod Pathol. 2007;20:435–444. doi: 10.1038/modpathol.3800756. [DOI] [PubMed] [Google Scholar]
- 53.Croce S, Chibon F. MED12 and uterine smooth muscle oncogenesis: state of the art and perspectives. Eur J Cancer. 2015;51:1603–1610. doi: 10.1016/j.ejca.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Je EM, Kim MR, Min KO, Yoo NJ, Lee SH. Mutational analysis of MED12 exon 2 in uterine leiomyoma and other common tumors. Int J Cancer. 2012;131:E1044–E1047. doi: 10.1002/ijc.27610. [DOI] [PubMed] [Google Scholar]
- 55.Tan J, Ong CK, Lim WK, et al. Genomic landscapes of breast fibroepithelial tumours. Nat Genet. 2015 Oct 5; doi: 10.1038/ng.3409. (epub) [DOI] [PubMed] [Google Scholar]
- 56.Lawton TJ, Acs G, Argani P, et al. Interobserver variability by pathologists in the distinction between cellular fibroadenomas and phyllodes tumors. Int J Surg Pathol. 2014;22:695–698. doi: 10.1177/1066896914548763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tay TKY, Chang KTE, Thike AA, Tan PH. Paediatric fibroepithelial lesions revisited: pathological insights. J Clin Pathol. 2015;68:633–641. doi: 10.1136/jclinpath-2015-202956. [DOI] [PubMed] [Google Scholar]
- 58.Ross DS, Giri DD, Akram MM, Catalano J, Van Zee KJ, Brogi E. Fibroepithelial lesions in the breast of adolescent females: a clinicopathological profile of 35 cases. Mod Pathol. 2012;25(Suppl 2):64a. doi: 10.1111/tbj.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faiz S, Tudor V, Yasim G-P, Badve S. Fibroadenomatous lesions in pediatric age group. Mod Pathol. 2013;26(Suppl 2):39A. [Google Scholar]
- 60.Giri D. Recurrent challenges in the evaluation of fibroepithelial lesions. Arch Pathol Lab Med. 2009;133:713–721. doi: 10.5858/133.5.713. [DOI] [PubMed] [Google Scholar]
- 61.Tsang AKH, Chan SK, Lam CCF, et al. Phyllodes tumours of the breast—differentiating features in core needle biopsy. Histopathology. 2011;59:600–608. doi: 10.1111/j.1365-2559.2011.03939.x. [DOI] [PubMed] [Google Scholar]
- 62.Lee AHS, Hodi Z, Ellis IO, Elston CW. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology. 2007;51:336–344. doi: 10.1111/j.1365-2559.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 63.Aiyer HM, Jain M, Thomas S, Logani KB. Diagnostic stromal histomorphology in fibroepithelial breast lesions: a fresh perspective. Indian J Pathol Microbiol. 2000;43:5–12. [PubMed] [Google Scholar]
- 64.Yasir S, Gamez R, Jenkins S, Visscher DW, Nassar A. Significant histologic features differentiating cellular fibroadenoma from phyllodes tumor on core needle biopsy specimens. Am J Clin Pathol. 2014;142:362–369. doi: 10.1309/AJCPZUZ96RESGPUP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi J, Koo JS. Comparative study of histological features between core needle biopsy and surgical excision in phyllodes tumor. Pathol Int. 2012;62:120–126. doi: 10.1111/j.1440-1827.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 66.Gould DJ, Salmans JA, Lassinger BK, et al. Factors associated with phyllodes tumor of the breast after core needle biopsy identifies fibroepithelial neoplasm. J Surg Res. 2012;178:299–303. doi: 10.1016/j.jss.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 67.Resetkova E, Khazai L, Albarracin CT, Arribas E. Clinical and radiologic data and core needle biopsy findings should dictate management of cellular fibroepithelial tumors of the breast. Breast J. 2010;16:573–580. doi: 10.1111/j.1524-4741.2010.01013.x. [DOI] [PubMed] [Google Scholar]
- 68.Yang X, Kandil D, Cosar EF, Khan A. Fibroepithelial tumors of the breast: pathologic and immunohistochemical features and molecular mechanisms. Arch Pathol Lab Med. 2014;138:25–36. doi: 10.5858/arpa.2012-0443-RA. [DOI] [PubMed] [Google Scholar]
- 69.Heneghan HM, Martin ST, Casey M, Tobbia I, Benani F, Barry KM. A diagnostic dilemma in breast pathology—benign fibroadenoma with multinucleated stromal giant cells. Diagn Pathol. 2008;3:33. doi: 10.1186/1746-1596-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan PH, Ellis IO. Myoepithelial and epithelial–myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. J Clin Pathol. 2013;66:465–470. doi: 10.1136/jclinpath-2012-201078. [DOI] [PubMed] [Google Scholar]
- 71.Grady I, Gorsuch H, Wilburn-Bailey S. Long-term outcome of benign fibroadenomas treated by ultrasound-guided percutaneous excision. Breast J. 2008;14:275–278. doi: 10.1111/j.1524-4741.2008.00574.x. [DOI] [PubMed] [Google Scholar]
- 72.Organ CH, Organ BC. Fibroadenoma of the female breast: a critical clinical assessment. J Natl Med Assoc. 1983;75:701–704. [PMC free article] [PubMed] [Google Scholar]
- 73.Nigro DM, Organ CH. Fibroadenoma of the female breast. Some epidemiologic surprises. Postgrad Med. 1976;59:113–117. doi: 10.1080/00325481.1976.11714358. [DOI] [PubMed] [Google Scholar]
- 74.Kim S, Kim J-Y, Kim DH, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013;141:353–363. doi: 10.1007/s10549-013-2684-x. [DOI] [PubMed] [Google Scholar]
- 75.Teo JY, Cheong CS-J, Wong CY. Low local recurrence rates in young Asian patients with phyllodes tumours: less is more. ANZ J Surg. 2012;82:325–328. doi: 10.1111/j.1445-2197.2012.06045.x. [DOI] [PubMed] [Google Scholar]
- 76.Rakha EA, Tan PH, Shaaban A, et al. Do primary mammary osteosarcoma and chondrosarcoma exist? A review of a large multi-institutional series of malignant matrix-producing breast tumours. Breast. 2013;22:13–18. doi: 10.1016/j.breast.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol. 2004;28:1506–1512. doi: 10.1097/01.pas.0000138183.97366.fd. [DOI] [PubMed] [Google Scholar]
- 78.Tse GM, Tan P-H, Chaiwun B, et al. p63 is useful in the diagnosis of mammary metaplastic carcinomas. Pathology. 2006;38:16–20. doi: 10.1080/00313020500444625. [DOI] [PubMed] [Google Scholar]
- 79.Chia Y, Thike AA, Cheok PY, Yong-Zheng Chong L, Man-Kit Tse G, Tan PH. Stromal keratin expression in phyllodes tumours of the breast: a comparison with other spindle cell breast lesions. J Clin Pathol. 2012;65:339–347. doi: 10.1136/jclinpath-2011-200377. [DOI] [PubMed] [Google Scholar]
- 80.Cimino-Mathews A, Sharma R, Illei PB, Vang R, Argani P. A subset of malignant phyllodes tumors express p63 and p40: a diagnostic pitfall in breast core needle biopsies. Am J Surg Pathol. 2014;38:1689–1696. doi: 10.1097/PAS.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SK, Jung WH, Koo JS. p40 (ΔNp63) expression in breast disease and its correlation with p63 immunohistochemistry. Int J Clin Exp Pathol. 2014;7:1032–1041. [PMC free article] [PubMed] [Google Scholar]
- 82.D’Alfonso TM, Ross DS, Liu Y-F, Shin SJ. Expression of p40 and laminin 332 in metaplastic spindle cell carcinoma of the breast compared with other malignant spindle cell tumours. J Clin Pathol. 2015;68:516–521. doi: 10.1136/jclinpath-2015-202923. [DOI] [PubMed] [Google Scholar]
- 83.Noronha Y, Raza A, Hutchins B, et al. CD34, CD117, and Ki- 67 expression in phyllodes tumor of the breast: an immunohistochemical study of 33 cases. Int J Surg Pathol. 2011;19:152–158. doi: 10.1177/1066896910382009. [DOI] [PubMed] [Google Scholar]
- 84.Moore T, Lee AH. Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology. 2001;38:62–67. doi: 10.1046/j.1365-2559.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 85.Cîmpean AM, Raica M, Nariţa D. Diagnostic significance of the immunoexpression of CD34 and smooth muscle cell actin in benign and malignant tumors of the breast. Rom J Morphol Embryol. 2005;46:123–129. [PubMed] [Google Scholar]
- 86.Lee AHS. Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology. 2008;52:45–57. doi: 10.1111/j.1365-2559.2007.02893.x. [DOI] [PubMed] [Google Scholar]
- 87.Dunne B, Lee AHS, Pinder SE, Bell JA, Ellis IO. An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol. 2003;34:1009–1015. doi: 10.1053/s0046-8177(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 88.Esposito NN, Mohan D, Brufsky A, Lin Y, Kapali M, Dabbs DJ. Phyllodes tumor: a clinicopathologic and immunohistochemical study of 30 cases. Arch Pathol Lab Med. 2006;130:1516–1521. doi: 10.5858/2006-130-1516-PTACAI. [DOI] [PubMed] [Google Scholar]
- 89.Tan P-H, Jayabaskar T, Yip G, et al. p53 and c-kit (CD117) protein expression as prognostic indicators in breast phyllodes tumors: a tissue microarray study. Mod Pathol. 2005;18:1527–1534. doi: 10.1038/modpathol.3800488. [DOI] [PubMed] [Google Scholar]
- 90.Lacroix-Triki M, Geyer FC, Lambros MB, et al. β-catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Mod Pathol. 2010;23:1438–1448. doi: 10.1038/modpathol.2010.141. [DOI] [PubMed] [Google Scholar]
- 91.Sawyer EJ, Hanby AM, Rowan AJ, et al. The Wnt pathway, epithelial–stromal interactions, and malignant progression in phyllodes tumours. J Pathol. 2002;196:437–444. doi: 10.1002/path.1067. [DOI] [PubMed] [Google Scholar]
- 92.Sawyer EJ, Hanby AM, Poulsom R, et al. Beta-catenin abnormalities and associated insulin-like growth factor overexpression are important in phyllodes tumours and fibroadenomas of the breast. J Pathol. 2003;200:627–632. doi: 10.1002/path.1391. [DOI] [PubMed] [Google Scholar]
- 93.Tsang JYS, Mendoza P, Lam CCF, et al. Involvement of α- and β-catenins and E-cadherin in the development of mammary phyllodes tumours. Histopathology. 2012;61:667–674. doi: 10.1111/j.1365-2559.2012.04271.x. [DOI] [PubMed] [Google Scholar]
- 94.Hayes MJ, Thomas D, Emmons A, Giordano TJ, Kleer CG. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin Cancer Res. 2008;14:4038–4044. doi: 10.1158/1078-0432.CCR-07-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sawhney N, Garrahan N, Douglas-Jones AG, Williams ED. Epithelial–stromal interactions in tumors. A morphologic study of fibroepithelial tumors of the breast. Cancer. 1992;70:2115–2120. doi: 10.1002/1097-0142(19921015)70:8<2115::aid-cncr2820700818>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 96.Sawyer EJ, Hanby AM, Ellis P, et al. Molecular analysis of phyllodes tumors reveals distinct changes in the epithelial and stromal components. Am J Pathol. 2000;156:1093– 1098. doi: 10.1016/S0002-9440(10)64977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsang JYS, Mendoza P, Putti TC, et al. E-cadherin expression in the epithelial components of mammary phyllodes tumors. Hum Pathol. 2012;43:2117–2123. doi: 10.1016/j.humpath.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 98.Feakins RM, Wells CA, Young KA, Sheaff MT. Platelet-derived growth factor expression in phyllodes tumors and fibroadenomas of the breast. Hum Pathol. 2000;31:1214–1222. doi: 10.1053/hupa.2000.18481. [DOI] [PubMed] [Google Scholar]
- 99.Dietrich CU, Pandis N, Rizou H, et al. Cytogenetic findings in phyllodes tumors of the breast: karyotypic complexity differentiates between malignant and benign tumors. Hum Pathol. 1997;28:1379–1382. doi: 10.1016/s0046-8177(97)90227-6. [DOI] [PubMed] [Google Scholar]
- 100.Dacic S, Kounelis S, Kouri E, Jones MW. Immunohistochemical profile of cystosarcoma phyllodes of the breast: a study of 23 cases. Breast J. 2002;8:376–381. doi: 10.1046/j.1524-4741.2002.08608.x. [DOI] [PubMed] [Google Scholar]
- 101.Karim RZ, Gerega SK, Yang YH, et al. Proteins from the Wnt pathway are involved in the pathogenesis and progression of mammary phyllodes tumours. J Clin Pathol. 2009;62:1016–1020. doi: 10.1136/jcp.2009.066977. [DOI] [PubMed] [Google Scholar]
- 102.Logullo AF, Nonogaki S, Do Socorro Maciel M, Mourão-Neto M, Soares FA. Stromal and epithelial cells react differentially to c-kit in fibroepithelial tumors of the breast. Mol Med Rep. 2008;1:857–861. doi: 10.3892/mmr_00000041. [DOI] [PubMed] [Google Scholar]
- 103.Rao BR, Meyer JS, Fry CG. Most cystosarcoma phyllodes and fibroadenomas have progesterone receptor but lack estrogen receptor: stromal localization of progesterone receptor. Cancer. 1981;47:2016–2021. doi: 10.1002/1097-0142(19810415)47:8<2016::aid-cncr2820470819>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 104.Mechtersheimer G, Krüger KH, Born IA, Möller P. Antigenic profile of mammary fibroadenoma and cystosarcoma phyllodes. A study using antibodies to estrogen- and progesterone receptors and to a panel of cell surface molecules. Pathol Res Pract. 1990;186:427–438. doi: 10.1016/S0344-0338(11)80460-7. [DOI] [PubMed] [Google Scholar]
- 105.Singh Y, Hatano T, Uemura Y, et al. Immunohistochemical profile of phyllodes tumors of the breast. Oncol Rep. 1996;3:677–681. [PubMed] [Google Scholar]
- 106.Shpitz B, Bomstein Y, Sternberg A, et al. Immunoreactivity of p53, Ki-67, and c-erbB-2 in phyllodes tumors of the breast in correlation with clinical and morphologic features. J Surg Oncol. 2002;79:86–92. doi: 10.1002/jso.10049. [DOI] [PubMed] [Google Scholar]
- 107.Tse GMK, Lee CS, Kung FYL, et al. Hormonal receptors expression in epithelial cells of mammary phyllodes tumors correlates with pathologic grade of the tumor: a multicenter study of 143 cases. Am J Clin Pathol. 2002;118:522–526. doi: 10.1309/D206-DLF8-WDNC-XJ8K. [DOI] [PubMed] [Google Scholar]
- 108.Sapino A, Bosco M, Cassoni P, et al. Estrogen receptor-beta is expressed in stromal cells of fibroadenoma and phyllodes tumors of the breast. Mod Pathol. 2006;19:599–606. doi: 10.1038/modpathol.3800574. [DOI] [PubMed] [Google Scholar]
- 109.Kim Y-H, Kim G-E, Lee JS, et al. Hormone receptors expression in phyllodes tumors of the breast. Anal Quant Cytol Histol. 2012;34:41–48. [PubMed] [Google Scholar]
- 110.Onkendi EO, Jimenez RE, Spears GM, Harmsen WS, Ballman KV, Hieken TJ. Surgical treatment of borderline and malignant phyllodes tumors: the effect of the extent of resection and tumor characteristics on patient outcome. Ann Surg Oncol. 2014;21:3304–3309. doi: 10.1245/s10434-014-3909-x. [DOI] [PubMed] [Google Scholar]
- 111.Lin C-C, Chang H-W, Lin C-Y, Chiu C-F, Yeh S-P. The clinical features and prognosis of phyllodes tumors: a single institution experience in Taiwan. Int J Clin Oncol. 2013;18:614–620. doi: 10.1007/s10147-012-0442-4. [DOI] [PubMed] [Google Scholar]
- 112.Grimes MM. Cystosarcoma phyllodes of the breast: histologic features, flow cytometric analysis, and clinical correlations. Mod Pathol. 1992;5:232–239. [PubMed] [Google Scholar]
- 113.Liang MI, Ramaswamy B, Patterson CC, et al. Giant breast tumors: surgical management of phyllodes tumors, potential for reconstructive surgery and a review of literature. World J Surg Oncol. 2008;6:117. doi: 10.1186/1477-7819-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ciatto S, Bonardi R, Cataliotti L, Cardona G. Phyllodes tumor of the breast: a multicenter series of 59 cases. Coordinating Center and Writing Committee of FONCAM (National Task Force for Breast Cancer), Italy. Eur J Surg Oncol. 1992;18:545–549. [PubMed] [Google Scholar]
- 115.Reinfuss M, Mituś J, Duda K, Stelmach A, Ryś J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77:910–916. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 116.Barth RJ. Histologic features predict local recurrence after breast conserving therapy of phyllodes tumors. Breast Cancer Res Treat. 1999;57:291–295. doi: 10.1023/a:1006260225618. [DOI] [PubMed] [Google Scholar]
- 117.Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, Souba WW. Surgical management of phyllodes tumors. Arch Surg. 1999;134:487–492. doi: 10.1001/archsurg.134.5.487. discussion 492–493. [DOI] [PubMed] [Google Scholar]
- 118.Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer. 2006;107:2127–2133. doi: 10.1002/cncr.22228. [DOI] [PubMed] [Google Scholar]
- 119.Mituś J, Reinfuss M, Mituś JW, et al. Malignant phyllodes tumor of the breast: treatment and prognosis. Breast J. 2014;20:639–644. doi: 10.1111/tbj.12333. [DOI] [PubMed] [Google Scholar]
- 120.Yom CK, Han W, Kim S-W, Park SY, Park IA, Noh D-Y. Reappraisal of conventional risk stratification for local recurrence based on clinical outcomes in 285 resected phyllodes tumors of the breast. Ann Surg Oncol. 2015;22:2912– 2918. doi: 10.1245/s10434-015-4395-5. [DOI] [PubMed] [Google Scholar]
- 121.Wood WC. Close/positive margins after breast-conserving therapy: additional resection or no resection? Breast. 2013;22(Suppl 2):S115–S117. doi: 10.1016/j.breast.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 122.Geisler DP, Boyle MJ, Malnar KF, et al. Phyllodes tumors of the breast: a review of 32 cases. Am Surg. 2000;66:360– 366. [PubMed] [Google Scholar]
- 123.Kapiris I, Nasiri N, A’Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol. 2001;27:723–730. doi: 10.1053/ejso.2001.1207. [DOI] [PubMed] [Google Scholar]
- 124.Fajdić J, Gotovac N, Hrgović Z, Kristek J, Horvat V, Kaufmann M. Phyllodes tumors of the breast diagnostic and therapeutic dilemmas. Onkologie. 2007;30:113–118. doi: 10.1159/000099580. [DOI] [PubMed] [Google Scholar]
- 125.Khosravi-Shahi P. Management of non metastatic phyllodes tumors of the breast: review of the literature. Surg Oncol. 2011;20:e143–e148. doi: 10.1016/j.suronc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 127.August DA, Kearney T. Cystosarcoma phyllodes: mastectomy, lumpectomy, or lumpectomy plus irradiation. Surg Oncol. 2000;9:49–52. doi: 10.1016/s0960-7404(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 128.Pandey M, Mathew A, Kattoor J, et al. Malignant phyllodes tumor. Breast J. 2001;7:6. doi: 10.1046/j.1524-4741.2001.07606.x. [DOI] [PubMed] [Google Scholar]
- 129.Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998–2009. Ann Surg Oncol. 2014;21:1222–1230. doi: 10.1245/s10434-013-3395-6. [DOI] [PubMed] [Google Scholar]