Abstract
Background
Negative outcomes of alcoholism are progressively more severe as the duration of problem alcohol use increases. Additionally, alcoholics demonstrate tendencies to neglect negative consequences associated with drinking and/or to choose to drink in the immediate presence of warning factors against drinking. The recently derived crossed High-Alcohol Preferring (cHAP) mice, which volitionally drink to heavier intoxication (as assessed by BEC) than other alcohol-preferring populations, as well as spontaneously escalating their intake, may be a candidate to explore mechanisms underlying long-term excessive drinking. Here we hypothesized that an extended drinking history would reduce the ability of two manipulations (forced abstinence and conditioned taste aversion) to attenuate drinking.
Methods
Experiment 1 examined differences between groups drinking for either 14 or 35 days, half of each subjected to 7 days of forced abstinence and half not, to characterize potential changes in post-abstinence drinking resulting from an extended drinking history. Experiment 2 used a conditioned taste aversion procedure to assess stimulus specificity of the ability of an aversive flavorant to decrease alcohol consumption. Experiment 3 used this taste aversion procedure to assess differences among groups drinking for 1, 14, or 35 days in their propensity to overcome this aversion when the flavorant was mixed with either ethanol or water.
Results
Experiment 1 demonstrated that although forced abstinence decreased alcohol consumption in mice with a 14-day drinking history, it failed to do so in mice drinking alcohol for 35 days. Experiment 2 showed that the addition of a flavorant only suppressed alcohol drinking if an aversion to the flavorant was previously established. Experiment 3 demonstrated that an extended drinking history expedited extinction of suppressed alcohol intake caused by a conditioned aversive flavor.
Conclusions
These data show that a history of long-term drinking in cHAP mice attenuates the efficacy of interventions that normally reduce drinking. Analogous to alcoholics who may encounter difficulties in limiting their intake, cHAP mice with long drinking histories are relatively insensitive both to abstinence and signals of harmful consequences. We propose that the cHAP line may be a valid model for adaptations that occur following extended heavy alcohol drinking.
Keywords: ethanol, abstinence, conditioned taste aversion, escalation, animal model
Introduction
Alcoholism is considered to be a chronic disease of excessive and uncontrollable alcohol use characterized by poor relational/occupational functioning and physical illness, representing a leading cause of preventable death (Turner et al., 1993; Danaei et al., 2009). Chronic alcoholics often demonstrate resistance to treatment, negligence of negative life consequences caused by drinking, and continued relapse following bouts of successful abstinence (Krystal et al., 2001; Kirshenbaum et al., 2009). “Lifetime” drinking problems, as compared to shorter bouts of alcohol use disorders, are predictive of very poor outcomes even under intention to quit (Moos and Moos, 2006). A better understanding of the neurological and behavioral factors preceding and resulting from long-term uncontrolled alcohol abuse and addiction is necessary to improve treatment. Unfortunately, human studies lack control of etiological factors including genetics and many environmental variables, and certain experimental manipulations of drinking are either impossible or unethical. Therefore, researchers should strive to establish an ecologically and neurologically valid animal model of long-term alcoholism and use it to further understanding of isolated behavioral and/or neurological factors contributing to the disease. Parallels to the tendency among humans with alcohol use disorders to disregard negative, or aversive, outcomes to obtain and consume alcohol have received recent focus in basic research. Indeed, the need for an improved model of aversion-resistant alcohol intake to facilitate identification of molecular mechanisms and assist in the development of therapies is elegantly described in a recent review by Hopf and Lesscher (2014).
Common genetic animal models of alcoholism include the reasonably high-drinking inbred C57BL/6J (B6) mouse, the outbred Wistar rat, and the selectively bred alcohol-preferring (P) and High-Alcohol Drinking (HAD) lines of rats (reviewed in McBride et al., 1998 and Spanagel et al., 2000). Volitional alcohol drinking despite adulteration using quinine (an aversive flavor) develops with prior extended alcohol drinking in rats (Hopf et al., 2010) and mice (Lesscher et al., 2010). However, free-choice drinking in these animal models does not yield intake levels similar to human alcoholics (Leeman et al., 2010). Indeed, these intakes would not be expected to result in the blood ethanol concentrations (BECs) that lend themselves to translational inferences regarding the effect of long-term heavy drinking (see Sanchis-Segura and Spanagel, 2006). We propose that a rodent model that, following long-term alcohol drinking, demonstrates high intakes of alcohol despite manipulations that would otherwise reduce intake may be useful alcoholism research. The crossed High-Alcohol Preferring (cHAP) mice, derived by crossing and subsequently selecting from the High-Alcohol Preferring replicate 1 (HAP1) and replicate 2 (HAP2) lines, consumes more alcohol than either parent line (Oberlin et al., 2011), and achieves BECs averaging 260 mg/dl during daily free-choice access to 10% alcohol and water (Matson and Grahame, 2013). Such BECs are comparable to those shown in a classic study of alcoholics given unlimited alcohol in an inpatient setting (Mello and Mendelson, 1970). We acknowledge that limitations to this model exist; for example, the parent lines show little or no physical withdrawal signs after repeated alcohol vapor exposure, and cHAPs would be expected to replicate this finding (Lopez et al., 2011). Nonetheless, we believe that the cHAP mouse is a strong candidate for an animal model of the difficulties of reducing drinking in chronic alcoholism (Sanchis-Segura and Spanagel, 2006).
Here we explore forced abstinence (FA) and conditioned taste aversion (CTA), two manipulations that we hypothesized to decrease alcohol intakes, to assess whether a longer drinking history may lead to otherwise undetectable adaptations in the mechanisms that regulate intakes. In many rodent lines, forced alcohol abstinence, or alcohol deprivation, is shown to produce a transient increase in drinking known as the alcohol deprivation effect, or ADE (Sinclair and Li, 1989; Bell et al., 2004; Melendez et al., 2006). However, we hypothesized that cHAP mice would show a decrease in alcohol intakes following abstinence, given that they increase alcohol intake over 7–10 days following alcohol presentation, which is correlated with the acquisition of functional (ataxia-resisting) and metabolic (alcohol-clearing) tolerance (Matson et al., 2013; Matson et al., 2014; Hall et al., 2001). Accordingly, if increases in intake are related to the development of tolerance, we hypothesized abstinence (here, we tested 7 days of abstinence) would diminish tolerance, which would be expected to decrease alcohol intake upon representation. Indeed, we observed this effect in a pilot study in our lab, in which mice that had 14 days of access to alcohol showed abstinence-induced decreases in drinking; however, no decrease was observed after 35 days of drinking. This pilot study was done as a within-subjects assessment, confounding the number of deprivations with duration of drinking, so the current study examined drinking duration effects in different mice. Additionally, a CTA paradigm involving the pairing of lithium chloride (LiCl)-induced gastric malaise with a flavorant would be an effective supplement to the previously-mentioned quinine aversion work because emetic aversion therapy has proven to be unsuccessful in chronic alcoholics (Hopf et al., 2010; Lesscher et al., 2010; Cannon et al., 1981). Although addition of a conditioned aversive flavorant should suppress alcohol intake in cHAP mice, a history of chronic drinking may attenuate the magnitude of this effect.
Thus, Experiment 1 was designed to assess effects of short- and long-term histories of free-choice drinking (14 or 35 days, building upon our pilot study) on the hypothesized efficacy of 7-day abstinence to reduce drinking and functional tolerance in cHAP mice. This experiment employed four groups; a forced-abstinence group and its continuous-access control group for both durations of drinking. Experiment 2 was designed to assess the hypothesized efficacy of reducing alcohol consumption by compounding the ethanol with a conditioned aversive stimulus. Because an added flavorant could reduce alcohol consumption in cHAP mice due to gustatory effects alone, two flavorants were used but only one was paired with LiCl. After this procedure was shown to be successful, Experiment 3 expanded upon it by comparing groups drinking for 1, 14, or 35 days on their propensity to overcome aversion when the LiCl-paired flavorant was mixed with either alcohol or water. We hypothesized that longer histories of drinking would reduce the ability of an aversive conditioned stimulus to decrease alcohol intake, while not affecting its ability to decrease intake of water.
Materials and Methods
Experiment 1 – Effects of Drinking Duration on Alcohol Intakes Following Forced Abstinence
Subjects
Sixty-four cHAP mice from S20 were counterbalanced across sex and family into 4 groups (described below). Mice were housed in a reverse-light cycle colony room in which lights were on from 20:00 to 08:00 hours, and drinking was measured during the dark part of the cycle using red illumination. Animals were brought to the colony room 14 days before commencement of the experiment, and single-housed 7 days later. All mice had ad lib access to food and water throughout in each experiment. All experimental procedures were approved by the IACUC of IUPUI, and were conducted in strict adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Procedure
Mice were provided with two 25-ml tubes with water only or 10% ethanol (v/v) in water. Intakes were read 6–7 days per week, and mice had bottles side-switched 3 times a week and were weighed once a week. Prior to initiation of abstinence, the 35-day free-choice alcohol access, 7-day forced abstinence group (35FC7A) had 35 days of access to 10% ethanol and water, followed by 7 days of water only access. The 35-day continuous free-choice access group (35FC) had access to water only for 7 days, followed by 35 days of access to 10% ethanol and water. The 14-day free-choice alcohol access, 7-day forced abstinence group (14FC7A) had 21 days of water only access, 14 days of access to 10% v/v ethanol and water, then 7 days of water only access. Finally, the 14-day continuous free-choice access group (14FC) had access to water for 28 days, followed by 10% ethanol and water access for 14 days. All animals experienced the initiation of abstinence on the same calendar day and had post-abstinence drinking assessed concurrently. For a schematic on this and the other two experiments, please refer to Figure 1.
Figure 1.
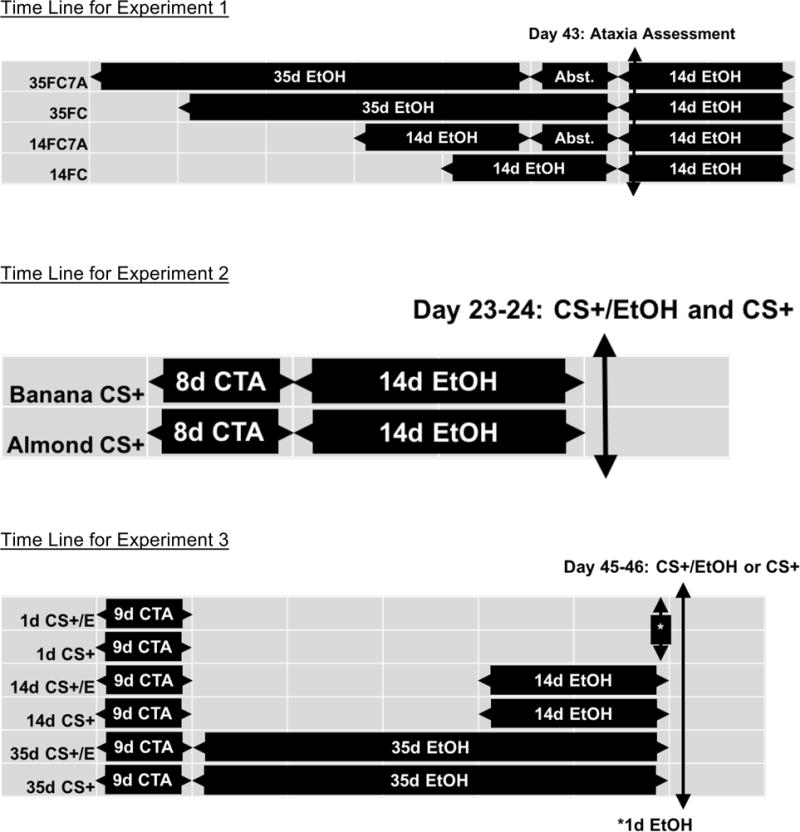
Time line for taste aversion conditioning (CTA), free-choice alcohol access (EtOH), and Abstinence (Abst.) for Experiments 1–3. In all experiments, the critical ataxia or alcohol drinking testing was conducted on the same calendar day for all groups. In Experiment 3, mice were tested with the CTA flavor on Days 45–46 either mixed with ethanol (CS+/E) or water (CS+), but in both cases, mice had free-choice access to unflavored water during training and testing.
Alcohol was removed from all mice at 20:00 hours on Day 42 in order to ensure that BECs were zero at the commencement of ataxia testing on Day 43. On Day 43, at 08:00 hours, half of the mice in each group were moved to a testing room using a light-shielded transporter and gently prodded to traverse a balance beam in both directions using a pencil. This procedure is shown to be sufficient in training mice to traverse the beam without difficulty during data collection (Crabbe et al., 2003). This half of the mice in each group were then given a 1.75 g/kg injection of ethanol and allowed to traverse the beam in both directions while an observer (blind to the experimental condition) counted number of slips by the hind feet (“footslips”). The balance beam consisted of a 122 cm × 2 cm × 4 cm wood block attached at both ends to two 48-cm ring stands. These animals were not used for any further manipulations or data. Mice in the other halves of each group were given free-choice alcohol access for an additional 14 days, with intakes measured 6 days per week. This served to ensure that alcohol injection would not affect intake data. During the first day of access on Day 43, intake of alcohol and water was assessed at 2, 4, 6, and 7 hours following onset of light using 8-ml sipper tubes readable to ± 0.1 ml.
Statistical Analysis
Data from all experiments were organized using Excel spreadsheets (Microsoft, 2010 Edition, Redmond, WA), analyzed using SPSS statistical software (IBM, Version 22, Armonk, NY), and graphed using Prism (GraphPad, Version 6, San Diego, CA). See Supplemental Methods for details specific to each experiment.
Experiment 2 – Pilot of a Novel Conditioned Taste Aversion Procedure
Subjects
Fourteen cHAP mice from S23 were counterbalanced across sex and family into two groups (ns = 7), differing on whether the LiCl unconditioned stimulus was paired with the banana or almond conditioned stimulus. The details of housing were the same as in Experiment 1 except that animals were deprived of water 22 h a day, receiving their daily 2-h water access at least 2 h after the LiCl injection.
Solutions
Flavorant solution parameters were modeled after Cunningham and Niehus (1997). Imitation almond extract and banana flavoring flavorants were obtained from Farmer Brothers Coffee Company (Torrence, CA). Almond solution was made by diluting 1.5% v/v imitation almond extract in distilled water. Banana solution was made by diluting 1.0% v/v banana flavoring and 0.5% EtOH in distilled water to equalize alcohol content between the flavorant solutions, as the almond flavoring was 45% alcohol (v/v) while the banana flavoring was 15% alcohol. Flavor compounds for testing alcohol preference drinking after CTA conditioning were composed of 9.5% EtOH and 1.5% v/v almond flavorant (10.175% total v/v ethanol) or 10% EtOH and 1% v/v banana flavorant (10.15% total v/v ethanol) in distilled water, rounded to 10% for g/kg intake calculations.
Procedure
Eight days of differential taste aversion conditioning, 4 with each flavor, were performed. Beginning at 11:00 AM on Day 1, groups of water-deprived mice received 40 ml/kg i.p injections of 0.15 M lithium chloride (LiCl) in distilled water following 30 minutes of free-choice access to the CS+ and water: banana extract or almond extract. The next day, flavorants were switched in each group to create the CS−, and isotonic saline injections were given after solution access. We used free-choice access to the flavorant instead of forced flavorant drinking to equate procedures to those of testing, in which we required free-choice access to the flavored alcohol solution and water. This procedure was performed 4 times over 8 total days. Mice were then given 14 days of free-choice 10% alcohol access, using the procedure described above except that intake readings were taken 3 times per week (Monday, Wednesday, and Friday).
A reading was taken at 8 PM on the night prior to target data collection, and the EtOH bottle was removed. Ten-ml sipper tubes with 10% EtOH/CS+ or 10% EtOH/CS−, counterbalanced across flavorant (i.e., mice from the banana CS+ and almond CS+ groups were evenly distributed), were placed on mice’s cages from 8:00 hours to 20:00 hours the following day. This 12-h time period corresponded to the high-activity dark cycle. Ten-ml sipper tubes with distilled water were also placed on the cages. Intake readings were taken bihourly; six were taken in total. The procedure was repeated the next day, with the flavorants switched so that the other half of the mice received the CS+ or CS−.
Statistical Analysis
CTA induction data were analyzed using a mixed-model ANOVA with between-subjects factors of Flavor and Sex and a within-subjects factor of Day (2 × 2 × 4) for the CS+ and CS− separately. Paired-samples t-tests were performed to test the expected reduction of intake between Day 1 and Day 4 in the CS+ condition and the expected maintenance of intake in the CS− condition. Bihourly g/kg intake data were analyzed using a mixed-model ANOVA testing between-subjects factors of Flavor (of CS+), Order, and Sex across the 6 bihourly Bins of each Day (2 × 2 × 2 × 6 × 2).
Experiment 3 – Effects of Drinking Duration on Alcohol Intakes In the Presence of a Conditioned Aversive Stimulus
Subjects
Sixty-six cHAP mice from S24 were counterbalanced across sex and family into six groups (ns = 11), differing on whether they were given 1 day (to prevent neophobic effects), 14 days, or 35 days of free-choice alcohol drinking, factorially manipulated by whether alcohol or water was mixed with the CS+ during target data collection; additionally, banana or almond flavorant as the CS+ was counterbalanced within each experimental group. Mice were and housed and treated identically to the subjects of Experiment 2.
Solutions
See Experiment 2.
Procedure
Eight days of taste aversion conditioning, 4 each with each flavor, were performed as in Experiment 2, except that both flavorants were paired with LiCl injections (i.e., were both a CS+) and 1 day of recovery was given at the midpoint of the procedure due to the stress of daily LiCl injection. Therefore, aversion conditioning was 9 days total. We used 2 CS+s as we had intended to gain more power by testing animals with both of them, allowing us to repeatedly assess the effect of an aversive CS in alcohol and water drinking. Over the next 5 weeks, beginning 3 days following the completion of CTA, mice were given either 35 days of free-choice alcohol access, 21 days of water-only access followed by 14 days of alcohol access, or 34 days of water-only access followed by 1 day of free-choice alcohol access. Following removal of alcohol bottles, mice were given free-choice access to a CS+ mixed with water or plain water for two days. On an additional 2 days, mice received free choice access to the other, not yet extinguished CS+ mixed with 10% ethanol or plain water, on 2 blocks of 2 days for each testing condition. We counterbalanced whether mice had access to FC alcohol or FC water first, and assessed intake every 2 h during the dark cycle to acquire a time course for extinction of the taste aversion. We measured intake of the CS+ in ml/kg for both ethanol and water-drinking mice, and g/kg for ethanol-drinking mice. Preference for the CS+ in both water and ethanol was calculated as (ml intake of CS+/total fluid intake).
Results
Experiment 1
A single mouse in the 14-day abstinent group was eliminated as a low outlier, with post-abstinence intakes averaging below 3 g/kg.
Pre-Abstinence Alcohol Intakes
There was no main effect of sex or interactions between Sex, Duration or Abstinence (ps > 0.2), so subsequent analyses collapsed across this factor. Pre-abstinence drinking was somewhat higher in 35-day groups than 14-day groups (28.18 ± 0.94 and 25.52 ± 0.95 g/kg, F[1, 27] = 4.42, p < 0.05). Importantly, there was neither a main effect of Abstinence nor an Abstinence X Duration interaction, ps > 0.05.
Post-Abstinence Alcohol Intakes
Hourly data on the first day of access after abstinence, Day 42 (data not shown), indicated no Abstinence or Duration effects (ps > 0.10). The pattern of intakes during the dark part of the cycle was similar to what has been observed previously (Matson and Grahame, 2013), demonstrating rates substantially above the ~1-g/kg per hour metabolic capacity of these animals, insuring pharmacologically relevant BECs.
Simultaneously, daily intake averaged across Days 2–14 of post-abstinence drinking (See Figure 2) indicated that alcohol consumption was only lowered in mice with 14 days of access, while abstinence had no effect on mice with 35 days of drinking experience. This conclusion was supported by Abstinence X Duration ANOVA indicating an interaction (F[1, 27] = 7.33, p = 0.01). Follow-up t-tests indicated a significant effect of abstinence in 14-day mice, t(13) = 12.74, p = 0.013, but not 35-day mice, p > 0.25. Similar patterns of post-abstinence intake were seen within each sex. In mice with a 14-day history of drinking, female intakes were 26.82 ± 1.34 and 20.82 ± 2.15 g/kg in free-choice and abstinent mice, respectively, while male intakes were 24.32 ± 1.21 and 22.37 ± 0.69 g/kg. In mice with a 35-day drinking history, female intakes were 25.81 ± 1.24 and 27.31 ± 1.7 g/kg in free-choice and abstinent mice, respectively, while male intakes were 23.88 ± 1.76 and 25.7 ± 0.96 g/kg.
Figure 2.
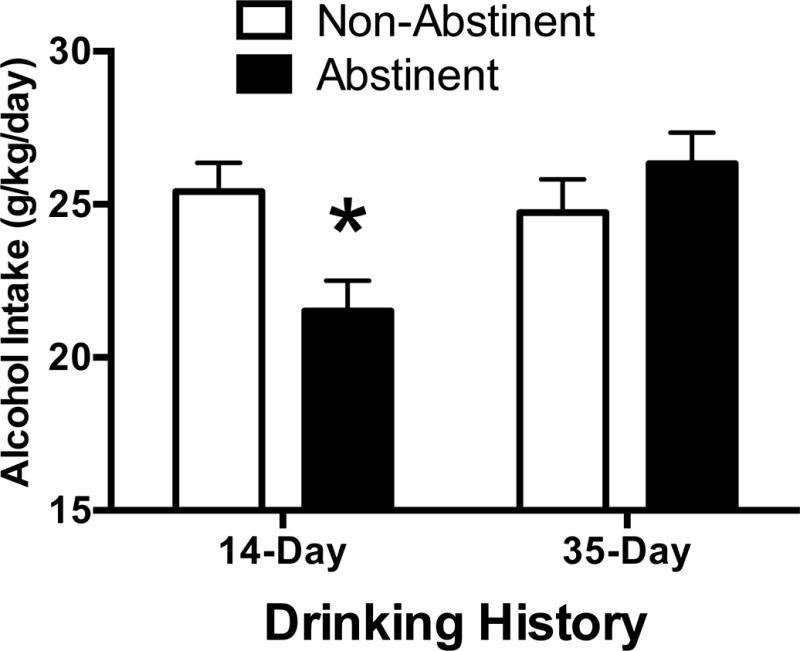
Forced abstinence results in lessened alcohol intake compared to a continuous access group only in mice with a 14-day drinking history. ns = 7–8, * p < 0.05 compared to deprived mice, independent-samples t-test
Post-Abstinence Motor Ataxia
Balance beam data revealed fewer footslips, and thus lower ataxia, in mice not subjected to forced abstinence. Deprived mice had 19.00 ± 2.71 footslips, while non-Deprived mice had 11.44 ± 4.13 footslips. The factorial ANOVA on footslips data revealed a main effect of Abstinence (F[1,28] = 7.79, p < .01), but no main effect nor interactions with drinking Duration (ps > 0.10), so we collapsed across this factor. Additionally, female mice had fewer footslips than male mice, but this factor did not interact with any of the others (p >0.10). These data indicate that continuous drinking decreases sensitivity to injected alcohol, consistent with tolerance being maintained by free-choice drinking, independent of the duration of alcohol access manipulation.
Experiment 2
Conditioned Taste Aversion
One mouse died due to injection-related complications during CTA induction, and its data were excluded from all analyses. CTA to both almond and banana was successfully established in mice that had each paired with LiCl, but not with saline (Figure 3). Mixed-model ANOVAs revealed main effects of Day in the CS+ data (F[3,30] = 33.82, p < .001), but no Flavor or Sex effects; Day 4 intake significantly differed from Day 1 using a paired-samples t-test (t[13] = 8.04, p < .001). A modest Day (F[3,30] = 3.993, p < .05) effect was seen in the CS- data; however, Day 4 intake did not differ from Day 1 (t[13] = .557, n.s.).
Figure 3.
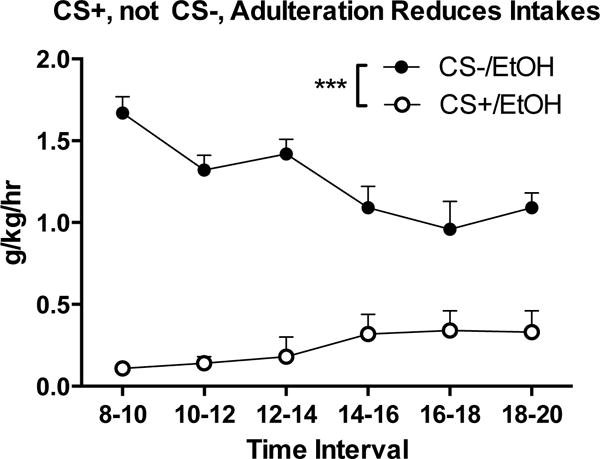
Adulteration using a CS+, but not a CS−, reduces intake of 10% ethanol in Experiment 2. All subjects are represented once per time interval, data collapsed across consecutive days. n = 13, *** p < .001, mixed-model ANOVA
Alcohol Intakes
The averaged mean and standard error of g/kg/day alcohol intakes over the final week of the free-choice drinking period was 20.80 ± 1.01 (n = 13). A mixed-model ANOVA considering the 2 days of alcohol drinking in the presence of the conditioned stimuli revealed no main effects of Day, Order, Sex, or Flavor (of CS+). However, a significant Day X Order interaction (F[1,6] = 353.19, p < .001) indicates that the direction of the within-subject modification between days was dependent upon the order that the CS+ and CS− were presented; intakes were heavily reduced on the day that mice were given the CS+, but not the CS− (Fig. 3). Overall, the CS+ greatly reduced alcohol intake, while the CS− appeared to have little effect on drinking, indicating that the association between the flavor and LiCl, rather than the flavor itself, attenuated free-choice alcohol consumption.
Experiment 3
Taste Aversion Conditioning
See Supplemental Results.
Baseline Alcohol Intakes
As in Experiment 1, we observed escalation of alcohol intake over time. Baseline alcohol intake (3-day means for 14- and 35-day drinkers and the single day of intake for the 1-day drinkers) were compared using a Duration × Sex ANOVA indicating no interaction (p > 0.5), so intakes were collapsed across sex. Intake (g/kg/day) differed across groups: 28.04 ± 0.91 for 35-day mice, 24.51 ± 0.83 for 14-day mice, and 20.88 (± 0.73) for mice with only 1 day of drinking experience (ns = 20–21; F[2, 56] = 18.72, p < 0.001). Follow-up t-tests indicated that 35-day mice drank more than 14-day, which in turn drank more than 1-day (ps < 0.01).
CS+ Effects on Alcohol Intake and Preference
Initial analysis using Sex as a factor showed no effects nor interactions, so subsequent analyses collapsed across this factor. Initial analyses also indicated that after the first 2 days of CTA extinction, there was in general a great deal of extinction to the CS+ when it was put into the alcohol solution, but not when it was mixed with water, so we used only the first two days of CS+ intake to avoid strong order effects. Alcohol consumption in alcohol-drinking mice showed a bihourly Bin × Duration interaction (F[22, 319] = 1.78, p = 0.018), consistent with faster extinction in the 35-day drinking mice (Fig. 4). A main effect of bihourly Bin was also observed (p < 0.001). There were no effects or interactions with drinking Duration on Day 1 (ps > 0.07). Therefore, to establish the source of the interaction, we focused on the second day and collapsed across the 1-day and 14-day Duration groups that did not differ in alcohol consumption (p > 0.2). T-tests indicated that 35-day mice drank more than other mice on the second and sixth bihourly bins (t[30] ≥ 2.08, ps ≤ 0.05). In female mice, Day 2 alcohol consumption averaged 0.87 ± 0.13, 1.22 ± 0.12, and 1.25 ± 0.13 g/kg in the 1, 14, and 35 Day history groups respectively, while males drank 0.65 ± 0.12, 0.71 ± 0.16, and 1.00 ± 0.10 g/kg in the same groups. Mice drinking water mixed with the CS+ showed no effects nor interactions with Duration (see Table 1 for means), indicating that Duration did not have a general effect on CTA learning, (ps > 0.5), although there was a main effect of bihourly Bin (p < 0.01).
Figure 4.
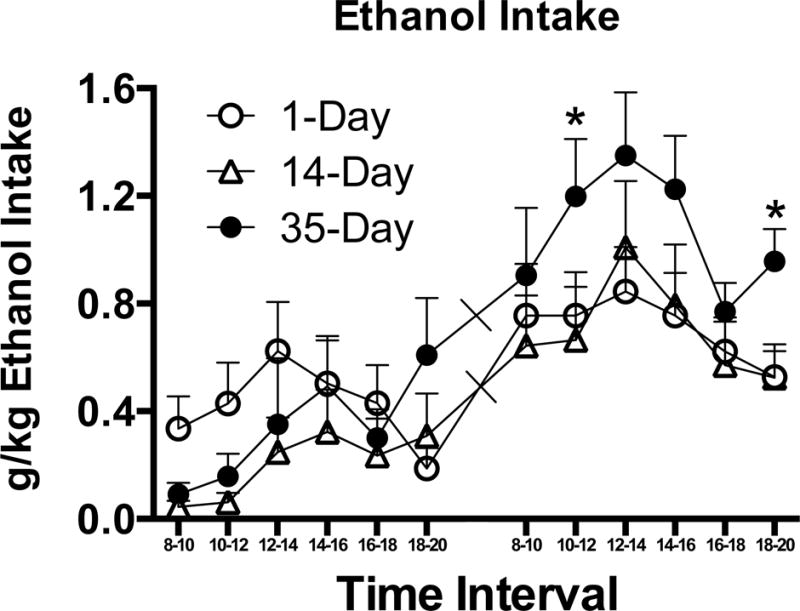
Ethanol intake per Time Interval (i.e., bihourly Bin) on the first two days of ethanol drinking mixed with the aversive CS+ in Experiment 3. Mice had previously had either 1 day, 14 days, or 35 day of alcohol drinking experience. ns = 10–11, * p < .05 indicates higher intake in 35-Day, one-way ANOVA.
Table 1.
ml/kg CS+/ethanol or CS+ alone intakes across two days in Experiment 3. Animals with a 35-day drinking history demonstrate more CS+/ethanol intakes elevated above CS+ alone than animals with a 14-day or 1-day drinking history.
| ml/kg, Day 1 | ml/kg, Day 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8–10 | 10–12 | 12–14 | 14–16 | 16–18 | 18–20 | 8–10 | 10–12 | 12–14 | 14–16 | 16–18 | 18–20 | ||
|
| |||||||||||||
| 1d CS+/E | M | 4.25 | 5.44 | 7.90 | 6.39 | 5.44 | 2.37 | 9.57 | 9.57 | 10.71 | 9.58 | 7.89 | 6.68 |
|
| |||||||||||||
| SEM | 1.52 | 1.92 | 2.31 | 2.01 | 1.80 | 0.97 | 2.43 | 2.06 | 2.09 | 2.01 | 1.40 | 1.22 | |
|
| |||||||||||||
| 1d CS+ | M | 2.32 | 5.38 | 5.94 | 4.28 | 5.10 | 2.39 | 6.36 | 5.74 | 7.26 | 9.56 | 4.93 | 4.54 |
|
| |||||||||||||
| SEM | 0.88 | 2.84 | 3.36 | 2.25 | 2.31 | 1.25 | 2.09 | 1.86 | 3.23 | 3.23 | 1.85 | 1.98 | |
|
| |||||||||||||
| 14d CS+/E | M | 0.57 | 0.79 | 3.15 | 4.11 | 2.98 | 3.91 | 8.15 | 8.43 | 12.78* | 10.07 | 7.24 | 6.65 |
|
| |||||||||||||
| SEM | 0.29 | 0.45 | 1.63 | 1.99 | 1.75 | 1.99 | 2.37 | 2.50 | 3.13 | 2.85 | 2.24 | 1.58 | |
|
| |||||||||||||
| 14d CS+ | M | 1.85 | 2.79 | 5.80 | 4.94 | 4.95 | 2.33 | 7.97 | 6.73 | 4.32 | 4.74 | 6.22 | 2.84 |
|
| |||||||||||||
| SEM | 1.08 | 2.56 | 3.74 | 2.64 | 3.04 | 1.14 | 3.38 | 3.29 | 1.62 | 2.35 | 2.93 | 1.52 | |
|
| |||||||||||||
| 35d CS+/E | M | 1.16 | 1.99 | 4.45 | 6.24 | 3.81 | 7.71* | 11.47* | 15.18** | 17.11* | 15.53* | 9.76*** | 12.13*** |
|
| |||||||||||||
| SEM | 0.53 | 1.07 | 3.10 | 2.37 | 1.36 | 2.68 | 3.18 | 2.70 | 2.97 | 2.51 | 1.35 | 1.52 | |
|
| |||||||||||||
| 35d CS+ | M | 0.80 | 1.14 | 2.35 | 2.94 | 1.51 | 1.71 | 2.20 | 5.41 | 7.62 | 6.79 | 2.83 | 1.65 |
|
| |||||||||||||
| SEM | 0.33 | 0.74 | 1.30 | 1.25 | 0.98 | 0.93 | 1.08 | 1.64 | 2.89 | 3.17 | 0.88 | 0.73 | |
ns = 10–11,
p < .05.
p < .01,
p < .001 compared to control CS+ alone group, independent-samples t-test
Four of the 744 preference data points (0.54%) had zero intake of either fluid, resulting in undefined preference scores, so the average of the preceding and succeeding bihourly bins was substituted to avoid a loss of subjects. ANOVAs of Duration × Fluid (CS+ in ethanol or CS+ in water) × Bin within each test day showed interactions of Bins × Fluid on Day 1 (F[5, 280] = 3.74, p < 0.005), and on Day 2 (F[5, 265] = 2.76, p < 0.02, indicating that across Duration groups, extinction of CS+ drinking was more rapid when the CS+ was in ethanol than when it was in water. Additionally, on both days we observed significant effects of Bin (ps < 0.001) and a main effect of of Fluid on Day 2 (F[1, 53] = 18.26, p < 0.001, but not Day 1, showing that over time, mice preferred drinking the ethanol mixed with the CS+ more than they preferred drinking water mixed with the CS+. There were no main effects or interactions with Duration on either day. However, inspection of Fig. 5 implies larger preference differences between ethanol and water-drinking mice in 35-Day drinking mice than in the other Duration groups. To investigate this, we used post-hoc t-tests Bonferroni-corrected for the 12 time bins over which we measured preference, resulting in an alpha level of 0.0042. Independent-samples t-tests revealed differences between CS+/ethanol and CS+ water consumption within 35-day drinking mice, but not any of the other Duration groups (Fig. 5). and in ml/kg/hr (Table 1). Together, the preference and intake data indicate that in alcohol-drinking mice only, longer-term drinking caused higher alcohol intake in the presence of the aversive CS+.
Figure 5.
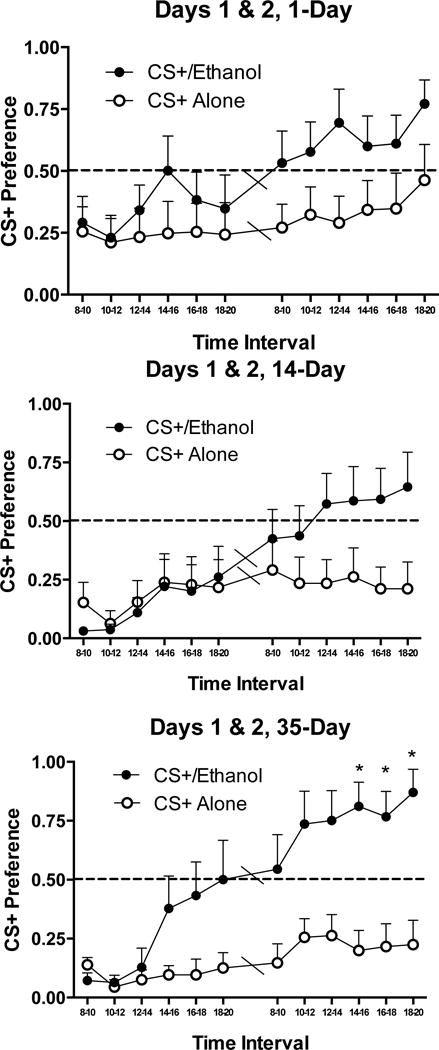
Preference of CS+ solutions assessed against water in Experiment 3. Animals with a 35-day drinking history demonstrate higher CS+/ethanol preferences, and uniquely show CS+/ethanol preferences that are elevated above CS+ in water, than animals with a 14-day or 1- day drinking history. ns = 10–11, * p < .0042 (Bonferroni corrected) compared to control CS+ alone group, independent-samples t-test
Discussion
Overall, although both abstinence and conditioned aversive flavors reduced alcohol consumption in cHAP mice, an extended drinking history lessened both of these effects, nullifying the abstinence effect and facilitating extinction of the taste aversion effect. Thus, using two very different tools, we were able to establish that long-term drinking leads to alcohol consumption that is less flexible than shorter-term drinking. These experiments suggest that long-term drinking in cHAP mice either recruits novel mechanisms that cause inflexible and persistent drinking behavior, or attenuates the influence of normal regulators of alcohol consumption. These mechanisms may include sensitization to hedonic effects, tolerance to aversive effects, a loss of behavioral flexibility, allostasis, disproportionate stimulus-response control of action, or others that would form the basis of future studies.
Interestingly, 7 days of abstinence increased ataxic sensitivity to alcohol regardless of the duration of baseline drinking, suggesting that a loss of tolerance occurs following abstinence, but not the 12-hours of abstinence required to train mice to use the balance beam without alcohol on board. In shorter-term drinking mice, these findings support previous work from our lab using cHAP mice indicating that alcohol tolerance drives escalating alcohol consumption (Matson et al., 2014). Here, we observed that after abstinence, footslips increase and alcohol intake declines in mice with a 14-day drinking history. Because reversible alcohol-induced neuroadaptations correlate with tolerance, our data demonstrate a phenomenological similarity between cHAPs and the clinical population that displays neurological recovery following abstinence (Bartsch et al, 2007; van Eijk et al., 2013). Furthermore, our results suggest that extended drinking does not “stamp in” functional tolerance; higher ataxia was observed in post-abstinence groups regardless of drinking duration. However, in long-term drinking mice, intake behavior may detach from the putative regulation of alcohol sensitivity, suggesting novel mechanisms unrelated to neurological correlates of tolerance. Interestingly, in mice drinking 14 days, intakes declined somewhat more in female mice than male mice after abstinence, although this difference did not reach significance. Females also showed lower alcohol sensitivity than males, regardless of drinking duration. Together, these findings are consistent with the idea that alcohol tolerance increases intakes, and that abstinence reverses both the alcohol insensitivity and concomitant high drinking levels more in females than in males.
In Experiments 2 and 3, we established a novel use of a CTA paradigm, and demonstrated that it may be sensitive to potential changes in motivation to drink depending upon duration of previous alcohol access. Experiment 2 demonstrated that these flavors only reduce alcohol intake when the flavor is an aversive conditioned stimulus, supporting that the reductions in drinking in Experiment 3 were not caused by novelty or any inherently aversive aspect of the flavorant. Experiment 3, then, demonstrated that duration of alcohol drinking caused no differences in the ability of the CS+ to inhibit water intake or initial alcohol intake, suggesting that long-term drinking does not impair retrieval of aversive associations in general, but expedites resumption of high alcohol intakes. That increasing durations of alcohol drinking caused no change in the rate of CTA extinction when the flavor was mixed with water indicates that an alcohol drinking history doesn’t affect efficacy of taste aversion conditioning. Also consistent with this idea is that even in mice drinking alcohol, the aversive flavor initially completely suppressed alcohol consumption. Similar to our results, Lesscher and colleagues (2010) demonstrated a reduction in the ability of quinine to inhibit alcohol drinking in B6 mice following long-term drinking. Alcohol intake levels in that study were fairly modest, and these authors did not report BECs; drinking peaked at about 0.7 g/kg per hour, roughly the same as the rate at which this strain can metabolize alcohol (Grisel et al., 2002). Other recent studies using rats surprisingly indicated a reduction in the flexibility of alcohol drinking at very modest BECs averaging below 50 mg/dl, below the widely-adopted “binge” drinking criteria of 80 mg/dl (Seif et al., 2013; Seif et al., 2015). However, alcohol intakes in our study were much higher and may therefore more directly model drinking patterns previously observed in human populations (e.g., Mello and Mendelson, 1970) while relating to studies indicating that alcoholics will overcome aversion to achieve intoxication (see McCrady et al., 2014).
Moreover, the development of relative inflexibility following long-term drinking in these mice parallels human epidemiological data. Specifically, chronic, lifetime drinking problems are a predictor of unsuccessful alcoholism remission and are characterized by greater resistance to treatment and/or susceptibility to relapse (Krystal et al. 2001; Pastor et al., 2012; Moos and Moos, 2006). We suggest that the cHAP line is a strong candidate for further experimentation in the area of persistent high alcohol intake despite intervention following prolonged access. Future abstinence research could explore other differences that precede and follow abstinence such as neural activity, metabolic capacity, and motivation to obtain alcohol in cHAP mice with varying durations of initial alcohol access. Future aversion studies, then, could explore other manipulations to volitional drinking such as quinine adulteration, establishing a CTA to alcohol itself, or paradigms such operant-based alcohol reinforcement paired with punishment. In either case, the examination of ecologically valid behavioral correlates and implicated neurological regions of chronic drinking in cHAP mice may be valuable. If the model is further established, cHAP mice with an extended alcohol history could be used in identifying neural circuits that promote compulsive drinking and testing of putative pharmacological therapies for nearly intractable alcoholism. Presently, the results of these experiments contribute a novel perspective on rodent models of alcohol intake following two distinct manipulations that were executed on mice that volitionally drink to BECs elevated beyond traditional high-drinking rodent lines.
Supplementary Material
Acknowledgments
This work was supported by a pilot proposal of the Indiana Alcohol Research Center (Overall PI: David Crabb) to NJG, AA07611. Thanks are due to Liana Matson and Evelyn Huffman for their assistance during these experiments.
Contributor Information
David Scott O’Tousa, Indiana University-Purdue University Indianapolis
Nicholas Joseph Grahame, Indiana University-Purdue University Indianapolis
References
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, Stefano ND, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin Exp Res. 2004;28:1867–1874. doi: 10.1097/01.alc.0000148101.20547.0a. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Baker TB, Wehl CK. Emetic and electric shock alcohol aversion therapy: Six- and twelve-month follow-up. J Consult Clin Psychol. 1981;49(3):360–368. doi: 10.1037//0022-006x.49.3.360. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Yu CH, Schlumbohm JP, Cameron AJ, Wahlsten D. Genotypic differences in ethanol sensitivity in two tests of motor incoordination. J Appl Physiol. 2003;95:1338–1351. doi: 10.1152/japplphysiol.00132.2003. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS. Flavor preference conditioning by oral self-administration of ethanol. Psychopharmacology. 1997;134(3):293–302. doi: 10.1007/s002130050452. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The preventable causes of death in the united states: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLOS Medicine. 2009;6(4):23. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoto CB, O’Donnell WE, Allred LJ, Lopes CE. Symptomatology in alcoholics at various stages of abstinence. Alcohol Clin Exp Res. 1985;9:505–512. doi: 10.1111/j.1530-0277.1985.tb05592.x. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Metten P, Wenger CD, Merrill CM, Crabbe JC. Mapping of quantitative trait loci underlying ethanol metabolism in BXD recombinant inbred mouse strains. Alcohol Clin Exp Res. 2002;26(5):610–616. [PubMed] [Google Scholar]
- Hall PDM, Lieber CS, DeCarli LM, French SW, Lindros KO, Jarvelainen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: A critical evaluation. Alcohol Clin Exp Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for Alcohol Becomes Resistant to Quinine Adulteration After 3 to 4 Months of Intermittent Alcohol Self-Administration. Alcohol Clin Exp Res. 2010;34(9):1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HMB. Rodent models for compulsive alcohol intake. Alcohol. 2014;48(3):253–264. doi: 10.1016/j.alcohol.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE. Adaptations underlying the development of excessive alcohol intake in selectively bred mice. Alcohol Clin Exp Res. 2014;38(1):36–39. doi: 10.1111/acer.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abus Treat. 2009;36:8–17. doi: 10.1016/j.jsat.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA, Vet Affairs Naltrexone C Naltrexone in the treatment of alcohol dependence. New Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcohol Clin Exp Res. 2010;34(7):1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- Limpens JH, Schut EH, Voorn P, Vanderschuren LJ. Using conditioned suppression to investigate compulsive drug seeking in rats. Drug Alcohol Depend. 2014;1(142):314–324. doi: 10.1016/j.drugalcdep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35(5):953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol. 2013;18:921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson L, Liangpunsakul S, Crabb D, Buckingham A, Ross RA, Halcomb M, Grahame N. Chronic free-choice drinking in crossed high alcohol preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol Clin Exp Res. 2013;37:194–201. doi: 10.1111/j.1530-0277.2012.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Kasten CR, Boehm SL, Grahame NJ. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res. 2014;38:267–274. doi: 10.1111/acer.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Owens MD, Borders AZ, Brovko JM. Psychosocial approaches to alcohol use disorders since 1940: a review. J Stud Alcohol Drugs. 2014;75S17:68–78. doi: 10.15288/jsads.2014.s17.68. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an Alcohol Deprivation and Escalation Effect in C57BL/6J Mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics a comparison between programed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173:101–116. [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa DS, Matson LM, Grahame NJ. Effects of intoxicating free-choice alcohol consumption during adolescence on drinking and impulsivity during adulthood in selectively bred high-alcohol preferring mice. Alcohol Clin Exp Res. 2013;37(1):141–149. doi: 10.1111/j.1530-0277.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor A, Jones DML, Currie J. High-dose baclofen for treatment-resistant alcohol dependence. J Clin Psychopharmacol. 2012;32:266–268. doi: 10.1097/JCP.0b013e31824929b2. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11(1):2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation - effects on aa and p alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Recent animal models of alcoholism. Alcohol Res Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Turner WM, Cutter HSG, Worobec TG, Ofarrell TJ, Bayog RD, Tsuang MT. Family history models of alcoholism - age of onset, consequences and dependence. J Stud Alcohol. 1993;54:164–171. doi: 10.15288/jsa.1993.54.164. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(586):1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res. 2013;37:67–74. doi: 10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


