Abstract
Two recent papers published in The Journal of Cell Biology (Borrego-Pinto et al., 2016) and Science ((Borrego-Pinto et al., 2016; Pimenta-Marques et al., 2016) have begun to shed light on the mechanism of centriole elimination during female oogenesis, highlighting a protective role for Polo kinase and the pericentriolar material.
Union of sperm and egg brings together two haploid genomes around which the first bipolar mitotic spindle is assembled. Each zygotic spindle pole contains one centrosome composed of a mother-daughter centriole pair surrounded by the microtubule (MT)-nucleating pericentriolar material (PCM). Most metazoans, including humans, flies, frogs and worms utilize a mechanism where the zygotic centrioles are paternally derived (mice are a notable exception) while the maternal centrioles are destroyed in a process known as Centriole Elimination (CE). CE is thought to ensure the correct number of centrioles to allow for normal mitosis and is thus thought to be a very fundamental developmental process. Mice, humans, flies and nematodes undergo CE prior to female meiosis leading to acentrosomal meiosis and gametes. Other species such as some echinoderms, mollusks and annelids undergo CE during and subsequent to meiosis (Manandhar et al., 2005). This difference suggests there may be distinct CE mechanisms (Figure 1). However, the nature of these mechanisms and why they occur remain a mystery. Recent studies from Borrego-Pinto et al. (2016) and Pimenta-Marques et al. (2016) shed light on this process using modern light microscopy in starfish and Drosophila oocytes to reveal surprising features of CE and its role in embryogenesis. Considered together, these studies highlight a conserved feature of CE, providing a beautiful example of how evolution has maintained some mechanistic aspects of CE while accommodating the unique biology of each system.
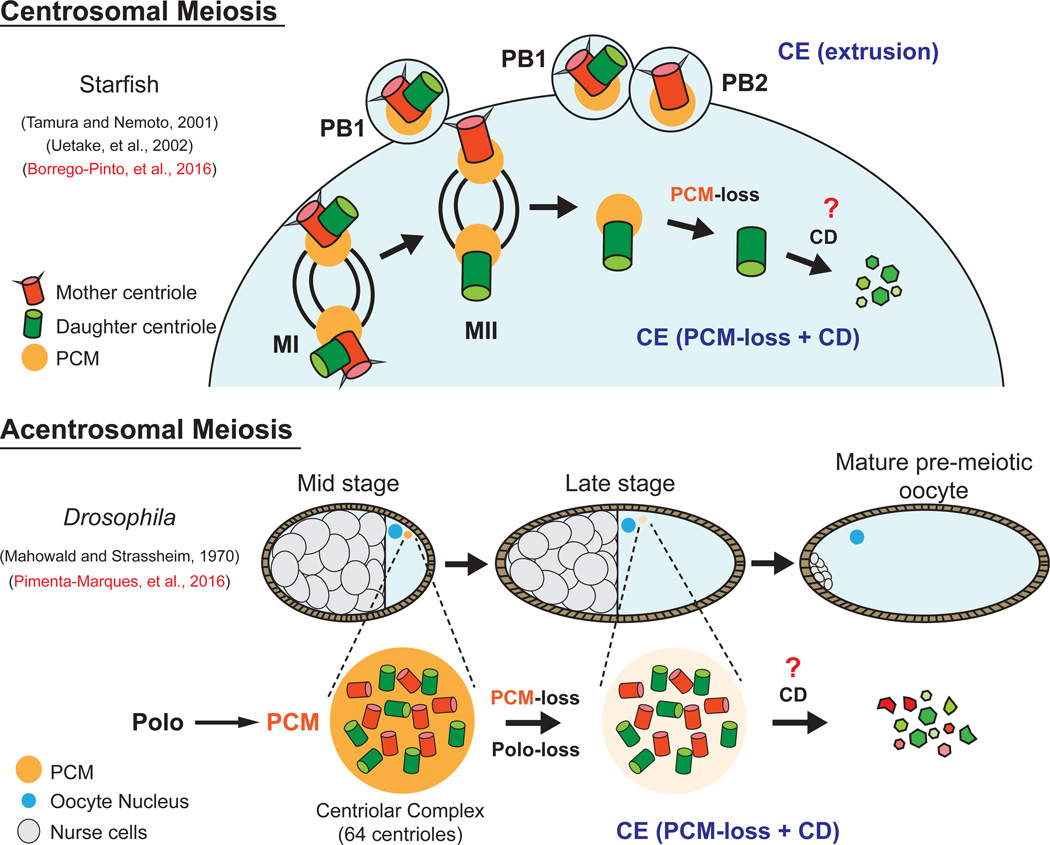
Figure 1. Mechanism of centriole elimination in centrosomal and acentrosomal meiosis.
In starfish, centriole elimination (CE) occurs during centrosomal meiosis via two mechanisms - polar body (PB) extrusion and PCM-loss + Centriole Destruction (CD). The fly shares the PCM-loss + CD mechanism of CE during late oogenesis that is dependent on downregulation of Polo kinase. In contrast to starfish, CE occurs prior to meiosis, resulting in acentrosomal meiosis. The mechanisms of CD remains unknown.
Borrego-Pinto and colleagues (2016) used starfish (Patiria miniata) oocytes as a model for CE that occurs during centrosomal female meiosis. The starfish orthologs of centriole proteins were identified and fluorescently labelled to follow mother/daughter centriole behavior by live cell imaging. This led to the discovery that a single daughter centriole is retained by the oocyte following meiosis II, while the other three centrioles (two mothers and one daughter) are extruded in polar bodies. Following anaphase II, this maternally contributed daughter centriole proceeded through CE by first shedding PCM and then degrading. To determine if retaining a daughter and not a mother centriole was biologically significant, the researchers abrogated polar-body extrusion and allowed the oocyte to inherit both mother and daughter centrioles. Amazingly, daughter centrioles followed the normal CE program, while mother centrioles maintained PCM, organized MT-asters that contributed to spindle assembly alongside paternal centrosomes, and finally resulted in detrimental multipolar spindle formation. These data help to explain previous studies in starfish that noted differences between meiotic centrioles, but could not assign the difference to mother vs daughter (Tamura and Nemoto, 2001; Uetake et al., 2002). In summary, two forms of CE occur sequentially during starfish meiosis. The first is CE via polar body extrusion, which eliminates the two mother centrioles and one daughter. The second CE mechanism involves PCM-loss and disassembly of the remaining daughter centriole. Together, these two forms of CE prevent spindle defects and chromosome segregation errors that would occur with multiple (>2) centrosomes (Figure 1).
In a more recent study, Pimenta-Marques and colleagues (2016) used Drosophila as a model for CE that occurs prior to meiosis. Fly eggs are formed from an oocyte that inherits the contents of 15-supportive nurse cells, including all their centrioles (Figure 1). A classic serial section EM study revealed the process of centriole dumping into the oocyte, which forms an aggregate termed a “centriolar complex” (Mahowald and Strassheim, 1970). The present study revisited CE in flies to show that centrioles are completely eliminated just prior to meiosis (Figure 1), later than previously thought. Through careful measurement of centrosome protein levels over time, the researchers show that PCM is lost first, followed by centriole proteins during later stages of oogenesis. This suggested that PCM might protect centrioles from elimination, a model they confirmed through RNAi studies of PCM components in cultured cells.
Importantly, the authors were able to provide mechanistic insight into how PCM protects centrioles. Given the known role of Polo in recruiting PCM to mitotic centrosomes, the authors reasoned that Polo might also be required for maintaining a protective PCM coat around centrioles during early- and mid-oogenesis. This was tested and supported through Polo loss-of function experiments, which led to early PCM-loss and accelerated CE. The authors then performed the reciprocal Polo gain-of-function experiment, which prevented PCM-loss and CE. The ectopic presence of centrioles led to abnormal centrosome numbers, irregular meiotic spindles, and chromosome segregation errors. Although these eggs could be fertilized, they rarely hatched and arrested at the first mitotic division in the zygote.
These studies highlight important features of CE that were alluded to in the starfish and furthered mechanistically in the fly. CE occurs through a series of ordered steps that begins with the down regulation of Polo, followed by PCM-loss and the degradation of centrioles (Figure 1). Together, these studies have now conclusively shown that CE is critical for maintaining proper centrosome copy number required for successful early development.
While both studies uncover aspects of CE that were previously unknown, many questions still remain. Does the starfish oocyte downregulate Polo kinase following anaphase II? The mechanism by which mother centrioles avoid CE also remains unclear. It could relate to the presence of centriole appendages on the mother that can retain a protective PCM layer (De Brabander et al., 1982; Gorgidze and Vorobjev, 1995), possibly via a Polo-independent mechanism. It also remains to be determined what ensures timely Polo downregulation and its removal from the centrioles to initiate CE, as well as which targets of Polo kinase activity in the PCM are responsible for its protective role. It’s possible that Polo kinase has other roles beyond maintaining PCM. This could be tested through RNAi of PCM components while driving active, targeted Polo to the centriole. Furthermore, it remains to be shown whether PCM-loss is coupled to a specialized “Centriole Destruction” (CD) mechanism that can target centriole components for degradation after PCM is removed (Figure 1). CD could involve targeted destruction of centriole components via an ubiquitin/proteasome mechanism. Alternatively, CD might involve MT depolymerization machinery that specifically attacks the centriole barrel, effectively reversing the normal centriole elongation machinery that occurs during centriole duplication. Novel proteins could also contribute to CD; the helicase CGH-1, for example, somehow delays CE in worms (Mikeladze-Dvali et al., 2012). Do other unsuspected mechanisms exist? A loss-of-function screen to suppress CE in flies or cultured cells could identify proteins involved in CD by evaluating centriole presence during late stages of oogenesis or in cell cycle arrested cultured cells.
Despite some specific differences, there remains a common thread: non-extrusion based CE involves the requisite PCM-loss followed by an unknown CD pathway (Figure 1). This CE mechanism has also been observed in worms (Mikeladze-Dvali et al., 2012), suggesting an ancient origin for CE maintained throughout evolution. More work will be required, but the footing has suddenly become more solid for future excursions into CE.
References
- Borrego-Pinto J, Somogyi K, Karreman MA, Konig J, Muller-Reichert T, Bettencourt-Dias M, Gonczy P, Schwab Y, Lenart P. Distinct mechanisms eliminate mother and daughter centrioles in meiosis of starfish oocytes. J Cell Biol. 2016;212:815–827. doi: 10.1083/jcb.201510083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J. Microtubule Stability and Assembly in Living Cells: The Influence of Metabolic Inhibitors, Taxol and pH. Cold Spring Harbor Symp Quant Biol. 1982;46:227–240. doi: 10.1101/sqb.1982.046.01.026. [DOI] [PubMed] [Google Scholar]
- Gorgidze LA, Vorobjev IA. Centrosome and microtubules behavior in the cytoplasts. J Submicrosc Cytol Pathol. 1995;27:381–389. [PubMed] [Google Scholar]
- Mahowald AP, Strassheim JM. Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J Cell Biol. 1970;45:306–320. doi: 10.1083/jcb.45.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, von Tobel L, Strnad P, Knott G, Leonhardt H, Schermelleh L, Gonczy P. Analysis of centriole elimination during C. elegans oogenesis. Development. 2012;139:1670–1679. doi: 10.1242/dev.075440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta-Marques A, Bento I, Lopes CA, Duarte P, Jana SC, Bettencourt-Dias M. A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster. Science. 2016 doi: 10.1126/science.aaf4866. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nemoto S. Reproductive maternal centrosomes are cast off into polar bodies during maturation division in starfish oocytes. Exp Cell Res. 2001;269:130–139. doi: 10.1006/excr.2001.5305. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Kato KH, Washitani-Nemoto S, Nemoto Si S. Nonequivalence of maternal centrosomes/centrioles in starfish oocytes: selective casting-off of reproductive centrioles into polar bodies. Dev Biol. 2002;247:149–164. doi: 10.1006/dbio.2002.0682. [DOI] [PubMed] [Google Scholar]