Abstract
Sentinel lymph node (SLN) biopsy has been established as the standard of care for axillary staging in patients with invasive breast carcinoma and clinically negative lymph nodes (cN0). Historically, all patients with a positive SLN underwent axillary lymph node dissection (ALND). The ACOSOG Z0011 trial showed that women with T1-T2 disease and cN0 who undergo breast conserving surgery and whole-breast radiotherapy can safely avoid ALND. The main goal of SLN examination should be to detect all macrometastases (>2mm). Gross sectioning SLNs at 2 mm intervals and microscopic examination of one H&E-stained section from each SLN block is the preferred method of pathologic evaluation of SLNs. The role and timing of SLN biopsy for patients having neoadjuvant chemotherapy is controversial and continues to be explored in clinical trials. SLN biopsies from patients with invasive breast carcinoma who have received neoadjuvant chemotherapy pose particular challenges for pathologists.
Keywords: Sentinel lymph node, breast carcinoma, neoadjuvant chemotherapy
INTRODUCTION
Axillary lymph node (ALN) status is an important prognostic factor and determinant of treatment for patients with breast carcinoma. Clinical trials have proven that SLN is equivalent to ALND for staging of the axilla in patients with clinically node-negative (cN0) disease and is associated with significantly less morbidity.1–6 The need for completion ALND in patients with limited SLN involvement treated with modern modalities has been investigated in recent clinical trials. The results suggest that ALND may be safely omitted in carefully selected cN0 patients with metastatic carcinoma limited to one or two SLNs. This has led to a change in clinical management of the axilla in many centres. The use and timing of SLN biopsy in patients with invasive breast carcinoma receiving neoadjuvant chemotherapy (NACT) is controversial and the approach to patients with biopsy-proven nodal disease before NACT is evolving. All of these changes in clinical practice have implications for how pathologists examine and report on SLNs.
AXILLARY LYMPH NODE STAGING
The American Joint Committee on Carcinoma (AJCC) and the Union for International Carcinoma Control (UICC) TNM (Tumour Node Metsatasis) staging systems classify nodal metastases based on size.7, 8 This classification and emphasis on reporting axillary nodal disease with greater precision was first introduced into the TNM in the sixth edition published in 2002 and was largely driven by increasing use of SLN biopsy and growing use of immunohistochemistry (IHC).9 Macrometastases are tumour deposits >2 mm [pN1], micrometastases range in size from greater than 0.2 mm to less or equal to 2 mm or consist of more than 200 carcinoma cells in a single lymph node section [pN1mi]. Isolated tumour cells (ITCs) are single cells or cell clusters each spanning less than 0.2mm in size and amounting to fewer than 200 carcinoma cells in one lymph node section [pN0(i+)], regardless of method of detection.
BIOLOGICAL AND CLINICAL SIGNIFICANCE OF OCCULT METASTASES
An “occult” metastasis is defined as any metastasis that is not identified on initial examination using a “standard” evaluation protocol.10 Following the introduction of SLN biopsy, many clinicians and pathologists pursued more extensive evaluation of the SLN(s), in an attempt to identify occult metastases, believing that this information would help in prediction of prognosis. “Enhanced pathology” protocols including use of additional H&E step-level sections and/or immunohistochemical stains for cytokeratins (CK-IHC) on all tissue blocks of any SLN without evidence of carcinoma in the initial H&E-stained section were employed. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 randomized prospective clinical trial demonstrated that in patients with T1-T2 cN0 tumors, and negative SLNs, staging by SLN biopsy is equivalent to ALND,1, 11 and also addressed the clinical significance of occult metastases in patients managed with modern treatment modalities.12 Participating sites were instructed to slice SLNs at 2 mm intervals, embed all tissue slices in paraffin blocks, and examine only one H&E-stained section from each tissue block. This approach aimed to identify all macrometastases (>2 mm) and these results were used for clinical treatment decisions. Based on clinical and tumour characteristics most patients in the study received systemic therapy, consisting of chemotherapy and/or hormonal therapy. The SLN blocks of patients with no evidence of SLN involvement in the initial H&E-stained section were then submitted to a central laboratory for additional evaluation using the “B-32 protocol”, designed to detect metastases larger than 1.0 mm in size,13 using one H&E-stained and CK-IHC stained sections at a depth of 0.5 mm and 1.0 mm into the paraffin block. Occult metastases were identified in 616/3884 (15.9%) patients (11.1% ITCs, 4.4% micrometastases, and 0.4% macrometastases).12 At five years follow-up, the differences in outcomes for patients with and without occult metastases were statistically significant, but amounted to a minimal percent increase with respect to overall survival (OS) (94.6% vs. 95.8%), disease free survival (DFS) (86.4% vs. 89.2%), and distant disease free interval (89.7% vs. 92.5%). A 10 year update of follow up has confirmed these results.11 In particular, not all the patients with occult metastases will necessarily develop recurrent disease and most of the patients with occult metastases are already treated using available modalities. Based on these findings, the use of enhanced pathology techniques to identify occult metastases in initially negative SLNs does not appear to translate into additional clinical benefit. The results of the NSABP B-32 clinical trial support the current guidelines for examination of SLNs from patients with breast carcinoma. The College of American Pathologists (CAP) does not recommend the use of routine multistep level sections and/or CK-IHC in the histologic evaluation of SLNs.14 The American Society of Clinical Oncology (ASCO) and National Comprehensive Carcinoma Network (NCCN) do not recommend the use of routine use of CK-IHC for evaluation of SLNs.15, 16 The European Society of Medical Oncology (ESMO) also endorses this approach.17 The National Health Service Breast Screening Programme (NHSBSP) and Royal College of Pathologists (RCPath) guidelines (2005) do not currently advocate routine use of ancillary techniques for assessment of SLNs.18
PREDICTION OF ADDITIONAL NODAL BURDEN AND NEED FOR ALND IN PATIENTS WITH POSITIVE SLNS
Studies have shown that the majority (approximately 60%) of patients with a positive SLN have no residual disease in the axilla,19–28 and derive no real benefit from ALND. In the first decade since the introduction of SLN biopsy most surgeons performed completion ALND in all patients with SLN involvement. Over time many surgeons modified their practice and did not always perform ALND in cases with limited SLN involvement.29 Furthermore, even in the most experienced hands, SLN biopsy is associated with a false-negative rate (FNR). An overview of 69 published studies of SLN biopsy validated with concurrent ALND confirms a SLN identification rate of 96%, with an average FNR of 7%.30 In a bid to aid clinical decision to perform or omit ALND, investigators evaluated various clinico-pathological parameters and developed mathematical predictive tools, also known as nomograms, for estimating the risk of additional LN metastases.31–42
Large clinical trials specifically questioned the need for completion ALND in cN0 patients with limited involvement of SLNs.43–46 The International Breast Carcinoma Study Group (IBCSG) 23-01 trial randomized 931 cN0 patients with T1-T2 breast carcinoma and SLN micrometastases to ALND or no further axillary surgery. Metastatic carcinoma was identified in non-SLNs in 13% of patients who underwent ALND in this study, but there was no significant difference in DFS between patients with and without ALND at a median follow-up of 5 years (92% vs. 87%, respectively).43 The AMAROS (After Mapping of the Axilla: Radiotherapy or Surgery?) trial randomized 1425 patients with T1-T2 cN0 breast carcinoma and 1 or 2 positive SLNs to ALND or axillary radiotherapy.46 Additional LN metastases were identified in 220/672 (33%) patients who underwent ALND and 52/672 (8%) patients had four or more additional metastatic nodes. With a median follow-up of 6.1 years, the axillary recurrence rate was extremely low in both groups, (0.43% in the ALND group, and 1.19% in the regional radiotherapy group) with no significant differences in DFS or OS between the two groups. AMAROS demonstrated that both treatment strategies provide excellent and comparable axillary control, but does not provide guidance on which SLN positive patients need further axillary treatment. The American College of Surgeons Oncology Group (ACOSOG) Z0011 prospective randomized trial looked at the benefit of ALND in patients with invasive breast carcinoma <5cm, no clinically palpable axillary adenopathy (T1-T2 cN0), and H&E-detected metastases limited to 1 or 2 SLNs, who were treated with BCT to negative margins followed by whole breast irradiation.44, 45 Adjuvant systemic therapy (chemotherapy and/or hormonal therapy) was as prescribed by the treating physician. Patients were randomly assigned to ALND or no further axillary treatment, and the clinical characteristics of the two groups were similar, including similar rates of adjuvant treatment. Additional LN involvement was documented in 27% of SLN-positive patients who underwent ALND. At 6.3 years median follow-up, there were no significant differences in regional LN recurrence between patients who underwent ALND and those who did not (0.9% vs. 0.5%, respectively). The two groups of patients had similar DFS (83.8% vs. 82.2%, respectively) and OS (92.5% vs. 91.5%, respectively). The results of the Z0011 study suggest that patients with T1-T2 tumors with ≤2 positive SLNs, who are treated with BCT and whole breast irradiation do not benefit from ALND.
The ACOSOG Z0011 trial has been influential and controversial. The results of this trial have been practice changing in many parts of the world,47–52 but are not universally accepted as such.53, 54 The criticisms of Z0011 include the failure to meet its accrual goal leading to early closure of the trial and lack of detail regarding radiation therapy. To address the latter issue, Jagsi et al.55 retrospectively analyzed available radiation therapy records of a subset of Z0011 patients. The authors found that most Z0011 patients received tangential field radiation therapy alone, with no significant differences in tangential field height between the two study arms, but 18.9% of patients received directed nodal irradiation via a third field, in violation of protocol.55 Although additional radiotherapy was administered with comparable frequency in Z0011 patients who did or did not undergo ALND, it is not possible to determine whether the additional radiation was beneficial, and how it might have influenced the rate of axillary recurrence in the SLN-only group. Authors working in healthcare systems where preoperative radiological assessment of the axilla is a routine practice also highlight the fact that Z0011 inclusion criterion was “no palpable adenopathy” and are critical of the lack of preoperative imaging to determine the axillary burden preoperatively.53, 56
Nevertheless, many national and international agencies have incorporated the Z0011 approach into their recent guidelines, to some extent. In 2014 ASCO published guidelines advising omission of completion ALND for patients with <3 positive SLNs if there is no evidence of bulky metastatic disease or gross ECE and the patient is treated with whole breast irradiation.15 The NCCN guidelines recommend considering level I and II ALND or no further axillary surgery for the patients who fulfill the aforementioned criteria.16 The St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015 concluded that ALND can be avoided in selected patients (i.e. those undergoing BCT followed by radiotherapy) with one or two macrometastatic lymph nodes.57 The Irish National Cancer Control Programme (NCCP) does not recommend ALND for patients with micrometastases in SLN and states that avoidance of ALND may be considered for patients undergoing BCT and radiotherapy who are clinically and radiologically node-negative at presentation and have 1 or 2 macrometastatic SLN(s), following a discussion at multidisciplinary team meeting and with the patient.58 In the U.K., the National Institute for Health and Care Excellence (NICE) guidelines for diagnosis and treatment of early and locally advanced breast cancer, published in 2009 and last reviewed in 2012, still recommend offering further axillary treatment, preferably ALND, to patients with early invasive breast cancer who have macrometastases or micrometastases in a SLN.59 However the NICE guidelines will be reviewed again in December 2015 and it is noted that following a multidisciplinary consensus meeting on further management of the malignant axillary node in January 2015, the Association of Breast Surgery, U.K., released a consensus statement on this issue and an updated guideline is in progress.60 If SLN(s) show ITCs or micrometastases no further axillary treatment is required. Further axillary management is no longer mandatory for patients with 1–2 SLN(s) with macrometastases in patients who are receiving BCT with whole breast radiotherapy, that are post-menopausal and have T1, grade 1 or 2, oestrogen receptor positive and HER2 negative tumours.60 There are still concerns about the limitations and the generalisability of Z0011 results and clinicians in the U.K. are encouraged to enter patients into clinical trials such as POSNOC (Positive Sentinel Node: Adjuvant Therapy Alone Versus Adjuvant Therapy Plus Clearance or Axillary Radiotherapy). POSNOC is a randomized controlled trial of axillary treatment in women with invasive breast carcinoma (< 5 cm), undergoing BCT or mastectomy, cN0 by clinical and ultrasound examination, who have macrometastases in 1 or 2 SLN(s) and no ECE.61 The accrual goal is 1900 women and all participants will be followed up for 5 years. The primary outcome is axillary recurrence at 5 years and secondary outcomes include loco-regional recurrence, OS and DFS.
INTRAOPERATIVE EVALUATION OF SLNS
Intraoperative detection of metastatic carcinoma in SLNs leads to immediate ALND, avoiding the need for a delayed second surgical procedure. Patients with a preoperative diagnosis of axillary nodal metastatic carcinoma often proceed to ALND, whereas intraoperative evaluation (IOE) of SLN at the time of primary breast surgery may be reserved for patients with clinically and radiologically negative axillae or suspicious intraoperative findings. The disadvantages of IOE of SLNs include an increase in operation time and possible false positive results. The use of IOE of SLNs varies greatly from centre to centre and is likely influenced by the extent to which preoperative ultrasound evaluation of the axilla, followed by fine needle aspiration (FNA) cytology or biopsy of suspicious LNs, is carried out.53, 62, 63 For example, in the U.K., where guidelines state that all patients with early invasive breast carcinoma should have axillary ultrasound in addition to clinical examination of the axilla,59 a national survey of pathologic evaluation of staging ALNs found that just 10% of laboratories used imprint cytology (IC) (5.6%) or frozen section (FS) (4.4%) for IOE of ALNs.64 A study from Memorial Sloan Kettering Cancer Center (MSKCC), New York, U.S.A., published in 2012, found that the use of SLN-FS decreased from 100% to 62% over a 10-year period.65 Rates of IOE of SLNs have also been influenced by the results of the Z0011 trial. A review of pre- and post-Z0011 practice patterns at the MD Anderson Cancer Center, Texas, U.S.A., found that surgeons were less likely to request IOE of SLNs in post-Z0011 patients (26% vs. 69%).47
FS, IC, or cytological smear (CS) can be used to evaluate SLNs intraoperatively. Cytologic techniques are faster than FS, and do not cause significant loss of nodal tissue, but it may be difficult to confirm findings limited to the cytology material, but not present in H&E-stained sections. FS is time-consuming; freezing introduces artifactual tissue distortion; sectioning of the frozen tissue block could potentially lead to the loss of critical tissue. Despite these disadvantages, FS is often the preferred method of IOE by most histopathologists. The sensitivity of SLN-FS ranges from 52% to 93% and specificity is 98.5% to 100%.66–76 A meta-analysis, including 47 FS studies, reported a pooled sensitivity of 73%, with higher sensitivity for macrometastases than micrometastases (94% vs. 40%).77 Wong and colleagues retrospectively studied 2202 SLN biopsies from 2174 patients with breast carcinoma, performed at Singapore General Hospital over a 7-year period and confirmed the relationship between the size of SLN metastasis and risk of false-negative FS result.78 While they identified an overall FNR of 13.5%, the FNR for detection of macrometastases was much lower, at 3.1%. In this study, the smaller the SLN metastases, the higher the odds of a false-negative diagnosis. In addition, non-ductal histological subtype and absence of lymphovascular invasion were identified as significant independent factors associated with a higher FNR. A wide range of sensitivity (from 34% to 96.9%), and specificity (from 96.3% to100%) has been reported for cytologic techniques.66, 69, 71–73, 79–91 A meta-analysis of 31 studies of IC identified an overall sensitivity of 63% and the pooled sensitivity for detection of macrometastases was higher than for micrometastases (81% vs. 22%).92 If required to perform IOE on SLNs pathologists should use the method they are most comfortable with to avoid false positive results.66
Rapid Molecular techniques for IOE are also available, but have somewhat questionable sensitivity. One-step nucleic acid amplification (OSNA) is a molecular assay used for IOE of SLNs. OSNA measures cytokeratin 19 (CK19) mRNA in homogenized SLN tissue and shows high sensitivity for the detection of metastases with increased identification of low volume nodal disease.93 Tsujimoto and colleagues quantified CK19 mRNA in histopathologically positive and negative ALNs from patients with invasive breast carcinoma using OSNA, used the mRNA copy numbers as a surrogate for metastatic LN positivity and determined cut-off values equivalent to micro-macrometastasis.94 Defined CK19 mRNA levels discriminate negative LNs (CK19 mRNA fewer than 250copies/μl or “−”) from micrometastases (CK19 mRNA 250–5000 copies/μl or “+”) and macrometastases (CK19 mRNA more than 5000 copies/μl or “++”).94, 95 Using the current TNM classification, the only category available for LNs containing metastatic carcinoma detected by molecular assay, but without histological or immunohistochemical evidence of LN involvement is pN0(mol+).7 The pN0(mol+) designation is a subset of the ITC category, may be potentially misleading, is probably not applicable in cases where OSNA results correspond to micro- or macrometastasis, and does not necessarily equate with pN0. Concerns about this technique relate to the fact that OSNA-based staging of LNs is not a recognized prognosticator and the homogenization of tissue required for analysis precludes the assessment of important morphologic features, such as size of the tumour deposit and extracapsular extension (ECE).93 Rare false-positive results can occur in cases of benign ectopic intranodal breast parenchyma.96, 97 The practice of using molecular methods without histologic confirmation of carcinoma has substantial limitations and is not advocated.14, 7 A recent meta-analysis of data from 12 studies that included 5057 SLNs from 2192 patients suggests that up to 21% of patients found to have macrometastases using OSNA would have had ALND whereas histology would have classified the deposits as non-macrometastases.98 A recent survey in the UK found that 13 laboratories used molecular assays for IOE of SLNs.64 While studies from around the world show that OSNA is a highly sensitive and specific technique for IOE of SLNs,94, 95, 97, 99–101 the question of whether molecular methods can verify the need for further axillary treatment, given changing clinical practice, is unclear.
Currently, IOE of SLNs of clinically “Z0011 eligible” patients is not routinely performed at many centres, and the decision whether to proceed to ALND is deferred to a later time, when all of the clinical and definitive pathological information is available.47, 49, 51, 102 At some centres IOE of SLNs of patients who fulfill the Z0011 criteria is still pursued, and pathologists are asked to report the number of LNs intraoperatively. IOE of SLNs continues to be performed routinely at many hospitals for cN0 patients undergoing mastectomy. The SLNs of patients who have had NACT are also evaluated intraoperatively at many institutions (see section on NACT).
PROTOCOLS FOR HISTOLOGICAL EVAUATION OF SLNS
A standardized SLN evaluation protocol combines careful gross and histologic evaluation.103 There is some variation in international guidelines and practice of pathologic examination of SLNs. CAP and ASCO recommend that each SLN is sectioned into 2 mm thick slices parallel to the long axis of the LN.14, 15 This approach is supported by the results of the NASBP B32 study.1, 12 The NHSBSP pathology reporting guidelines recommend histological examination of the SLN at intervals of approximately 3 mm or less and sectioning of the LN perpendicular to the long axis.18 A national survey of pathologic evaluation of staging ALNs in the U.K. revealed a variety of practices among respondents, with 64% of laboratories examining one section from each SLN block, 31% examining multiple sections taken at predefined intervals and 3.7% routinely performing CK-IHC on SLNs.64 Since the publication of the NSABP B32 study, at our institution we carefully count the number of SLNs grossly, and then section each SLN into 2 mm thick slices parallel to its longest axis, and embed all tissue slices of each SLN in one cassette. If two small SLNs are submitted in one cassette, each SLN is inked differentially, to ensure an accurate count of the SLNs, including those with metastatic disease, at the time of microscopic examination. We routinely evaluate only one H&E-stained section per block. The H&E-stained section should provide a full cross section of each SLN slice, including subcapsular space and SLN capsule. This protocol is used for all SLNs, independent of the subtype of the invasive mammary carcinoma, including invasive lobular carcinoma. Additional level sections and CK-IHC are obtained in selected cases to further investigate uncertain morphologic findings, but are not performed routinely. In the final report we include the total number of SLNs examined, the number of SLNs with metastatic carcinoma, and the span of the largest metastatic focus. We also report information on ECE (present, absent, or indeterminate) and its largest extent (<2 mm, 2 mm or > 2 mm). ECE is defined as the largest span of tumour deposit outside the LN capsule (Figure 1). In patients who meet Z0011 eligibility criteria the extent of ECE correlates with the likelihood of involvement of additional ALNs.104
Figure 1. Measuring extracapsular extension.
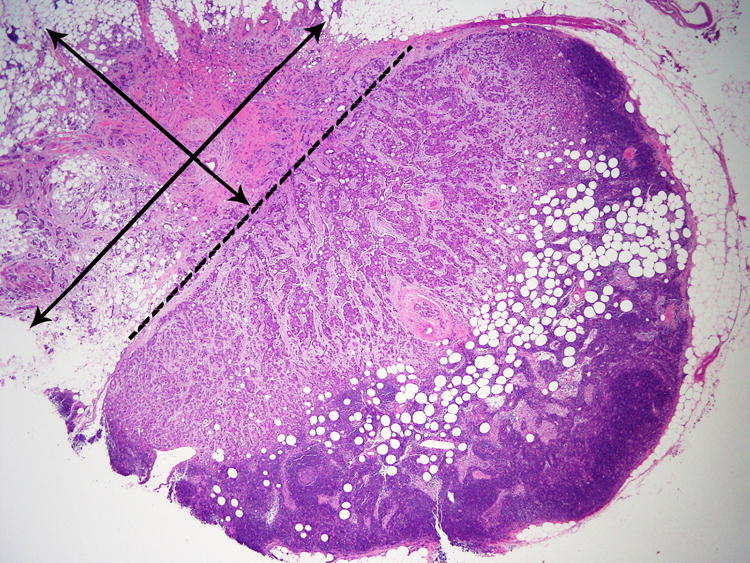
The dashed line represents the position of the lymph node capsule. Extracapsular extension measures 4 mm in the parallel plane and 3 mm in the perpendicular. We report the largest dimension of extracapsular extension.
Classification of LN metastasis is based on size and/or number of cells and it is recognized that these parameters can be difficult to apply in certain cases, e.g. dispersed single cell pattern of metastatic lobular carcinoma and cases with multiple cohesive clusters.14, 105–108 The size of the metastatic deposit for N classification is based on the largest contiguous cluster of tumour cells and the distance between clusters should not be included in the measurement.7, 14 However, pathologists may interpret these patterns in different ways and potentially assign such cases to different pN categories. CAP acknowledge this issue and advise that when the overall volume of tumour is similar to that of the higher nodal category (e.g., a node with 9 clusters of tumor cells, each measuring 1 mm), then the pathologist must use his/her judgment in assigning the N category.14 We also advocate this common sense approach and believe that the overall extent of tumour present in the LN be reflected in the report. These cases can be challenging and it may be beneficial to obtain additional sections deeper into the tissue block, and to review the case with colleagues. Per the CAP guidelines, we include a note in the report describing the distribution and pattern of carcinoma cells in the LN and conveying the difficulties of the case and the reason for assigning a particular N category.
NEOADJUVANT CHEMOTHERAPY AND SLN BIOPSY
Preoperative or neoadjuvant chemotherapy (NACT) has become the standard of care for locally advanced and inflammatory breast cancer, where the aim is to convert these patients to operable candidates. In addition, NACT is also used to reduce the size of the primary tumour and increase the rate of BCT in patients with operable breast cancer in whom mastectomy is initially indicated. Studies have shown no significant difference in OS or DFS between patients who received NACT versus those who received adjuvant chemotherapy.109, 110 The potential clinical advantages of NACT include extended opportunity for BCT, and ability to assess response to systemic therapy.111 Downstaging of ALN involvement occurs in 30% to 40% of patients treated with NACT.24, 112–115 Patients with triple-negative and Her2-positive breast carcinomas are most likely to have a complete pathological response (pCR), including no residual carcinoma in LNs. With the knowledge that NACT could potentially downstage the axilla, came the possibility that the extent of axillary surgery required for patients with no residual nodal disease after NACT could be decreased, leading clinicians to explore the use of SLN biopsy in this setting.
The accuracy of SLN biopsy in patients treated with NACT is a cause of concern, as tumour response is often heterogeneous. Furthermore, tumour response, inducing fibrosis, could alter lymphatic drainage, impeding lymphatic mapping and leading to decreased SLN identification rate and an increased FNR when compared to SLN biopsy performed in untreated patients. SLN biopsy performed prior to initiation of NACT achieves high SLN identification rates, 98% to 100%,116–119 comparable to the same procedure in primary surgical candidates. Three meta-analyses demonstrated SLN identification rates of 90% to 91% and FNRs of 8% to 10.5% after NACT.120–122 The studies included in the meta-analysis combined the results of cN0 and clinically node-positive patients and individual studies included in these analyses showed varying results. Several variables were reported to be associated with decreased SLN biopsy accuracy, including positive clinical nodal status at presentation.122
The timing of SLN, before versus after NACT, has been particularly controversial. There are advantages and disadvantages associated with both approaches. Upfront SLN biopsy carried out before starting chemotherapy provides information on axillary nodal status without the confounding effects of NACT and may allow more accurate initial staging. Furthermore, it is associated with high SLN identification rates.116–119 Proponents of this approach assert that pre-NACT pathological nodal status provides important information that can guide decisions regarding optimal treatment, particularly the need for and extent of locoregional radiotherapy. If the SLN is negative before NACT the patient will not have ALND at the time of definitive surgery. The need for two separate operations is a disadvantage of performing SLN biopsy before NACT. Patients with a positive SLN prior to NACT are committed to ALND, generally performed at the same time as their post-treatment BCT or mastectomy. A recent study showed that the proportion of patients receiving any axillary treatment was higher for those with SLN biopsy before NACT than after (45% vs. 33%).123 Pre-NACT SLN biopsy, however, does not exploit the potential downstaging effect of NACT, whereas if post-NACT SLN biopsy is negative, patients with involved ALNs at presentation may potentially be spared ALND and the complications associated with this procedure.
A major focus of investigation has been the role of SLN biopsy after NACT in patients who have axillary nodal metastases at presentation. A recent systematic review included 15 studies, with a total of 2,471 clinically node-positive early breast cancer patients treated with NACT and identified an overall SLN identification rate of 89% (range 78% to 98%) and FNR of 14% (range 5% to 25%).124 Three recent prospective clinical trials, discussed below, specifically investigated the use of SLN biopsy in this situation (Table 1).
Table 1.
Summary of clinical trials investigating sentinel lymph node (SLN) biopsy in clinically node-positive patients who received neoadjuvant chemotherapy (NACT)
| Nodal status | No. of patients | Pathological complete nodal response | SLN identification rate | False-negative rate | Biopsy/FNA pre-NACT | Pathological evaluation of SLN | |
|---|---|---|---|---|---|---|---|
| SENTINA125 | Arm A: cN0, pN0(sn) Arm B: cN0, pN1(sn) Upfront SLNB, repeat SLNB post-NACT Arm C: cN1–2, ycN0 Arm D: cN1–2, ycN1–N2 |
Arm A: 622 Arm B: 360 Arm C: 592 Arm D: 123 |
Arm B: 70.8%* Arm C: 52.3%* |
Arm A + B: 99.1% Arm B: 60.8% Repeat SLNB Arm C: 80.1% Arm D: NA |
Arm A: NA Arm B: 51.6% Arm C: Overall FNR 14.2% 1 SLN 24.3% 2 SLNs 18.5% 3 SLNs 7.3% Arm D: NA |
149/592 (25%) women in Arm C had pre-NACT FNA or LN core biopsy | SLNs sliced at intervals of 2–3 mm. H&E-stained step-sections at intervals of ≤500 μm, IHC not required Metastases classified with UICC TNM staging system8 |
| ACOSOGZ1071126 | cN1† | 649 | 41% | 92.9% | Overall FNR† 12.6% 1 SLN 31% 2 SLNs 21.1% ≥3 SLNs 9.1% |
All patients had pre-NACT LN core biopsy or FNA | H&E—no further details Metastases of >0.2 mm considered to be positive |
| SN FNAC128 | cN1–2 | 145 | 34.5% | 87.6% | Overall FNR 8.4% 1 SLN 18.2% ≥2 SLNs 4.9% |
All patients had pre-NACT LN core biopsy or FNA | SLNs sliced at 2-mm intervals H&E stain, if negative, IHC performed Metastases of any size, including <0.2 mm, considered to be positive |
cN0, Clinically node-negative; cN1–2, Clinically node-positive; FNA, Fine needle aspiration; FNR, False-negative rate; H&E, Haematoxylin and eosin; IHC, Immunohistochemistry; LN, Lymph node; NA, Not applicable; pN0(sn), SLN(s) pathologically negative; pN1(sn), SLN(s) pathologically positive; SLNB, SLN biopsy; TNM, Tumour Node Metastasis; UICC, Union for International Carcinoma Control; ycN0, Clini- cally node-negative after NACT; ycN1–2, Clinically node-positive after NACT.
Pathological complete nodal response calculated for patients with successful SLN mapping.
The primary endpoint was the FNR of SLN surgery after NACT in women who presented with cN1 disease, and the overall FNR is based on 525 patients with at least two SLNs identified.
SENTINA (SENTinel NeoAdjuvant), a prospective, multicentre study of SLN biopsy use in patients receiving NACT included 1737 patients, stratified by clinical axillary nodal status, as documented by clinical examination and axillary ultrasound (Table 1).125 Arm C (n=592) of the trial included women who were clinically node-positive at baseline, converted to cN0 after NACT and underwent SLN biopsy and ALND. The SLN identification rate in this cohort was 80.1% and the overall FNR was 14.2%. Further analysis showed a significant relationship between the number of resected SLNs and the FNR in the arm C patients with an FNR of less than 10% when 3 or more SLNs were identified.
The ACOSOG Z1071 (Alliance) Trial evaluated 649 patients with biopsy or FNA proven cN1 disease, who completed NACT and subsequently underwent SLN biopsy and ALND.126 At completion of NACT, 83% of patients were cN0, the SLN identification rate was 92.9% and the overall FNR was 12.6%, higher than the predetermined acceptable FNR of 10%. Once again, a relationship between the number of SLNs identified and FNR was evident, with a FNR of 9% when 3 or more SLNs were identified. A secondary end point of this study was to determine whether axillary ultrasound after NACT can aid patient selection for SLN biopsy.127 Post-NACT ultrasound studies were reviewed for 611 patients; 130/180 (71.8%) patients with suspicious ultrasound findings were node-positive at surgery compared with 243/430 (56.5%) patients with normal ultrasound post-NACT. The FNR estimate when using a combination of post-NACT ultrasound and SLN was 9.8%. However, the authors point out that the original FNR from the trial when using SLN alone was 12.6%, which falls within the rather wide 90% CI (7.1% to 13.2%), for ultrasound plus SLN. They recommend the use of axillary ultrasound after NACT to identify patients with the greatest likelihood of nodal response, who may be good candidates for SLN biopsy and the opportunity to avoid ALND.
The Sentinel Node Biopsy After Neoadjuvant Chemotherapy in Biopsy Proven Node-Positive Breast Cancer (SN FNAC) multicentre prospective study evaluated 145 patients with node-positive breast cancer who underwent SLN biopsy and ALND after NACT.128 IHC use was compulsory in this study and SLN metastases of any size were considered positive. The SLN identification rate was 87.6% and the overall FNR was 8.4%. Taken all together, these trials show that in patients who convert to cN0 disease, SLN biopsy after NACT has a FNR of <10% when ≥3 SLNs are identified. As pointed out in a recent review,129 just 57% of patients in the ACOSOG Z1071 trial had 3 or more SLNs,126 and only 34% of patients in arm C of the SENTINA trial.125 Thus, many patients who convert from clinically node-positive to cN0 status after NACT will not have ≥3 SLNs identified.129
Although SENTINA, ACOSOG Z1071 and SN FNAC provide us with information on the performance of SLN biopsy after NACT, they do not address the clinical significance of leaving metastatic disease behind after NACT or examine the modification of locoregional therapy based on the response to NACT. Two current ongoing randomised clinical trials specifically address axillary management following NACT in patients with proven ALN metastases and will provide us with more information on the longer term safety of SLN after NACT and the optimal management of these patients. In both trials, patients receive NACT followed by SLN biopsy. The NSABP B-51/Radiation Therapy Oncology Group (RTOG) 1304 (NRG 9353) is a phase III clinical trial evaluating the role of radiotherapy in patients with documented positive ALNs who convert to ypN0 after NACT.130 The Alliance A011202 trial also seeks to define the optimal management of the axilla in patients with a positive SLN following NACT.131 Patients with positive SLNs after NACT are randomly assigned to completion ALND or axillary radiation.
Sentinel lymph node biopsy is not recommended in patients with T4d/inflammatory breast cancer who have received NACT, regardless of clinical response to treatment, or in patients with T4abc breast cancer whose cancer has been clinically downstaged after receiving NACT.15 The 2014 ASCO guidelines state that SLN biopsy may be offered before or after NACT, but the procedure seems less accurate after NACT.15 The FNR reported with SLN biopsy after NACT appears to be higher than that reported before NACT and FNRs of 10% to 30% are, in the opinion of the ASCO guideline panelists, unacceptably high and may lead to inaccurate staging and under treatment of patients.15, 132 The 2015 St. Gallen panel considers SLN biopsy to be appropriate for patients who are clinically node-positive at presentation and downstage after NACT, but recommends that ALND should be performed even if one SLN is positive, emphasizing that FNRs remain high unless 3 or more SLNs are examined.57
PATHOLOGICAL ASSESSMENT OF SLN AFTER NACT
Evaluation of SLNs from patients who have had NACT can be challenging for the pathologist. Patients with pCR in the breast and ALNs have significantly improved OS and DFS.133, 134 One study also showed that no residual carcinoma in ALNs is associated with an excellent prognosis, even with residual disease in the breast.135 Women with residual disease in the ALNs post-NACT have a poorer prognosis.136, 137 While low volume SLN metastatic disease does not always mandate completion ALND in primary surgical patients, this is not the case in those who have had NACT. In this clinical context accurate assessment is crucial because just one positive SLN, even if metastatic carcinoma is of small size, influences the decision to proceed to ALND. The clinical significance of residual ITCs following NACT is unclear. The 2012 WHO classification takes the stance that small nodal metastases and ITCs are evidence of an incomplete response.138 Similarly the AJCC TNM staging manual alludes to the fact that ITCs may represent minimal nodal disease pre-treatment that did not respond to NACT or residual macroscopic nodal disease with a partial response.7 Despite the fact that the TNM says that the presence of ITCs precludes classifying the patient as having a pathologic complete response, it still recommends classifying these cases as ypN0(i+). While this approach allows standard definitions of N staging to be maintained, it is somewhat confusing.
Decisions regarding the extent of radiation therapy may be based on the combination of nodes with viable metastatic carcinoma as well as probable number of nodes involved by metastatic carcinoma prior to NACT. It follows that the presence of features indicative of regressed metastatic carcinoma should be reported.139 After NACT, LNs are often smaller in size, and often appear slightly lymphocyte depleted. In most cases, complete response is evident in the form of fibrosis and aggregates of foamy macrophages devoid of viable carcinoma cells, similar to the histological features seen in the primary tumour bed (Figure 2),139, 140 but in some cases the histologic evidence of prior tumour involvement can be very subtle.
Figure 2. Lymph node with complete pathological response to neoadjuvant chemotherapy.
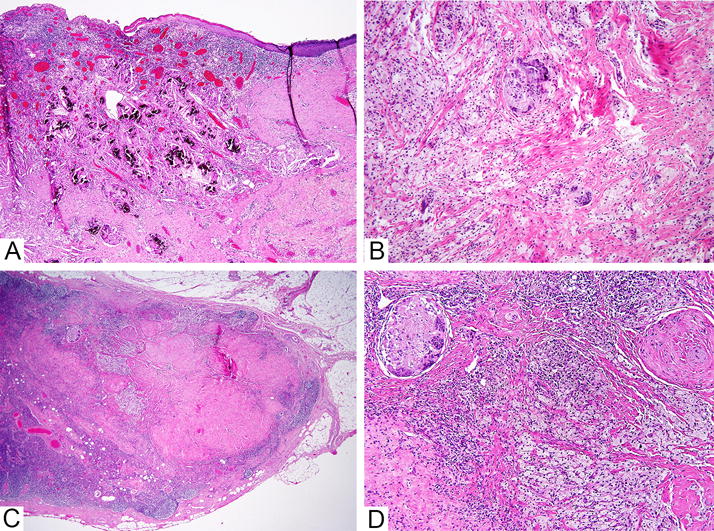
This patient had complete pathological response to neoadjuvant chemotherapy. A. Low power image of primary tumour bed in the breast. B. Medium power image of primary tumour bed in the breast. C. The lymph node shows marked fibrosis at low power. D. Foamy macrophages and multinucleated giant cells are present in the lymph node. The features are morphologically similar to those seen in the tumour bed in the breast.
Metastatic carcinoma cells may be scattered throughout a fibrotic lymph node (Figure 3) and CK-IHC may be useful to confirm suspicious morphology. Extramedullary haematopoeisis may occur in patients treated with chemotherapy and megakaryocytes in ALNs are a potential mimic of metastatic carcinoma.141–144 At some centres, ALNs biopsied before NACT are marked with a clip, enabling targeted excision of specific LNs.145 In these cases, the pathologist can confirm the presence of the marking clip.
Figure 3. Lymph nodes with residual metastatic carcinoma post neoadjuvant chemotherapy.
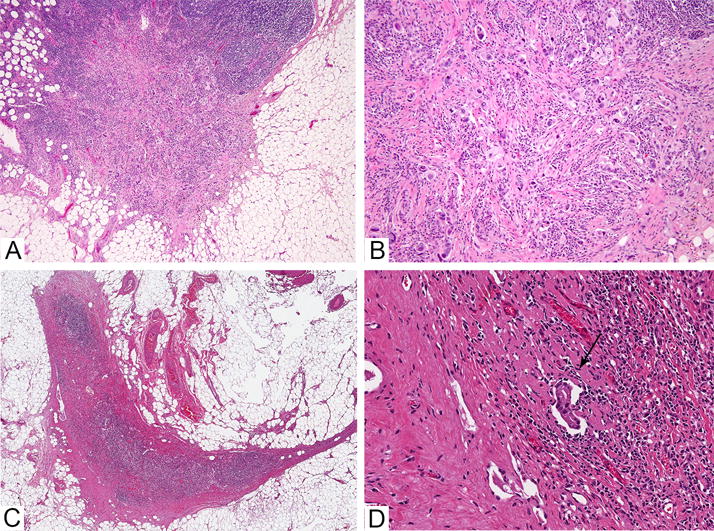
A. Low power image of lymph node with residual metastastic carcinoma composed of single cells in desmoplastic stroma. Extracapsular extension is present. B. Higher power image of the same lymph node. C. Low power image of a lymph node with a subtle focus of residual carcinoma after neoadjuvant chemotherapy, seen at higher power in image D (see arrow).
In post-NACT cases we report the total number of LNs with metastatic carcinoma, size of the largest metastatic focus, presence of ECE and the number of LNs with treatment related changes and no viable carcinoma. A 2006 study showed that the LN metastasis size and number of involved LNs after NACT were independent predictors of distant DFS and OS.146 The terms ITCs, and micro-macrometastasis are not usually adopted when reporting the size of the largest post-NACT metastatic carcinoma, and their use could be misleading. If the breast shows pCR but carcinoma is present in the LNs, oestrogen receptor, progesterone receptor and HER2 stains are performed on the largest focus of metastatic carcinoma. In our department, IOE of SLNs post-NACT is routinely performed.
The accuracy, sensitivity and specificity of IOE of SLNs post-NACT has been found to be comparable to non-NACT SLNs, in relatively small studies.72, 147–149 In our opinion, it is best to exercise caution in these cases and maintain a low threshold for deferral of final diagnosis to permanent section, in order to avoid false positive diagnosis and unnecessary ALNDs. Aggregates of macrophages, multinucleated giant cells and megakaryocytes may mimic metastatic carcinoma on FS. Uncertain or minimal morphologic findings are best assessed on permanent section and may require use of ancillary tests for confirmation.
SUMMARY
The results of recent clinical trials have substantially changed the clinical management of the axilla, resulting in fewer ALNDs in selected cN0, SLN-positive patients. The identification of occult metastases does not appear to be of clinical benefit in contemporary T1-T2 cN0 patients, who are treated with adjuvant hormonal treatment and/or chemotherapy in most cases. The goal of SLN examination should be to detect all macrometastases (>2mm) and the use of deeper level sections and CK-IHC is not warranted in routine practice. Further studies are needed to refine the management of the axilla in SLN-positive patients who were not included, under-represented or unspecified in the aforementioned clinical trials, such as patients undergoing mastectomy, and HER2-positive patients. The use and timing of SLN biopsy in patients with invasive breast carcinoma receiving NACT is evolving. SLN biopsy appears to be a suitable method of axillary staging for patients who are cN0 prior to initiation of chemotherapy and studies show that it may be suitable for selected clinically node-positive patients who convert to cN0 after treatment.
Acknowledgments
A.M. and E.B. co-wrote the review article. The authors thank Ms. Angelica Martin (Pathologist Office Assistant, Memorial Sloan Kettering Cancer Center), for her assistance with editing of the manuscript, and Ms. Lorraine Biedrzycki (Medical Phogographer, Memorial Sloan Kettering Cancer Center) for her assistance with preparation of the figures.
GRANT NUMBER: P30 CA008748
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors have no conflict of interest to declare.
References
- 1.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the nsabp b-32 randomised phase 3 trial. The Lancet Oncology. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canavese G, Catturich A, Vecchio C, et al. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: Results of randomized trial. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2009;20:1001–1007. doi: 10.1093/annonc/mdn746. [DOI] [PubMed] [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The almanac trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: Ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 6.Zavagno G, De Salvo GL, Scalco G, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: Results of the sentinella/givom trial. Ann Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 7.Edge SBD, Compton C, Fritz AG, Greene FL, Trotti A, editors. Ajcc cancer staging manual. 7th. New York: Springer; 2010. [Google Scholar]
- 8.Sobin LH, Gospodarowic MK, Wittekind C, editors. International union against cancer (uicc) Tnm classification of malignant tumours. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 9.Singletary SE, Connolly JL. Breast cancer staging: Working with the sixth edition of the ajcc cancer staging manual. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. quiz 50–31. [DOI] [PubMed] [Google Scholar]
- 10.Weaver DL. Sentinel lymph nodes and breast carcinoma: Which micrometastases are clinically significant? Am J Surg Pathol. 2003;27:842–845. doi: 10.1097/00000478-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Julian T, Anderson SJ, Krag DN, editors. 2013 ASCO Annual Meeting; 2013: Journal of Clinical Oncology. 2013. 10-yr follow-up results of nsabp b-32, a randomized phase iii clinical trial to compare sentinel node resection (snr) to conventional axillary dissection (ad) in clinically node-negative breast cancer patients. [Google Scholar]
- 12.Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–421. doi: 10.1056/NEJMoa1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver DL, Le UP, Dupuis SL, et al. Metastasis detection in sentinel lymph nodes: Comparison of a limited widely spaced (nsabp protocol b-32) and a comprehensive narrowly spaced paraffin block sectioning strategy. Am J Surg Pathol. 2009;33:1583–1589. doi: 10.1097/PAS.0b013e3181b274e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester SC, Bose S, Chen Y-Y, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Hill KA, Kleer C, O’Malley FP, Page DL, Smith BL, Tan LK, Weaver DL, Winer E, Simpson JF. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. College of American Pathologists. 2013 doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 15.Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 16.National comprehensive cancer network clinical practice guidelines in oncology. Breast Cancer Version 320152015 [Google Scholar]
- 17.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24(Suppl 6):vi7–23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 18.Ellis IO. National Breast Screening Programme (NHSBSP) Publication, NHS Cancer Screening Programmes and The Royal College of Pathologists London. 2005. Pathology reporting of breast disease. (Publication No. 58). [Google Scholar]
- 19.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. Jama. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 20.Borgstein PJ, Pijpers R, Comans EF, van Diest PJ, Boom RP, Meijer S. Sentinel lymph node biopsy in breast cancer: Guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. Journal of the American College of Surgeons. 1998;186:275–283. doi: 10.1016/s1072-7515(98)00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. doi: 10.1097/00000658-199409000-00015. discussion 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 24.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the nsabp b-32 randomised phase iii trial. The Lancet Oncology. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 25.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surgical oncology. 1993;2:335–339. doi: 10.1016/0960-7404(93)90064-6. discussion 340. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH, Giuliano AE, Somerfield MR, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–581. doi: 10.1002/1097-0142(20000801)89:3<574::aid-cncr12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 29.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 30.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: A metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 31.Houvenaeghel G, Nos C, Giard S, et al. A nomogram predictive of non-sentinel lymph node involvement in breast cancer patients with a sentinel lymph node micrometastasis. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35:690–695. doi: 10.1016/j.ejso.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Katz A, Niemierko A, Gage I, et al. Can axillary dissection be avoided in patients with sentinel lymph node metastasis? J Surg Oncol. 2006;93:550–558. doi: 10.1002/jso.20514. [DOI] [PubMed] [Google Scholar]
- 33.Katz A, Smith BL, Golshan M, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008;26:2093–2098. doi: 10.1200/JCO.2007.11.9479. [DOI] [PubMed] [Google Scholar]
- 34.Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255:109–115. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio IT, Espinosa-Bravo M, Rodrigo M, et al. Nomogram including the total tumoral load in the sentinel nodes assessed by one-step nucleic acid amplification as a new factor for predicting nonsentinel lymph node metastasis in breast cancer patients. Breast Cancer Res Treat. 2014;147:371–380. doi: 10.1007/s10549-014-3108-2. [DOI] [PubMed] [Google Scholar]
- 36.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95:302–309. doi: 10.1002/bjs.5943. [DOI] [PubMed] [Google Scholar]
- 38.Degnim AC, Reynolds C, Pantvaidya G, et al. Nonsentinel node metastasis in breast cancer patients: Assessment of an existing and a new predictive nomogram. American journal of surgery. 2005;190:543–550. doi: 10.1016/j.amjsurg.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–254. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Kohrt HE, Olshen RA, Bermas HR, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. doi: 10.1186/1471-2407-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91:113–119. doi: 10.1007/s10549-004-5781-z. [DOI] [PubMed] [Google Scholar]
- 42.Chue KM, Yong WS, Thike AA, et al. Predicting the likelihood of additional lymph node metastasis in sentinel lymph node positive breast cancer: Validation of the memorial sloan-kettering cancer centre (mskcc) nomogram. J Clin Pathol. 2014;67:112–119. doi: 10.1136/jclinpath-2013-201524. [DOI] [PubMed] [Google Scholar]
- 43.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (ibcsg 23–01): A phase 3 randomised controlled trial. The Lancet Oncology. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. Jama. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The american college of surgeons oncology group z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (eortc 10981–22023 amaros): A randomised, multicentre, open-label, phase 3 non-inferiority trial. The Lancet Oncology. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caudle AS, Hunt KK, Tucker SL, et al. American college of surgeons oncology group (acosog) z0011: Impact on surgeon practice patterns. Ann Surg Oncol. 2012;19:3144–3151. doi: 10.1245/s10434-012-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gainer SM, Hunt KK, Beitsch P, Caudle AS, Mittendorf EA, Lucci A. Changing behavior in clinical practice in response to the acosog z0011 trial: A survey of the american society of breast surgeons. Ann Surg Oncol. 2012;19:3152–3158. doi: 10.1245/s10434-012-2523-z. [DOI] [PubMed] [Google Scholar]
- 49.Massimino KP, Hessman CJ, Ellis MC, Naik AM, Vetto JT. Impact of american college of surgeons oncology group z0011 and national surgical adjuvant breast and bowel project b-32 trial results on surgeon practice in the pacific northwest. American journal of surgery. 2012;203:618–622. doi: 10.1016/j.amjsurg.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Yi M, Kuerer HM, Mittendorf EA, et al. Impact of the american college of surgeons oncology group z0011 criteria applied to a contemporary patient population. Journal of the American College of Surgeons. 2013;216:105–113. doi: 10.1016/j.jamcollsurg.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright GP, Mater ME, Sobel HL, et al. Measuring the impact of the american college of surgeons oncology group z0011 trial on breast cancer surgery in a community health system. American journal of surgery. 2014 doi: 10.1016/j.amjsurg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Joyce DP, Lowery AJ, McGrath-Soo LB, et al. Management of the axilla: Has z0011 had an impact? Irish journal of medical science. 2015 doi: 10.1007/s11845-015-1246-0. [DOI] [PubMed] [Google Scholar]
- 53.Guth U, Myrick ME, Viehl CT, Schmid SM, Obermann EC, Weber WP. The post acosog z0011 era: Does our new understanding of breast cancer really change clinical practice? European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2012;38:645–650. doi: 10.1016/j.ejso.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Nadeem R, Saidan Z. Implications of omitting axillary clearance in positive sentinel nodes: A retrospective application of the acosog z0011 and posnoc trials. European Journal of Surgical Oncology (EJSO) 2013;39:461. [Google Scholar]
- 55.Jagsi R, Chadha M, Moni J, et al. Radiation field design in the acosog z0011 (alliance) trial. J Clin Oncol. 2014;32:3600–3606. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed M, Douek M. What is the future of axillary surgery for breast cancer? Ecancermedicalscience. 2013;7:319. doi: 10.3332/ecancer.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies – improving the management of early breast cancer: St galleninternational expert consensus on the primary therapy of early breast cancer 2015. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2015 doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National cancer control programm. National clinical guideline. Diagnosis, staging and treatment of patients with breast cancer. National Clinical Guideline. 2015 Available from: http://www.hse.ie/eng/services/list/5/cancer/profinfo/guidelines/breast/breastguideline.pdf.
- 59.National institute for health and care excellence. clinical guideline 80. London: NICE; 2009. Early and locally advanced breast cancer diagnosis and treatment. Available from: www.nice.org/CG80. [Google Scholar]
- 60.Association of breast surgery. Consensus statement on management of the malignant axilla in early breast cancer. 2015 [cited 2015 25/07/15]. Available from: http://www.associationofbreastsurgery.org.uk/publications/guidelines/abs-consensus-statement-management-of-the-malignant-axilla-in-early-breast-cancer-(2015)/
- 61.Goyal A, Dodwell D, Reed MW, Coleman RE. Axillary treatment in women with one or two sentinel nodes with macrometastases: More evidence is needed to inform practice. J Clin Oncol. 2014;32:3902. doi: 10.1200/JCO.2014.57.3717. [DOI] [PubMed] [Google Scholar]
- 62.Carroll PA, O’Mahony D, McDermott R, et al. Perioperative diagnosis of the positive axilla in breast cancer: A safe, time efficient algorithm. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37:205–210. doi: 10.1016/j.ejso.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Ibrahim-Zada I, Grant CS, Glazebrook KN, Boughey JC. Preoperative axillary ultrasound in breast cancer: Safely avoiding frozen section of sentinel lymph nodes in breast-conserving surgery. Journal of the American College of Surgeons. 2013;217:7–15. doi: 10.1016/j.jamcollsurg.2013.01.064. discussion 15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma R, Sundara Rajan S, Verghese ET, Horgan K, Hanby AM, Lane S. Pathological evaluation of the staging axillary lymph nodes for breast cancer: A national survey in the united kingdom. Histopathology. 2014;65:707–711. doi: 10.1111/his.12440. [DOI] [PubMed] [Google Scholar]
- 65.Weber WP, Barry M, Stempel MM, et al. A 10-year trend analysis of sentinel lymph node frozen section and completion axillary dissection for breast cancer: Are these procedures becoming obsolete? Ann Surg Oncol. 2012;19:225–232. doi: 10.1245/s10434-011-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brogi E, Torres-Matundan E, Tan LK, Cody HS., 3rd The results of frozen section, touch preparation, and cytological smear are comparable for intraoperative examination of sentinel lymph nodes: A study in 133 breast cancer patients. Ann Surg Oncol. 2005;12:173–180. doi: 10.1245/ASO.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 67.Lu Q, Tan EY, Ho B, et al. Achieving breast cancer surgery in a single setting with intraoperative frozen section analysis of the sentinel lymph node. Clinical breast cancer. 2013;13:140–145. doi: 10.1016/j.clbc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Sabel MS, Jorns JM, Wu A, Myers J, Newman LA, Breslin TM. Development of an intraoperative pathology consultation service at a free-standing ambulatory surgical center: Clinical and economic impact for patients undergoing breast cancer surgery. American journal of surgery. 2012;204:66–77. doi: 10.1016/j.amjsurg.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Memar B, Sadeghi R, Ayati NK, et al. The value of touch imprint cytology and frozen section for intra-operative evaluation of axillary sentinel lymph nodes. Polish journal of pathology: official journal of the Polish Society of Pathologists. 2010;61:161–165. [PubMed] [Google Scholar]
- 70.Chao C. The use of frozen section and immunohistochemistry for sentinel lymph node biopsy in breast cancer. Am Surg. 2004;70:414–419. [PubMed] [Google Scholar]
- 71.Van Diest PJ, Torrenga H, Borgstein PJ, et al. Reliability of intraoperative frozen section and imprint cytological investigation of sentinel lymph nodes in breast cancer. Histopathology. 1999;35:14–18. doi: 10.1046/j.1365-2559.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 72.Komenaka IK, Torabi R, Nair G, et al. Intraoperative touch imprint and frozen section analysis of sentinel lymph nodes after neoadjuvant chemotherapy for breast cancer. Ann Surg. 2010;251:319–322. doi: 10.1097/SLA.0b013e3181ba845c. [DOI] [PubMed] [Google Scholar]
- 73.Motomura K, Inaji H, Komoike Y, et al. Intraoperative sentinel lymph node examination by imprint cytology and frozen sectioning during breast surgery. Br J Surg. 2000;87:597–601. doi: 10.1046/j.1365-2168.2000.01423.x. [DOI] [PubMed] [Google Scholar]
- 74.Ali R, Hanly AM, Naughton P, et al. Intraoperative frozen section assessment of sentinel lymph nodes in the operative management of women with symptomatic breast cancer. World J Surg Oncol. 2008;6:69. doi: 10.1186/1477-7819-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan SW, LaVigne KA, Port ER, et al. Does the benefit of sentinel node frozen section vary between patients with invasive duct, invasive lobular, and favorable histologic subtypes of breast cancer? Ann Surg. 2008;247:143–149. doi: 10.1097/SLA.0b013e3181581f41. [DOI] [PubMed] [Google Scholar]
- 76.Jensen AJ, Naik AM, Pommier RF, Vetto JT, Troxell ML. Factors influencing accuracy of axillary sentinel lymph node frozen section for breast cancer. American journal of surgery. 2010;199:629–635. doi: 10.1016/j.amjsurg.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Liu LC, Lang JE, Lu Y, et al. Intraoperative frozen section analysis of sentinel lymph nodes in breast cancer patients: A meta-analysis and single-institution experience. Cancer. 2011;117:250–258. doi: 10.1002/cncr.25606. [DOI] [PubMed] [Google Scholar]
- 78.Wong J, Yong WS, Thike AA, et al. False negative rate for intraoperative sentinel lymph node frozen section in patients with breast cancer: A retrospective analysis of patients in a single asian institution. J Clin Pathol. 2015;68:536–540. doi: 10.1136/jclinpath-2014-202799. [DOI] [PubMed] [Google Scholar]
- 79.Deo S, Samaiya A, Jain P, et al. Sentinel lymph node biopsy assessment using intraoperative imprint cytology in breast cancer patients: Results of a validation study. Asian journal of surgery/Asian Surgical Association. 2004;27:294–298. doi: 10.1016/S1015-9584(09)60054-3. [DOI] [PubMed] [Google Scholar]
- 80.Van Eetvelde E, Vanhoeij M, Verfaillie G, Bourgain C, Lamote J. Role of intra-operative touch imprint cytology in the treatment of breast cancer. Acta chirurgica Belgica. 2011;111:130–135. doi: 10.1080/00015458.2011.11680723. [DOI] [PubMed] [Google Scholar]
- 81.Cserni G. The potential value of intraoperative imprint cytology of axillary sentinel lymph nodes in breast cancer patients. Am Surg. 2001;67:86–91. [PubMed] [Google Scholar]
- 82.Hamidian Jahromi A, Narayanan S, MacNeill F, Osin P, Nerurkar A, Gui G. Testing the feasibility of intra-operative sentinel lymph node touch imprint cytology. Annals of the Royal College of Surgeons of England. 2009;91:336–339. doi: 10.1308/003588409X391758a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zgajnar J, Frkovic-Grazio S, Besic N, et al. Low sensitivity of the touch imprint cytology of the sentinel lymph node in breast cancer patients–results of a large series. J Surg Oncol. 2004;85:82–86. doi: 10.1002/jso.20011. discussion 87. [DOI] [PubMed] [Google Scholar]
- 84.Jeruss JS, Hunt KK, Xing Y, et al. Is intraoperative touch imprint cytology of sentinel lymph nodes in patients with breast cancer cost effective? Cancer. 2006;107:2328–2336. doi: 10.1002/cncr.22275. [DOI] [PubMed] [Google Scholar]
- 85.Lee A, Krishnamurthy S, Sahin A, Symmans WF, Hunt K, Sneige N. Intraoperative touch imprint of sentinel lymph nodes in breast carcinoma patients. Cancer. 2002;96:225–231. doi: 10.1002/cncr.10721. [DOI] [PubMed] [Google Scholar]
- 86.Chicken DW, Kocjan G, Falzon M, et al. Intraoperative touch imprint cytology for the diagnosis of sentinel lymph node metastases in breast cancer. Br J Surg. 2006;93:572–576. doi: 10.1002/bjs.5289. [DOI] [PubMed] [Google Scholar]
- 87.Chen JJ, Yang BL, Zhang JX, Xu WP, Shao ZM, Wu J. The evaluation and optimization of intraoperative touch imprint cytology for sentinel lymph nodes in early-stage breast cancer in china. World J Surg. 2010;34:2325–2332. doi: 10.1007/s00268-010-0684-0. [DOI] [PubMed] [Google Scholar]
- 88.Creager AJ, Geisinger KR, Perrier ND, et al. Intraoperative imprint cytologic evaluation of sentinel lymph nodes for lobular carcinoma of the breast. Ann Surg. 2004;239:61–66. doi: 10.1097/01.sla.0000103072.34708.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clarke D, Leung E, Chachlani N, et al. Intraoperative assessment of sentinel node using imprint cytology. World J Surg. 2010;34:55–61. doi: 10.1007/s00268-009-0301-2. [DOI] [PubMed] [Google Scholar]
- 90.Llatjos M, Castella E, Fraile M, et al. Intraoperative assessment of sentinel lymph nodes in patients with breast carcinoma: Accuracy of rapid imprint cytology compared with definitive histologic workup. Cancer. 2002;96:150–156. doi: 10.1002/cncr.10620. [DOI] [PubMed] [Google Scholar]
- 91.Shiver SA, Creager AJ, Geisinger K, Perrier ND, Shen P, Levine EA. Intraoperative analysis of sentinel lymph nodes by imprint cytology for cancer of the breast. American journal of surgery. 2002;184:424–427. doi: 10.1016/s0002-9610(02)01003-6. [DOI] [PubMed] [Google Scholar]
- 92.Tew K, Irwig L, Matthews A, Crowe P, Macaskill P. Meta-analysis of sentinel node imprint cytology in breast cancer. Br J Surg. 2005;92:1068–1080. doi: 10.1002/bjs.5139. [DOI] [PubMed] [Google Scholar]
- 93.Cserni G. Intraoperative analysis of sentinel lymph nodes in breast cancer by one-step nucleic acid amplification. J Clin Pathol. 2012;65:193–199. doi: 10.1136/jclinpath-2011-200301. [DOI] [PubMed] [Google Scholar]
- 94.Tsujimoto M, Nakabayashi K, Yoshidome K, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 95.Le Frere-Belda MA, Bats AS, Gillaizeau F, et al. Diagnostic performance of one-step nucleic acid amplification for intraoperative sentinel node metastasis detection in breast cancer patients. Int J Cancer. 2012;130:2377–2386. doi: 10.1002/ijc.26291. [DOI] [PubMed] [Google Scholar]
- 96.Maiorano E, Mazzarol GM, Pruneri G, et al. Ectopic breast tissue as a possible cause of false-positive axillary sentinel lymph node biopsies. Am J Surg Pathol. 2003;27:513–518. doi: 10.1097/00000478-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 97.Bernet L, Cano R, Martinez M, et al. Diagnosis of the sentinel lymph node in breast cancer: A reproducible molecular method: A multicentric spanish study. Histopathology. 2011;58:863–869. doi: 10.1111/j.1365-2559.2011.03836.x. [DOI] [PubMed] [Google Scholar]
- 98.Tiernan JP, Verghese ET, Nair A, et al. Systematic review and meta-analysis of cytokeratin 19-based one-step nucleic acid amplification versus histopathology for sentinel lymph node assessment in breast cancer. Br J Surg. 2014;101:298–306. doi: 10.1002/bjs.9386. [DOI] [PubMed] [Google Scholar]
- 99.Jara-Lazaro AR, Hussain IH, Thike AA, et al. Assessment of suitability of the one step nucleic acid amplification (OSNA) assay as an intraoperative procedure for detection of metasta- sis in sentinel lymph nodes of breast cancer. J Clin Pathol. 2014;67:1032–1037. doi: 10.1136/jclinpath-2014-202361. [DOI] [PubMed] [Google Scholar]
- 100.Pathmanathan N, Renthawa J, French JR, et al. Intraoperative sentinel lymph node assessment in breast cancer: a compar- ison of rapid diagnostic method based on CK19 mRNA expression and imprint cytology. ANZ J Surg. 2014;84:730–734. doi: 10.1111/ans.12668. [DOI] [PubMed] [Google Scholar]
- 101.Tamaki Y, Akiyama F, Iwase T, et al. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. 2009;15:2879–2884. doi: 10.1158/1078-0432.CCR-08-1881. [DOI] [PubMed] [Google Scholar]
- 102.Dengel LT, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014;21:22–27. doi: 10.1245/s10434-013-3200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol. 2010;23(Suppl 2):S26–S32. doi: 10.1038/modpathol.2010.36. [DOI] [PubMed] [Google Scholar]
- 104.Gooch J, King TA, Eaton A, et al. The extent of extracapsular extension may influence the need for axillary lymph node dis- section in patients with T1–T2 breast cancer. Ann Surg Oncol. 2014;21:2897–2903. doi: 10.1245/s10434-014-3752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cserni G, Amendoeira I, Bianchi S, et al. Distinction of isolated tumour cells and micrometastasis in lymph nodes of breast cancer patients according to the new tumour node metastasis (TNM) definitions. Eur J Cancer. 2011;47:887–894. doi: 10.1016/j.ejca.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 106.Turner RR, Weaver DL, Cserni G, et al. Nodal stage classifica- tion for breast carcinoma: improving interobserver repro- ducibility through standardized histologic criteria and image-based training. J Clin Oncol. 2008;26:258–263. doi: 10.1200/JCO.2007.13.0179. [DOI] [PubMed] [Google Scholar]
- 107.Walker RA, Hanby A, Pinder SE, Thomas J, Ellis IO, National Coordinating Committee for Breast Pathology Research Sub- group Current issues in diagnostic breast pathology. J Clin Pathol. 2012;65:771–785. doi: 10.1136/jclinpath-2012-200733. [DOI] [PubMed] [Google Scholar]
- 108.Sahin AA, Guray M, Hunt KK. Identification and biologic sig- nificance of micrometastases in axillary lymph nodes in patients with invasive breast cancer. Arch Pathol Lab Med. 2009;133:869–878. doi: 10.5858/133.6.869. [DOI] [PubMed] [Google Scholar]
- 109.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adju- vant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 110.Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–1200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 111.Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann Surg Oncol. 2015;22:1425–1433. doi: 10.1245/s10434-015-4406-6. [DOI] [PubMed] [Google Scholar]
- 112.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preop- erative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 113.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer Trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 114.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to pre- operative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 115.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 116.Menard JP, Extra JM, Jacquemier J, et al. Sentinel lym- phadenectomy for the staging of clinical axillary node-nega- tive breast cancer before neoadjuvant chemotherapy. Eur J Surg Oncol. 2009;35:916–920. doi: 10.1016/j.ejso.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 117.Sabel MS, Schott AF, Kleer CG, et al. Sentinel node biopsy prior to neoadjuvant chemotherapy. Am J Surg. 2003;186:102–105. doi: 10.1016/s0002-9610(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 118.Schrenk P, Hochreiner G, Fridrik M, Wayand W. Sentinel node biopsy performed before preoperative chemotherapy for axillary lymph node staging in breast cancer. Breast J. 2003;9:282–287. doi: 10.1046/j.1524-4741.2003.09406.x. [DOI] [PubMed] [Google Scholar]
- 119.Straver ME, Rutgers EJ, Russell NS, et al. Towards rational axillary treatment in relation to neoadjuvant therapy in breast cancer. Eur J Cancer. 2009;45:2284–2292. doi: 10.1016/j.ejca.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 120.Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preopera- tive chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–546. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 121.Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer sentinel node identification and classification after neoadju- vant chemotherapy – systematic review and meta analysis. Acad Radiol. 2009;16:551–563. doi: 10.1016/j.acra.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 122.van Deurzen CH, Vriens BE, Tjan-Heijnen VC, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer. 2009;45:3124–3130. doi: 10.1016/j.ejca.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 123.van der Heiden-van der Loo M, de Munck L, Sonke GS, et al. Population based study on sentinel node biopsy before or after neoadjuvant chemotherapy in clinically node negative breast cancer patients: identification rate and influence on axillary treatment. Eur J Cancer. 2015;51:915–921. doi: 10.1016/j.ejca.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 124.Fu JF, Chen HL, Yang J, Yi CH, Zheng S. Feasibility and accu- racy of sentinel lymph node biopsy in clinically node-positive breast cancer after neoadjuvant chemotherapy: a meta-analy- sis. PLoS ONE. 2014;9:e105316. doi: 10.1371/journal.pone.0105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoad- juvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 126.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boughey JC, Ballman KV, Hunt KK, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Sur- geons Oncology Group Z1071 trial (Alliance) J Clin Oncol. 2015 doi: 10.1200/JCO2014.57.8401. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 129.King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–343. doi: 10.1038/nrclinonc.2015.63. [DOI] [PubMed] [Google Scholar]
- 130.Mamounas EP, White JR, Bandos H, et al. NSABP B-51/RTOG 1304: randomized phase III clinical trial evaluating the role of postmastectomy chest wall and regional nodal XRT (CWRNRT) and post-lumpectomy RNRT in patients (PTS) with documented positive axillary (AX) nodes before neoadjuvant chemotherapy (NC) who convert to pathologically negative AX nodes after NC. J Clin Oncol. 2014;32:5s. (suppl; abstr TPS1141) [Google Scholar]
- 131.A Randomized Phase III Trial Comparing Axillary Lymph Node Dissection to Axillary Radiation in Breast Cancer Patients (cT1-3 N1) Who Have Positive Sentinel Lymph Node Disease After Neoadjuvant Chemotherapy. United States: Alliance for Clinical Trials in Oncology; 2015. Access date: 2nd October 2015. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01901094. [Google Scholar]
- 132.Lyman GH. Appropriate role for sentinel node biopsy after neoadjuvant chemotherapy in patients with early-stage breast cancer. J Clin Oncol. 2015;33:232–234. doi: 10.1200/JCO.2014.58.9838. [DOI] [PubMed] [Google Scholar]
- 133.Zhang GC, Zhang YF, Xu FP, et al. Axillary lymph node sta- tus, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr Oncol. 2013;20:e180–e192. doi: 10.3747/co.20.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawajiri H, Takashima T, Aomatsu N, et al. Prognostic signifi- cance of pathological complete response following neoadju- vant chemotherapy for operable breast cancer. Oncol Lett. 2014;7:663–668. doi: 10.3892/ol.2014.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 136.Penault-Llorca F, Abrial C, Raoelfils I, et al. Comparison of the prognostic significance of Chevallier and Sataloff’s pathologic classifications after neoadjuvant chemotherapy of operable breast cancer. Hum Pathol. 2008;39:1221–1228. doi: 10.1016/j.humpath.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 137.Gralow JR, Zujewski JA, Winer E. Preoperative therapy in invasive breast cancer: reviewing the state of the science and exploring new research directions. J Clin Oncol. 2008;26:696–697. doi: 10.1200/JCO.2007.15.9459. [DOI] [PubMed] [Google Scholar]
- 138.Symmans F, P S, Lester S, Kulka J. Post-therapy effects. In: Lakhani SR, EI Schnitt SJ, Tan PH, de van Vijver MJ, editors. World Health Organization classification of tumours of the breast. 4th. Lyons: IARC; 2012. pp. 24–26. [Google Scholar]
- 139.Pinder SE, Rakha EA, Purdie CA, et al. Macroscopic handling and reporting of breast cancer specimens pre- and post-neoad- juvant chemotherapy treatment: review of pathological issues and suggested approaches. Histopathology. 2015;67:279–293. doi: 10.1111/his.12649. [DOI] [PubMed] [Google Scholar]
- 140.Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med. 2009;133:633–642. doi: 10.5858/133.4.633. [DOI] [PubMed] [Google Scholar]
- 141.Wilsher MJ, Bonar SF. Megakaryocytes in axillary lymph nodes mimicking metastatic breast carcinoma following neoadjuvant chemotherapy and herceptin. Pathology. 2014;46:453–455. doi: 10.1097/PAT.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 142.Prieto-Granada C, Setia N, Otis CN. Lymph node extramedul- lary hematopoiesis in breast cancer patients receiving neoad- juvant therapy: a potential diagnostic pitfall. Int J Surg Pathol. 2013;21:264–266. doi: 10.1177/1066896913480831. [DOI] [PubMed] [Google Scholar]
- 143.Takhar AS, Ney A, Patel M, Sharma A. Extramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancer. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-008943. Access date: 21st September 2015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23704429. [DOI] [PMC free article] [PubMed]
- 144.Millar EK, Inder S, Lynch J. Extramedullary haematopoiesis in axillary lymph nodes following neoadjuvant chemotherapy for locally advanced breast cancer – a potential diagnostic pit- fall. Histopathology. 2009;54:622–623. doi: 10.1111/j.1365-2559.2009.03246.x. [DOI] [PubMed] [Google Scholar]
- 145.Caudle AS, Yang WT, Mittendorf EA, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015;150:137–143. doi: 10.1001/jamasurg.2014.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klauber-DeMore N, Ollila DW, Moore DT, et al. Size of residual lymph node metastasis after neoadjuvant chemotherapy in locally advanced breast cancer patients is prognostic. Ann Surg Oncol. 2006;13:685–691. doi: 10.1245/ASO.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 147.Shimazu K, Tamaki Y, Taguchi T, Tsukamoto F, Kasugai T, Noguchi S. Intraoperative frozen section analysis of sentinel lymph node in breast cancer patients treated with neoadju- vant chemotherapy. Ann Surg Oncol. 2008;15:1717–1722. doi: 10.1245/s10434-008-9831-3. [DOI] [PubMed] [Google Scholar]
- 148.Rubio IT, Aznar F, Lirola J, Peg V, Xercavins J. Intraoperative assessment of sentinel lymph nodes after neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol. 2010;17:235–239. doi: 10.1245/s10434-009-0695-y. [DOI] [PubMed] [Google Scholar]
- 149.Han A, Moon HG, Kim J, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16:378–385. doi: 10.4048/jbc.2013.16.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]


