Abstract
A case of late-infantile Krabbe disease in a patient who presented with developmental regression and spastic quadriplegia in late infancy is reported. Brain magnetic resonance imaging (MRI) at 11 months of age showed predominant corticospinal tract involvement, which usually appears in adult Krabbe disease. Galactocerebrosidase activity in lymphocytes and skin fibroblasts was very low. Genetic testing revealed compound heterozygous mutations of the galactocerebrosidase (GALC) gene, c.635_646 delinsCTC and c.1901T>C [p.L618S], both of which are known pathogenic mutations. It has been reported that the c.1901T>C [p.L618S] mutation is associated with the late-onset phenotype and, in a past case, a homozygous mutation at this location showed predominant corticospinal tract involvement on MRI. Although further analysis is needed to identify the pathophysiological mechanism, this combination of mutations is likely to be associated with this unusual MRI finding in late-infantile Krabbe disease. Because these types of mutations are common for Japanese patients, it is possible that there are more undiagnosed and late-diagnosed patients of late-infantile Krabbe disease who display limited lesions on MRI. Pediatricians should be aware that patients with late-infantile Krabbe disease can present with predominant corticospinal tract involvement on MRI.
Keywords: Krabbe disease, late-infantile form, magnetic resonance imaging, unusual images, predominant corticospinal tract involvement
Introduction
Krabbe disease is a rare neurodegenerative disorder caused by galactocerebrosidase (GALC) deficiency due to GALC gene mutations. Four clinical forms of Krabbe disease are classified based on the age of onset and severity of disease: early-infantile (onset before six months), late-infantile (onset six months to three years), juvenile (onset three to eight years), and adult (onset after nine years) forms. The latter three forms are also categorized into late-onset form in contrast to the typical early-infantile form.1,2 To date, more than 120 mutations in the GALC gene have been found in patients with Krabbe disease, and the pattern of mutations is partly reflected in the phenotypes of the disease.3
Magnetic resonance imaging (MRI) pattern recognition studies have indicated characteristic radiologic phenotypes for this disorder.4 Several reports have identified unusual radiologic findings in Krabbe disease patients.5,6 However, genetic mutations in cases of those exhibiting unusual images have not been reported.
A case of late-infantile Krabbe disease in which brain MRI findings showed predominant corticospinal tract involvement in white matter, which usually appears in the adult form is described; genetic testing revealed two GALC mutations (c.635_646 delinsCTC and c.1901T>C [p.L618S]). The patient’s next of kin gave consent for publication of images and details of the case.
Case
This female patient was born after an uncomplicated 40-week gestation period. She was the first child of healthy, nonconsanguineous parents. There was no family history of neurodegenerative disorders. Her development was normal during early infancy, and she could sit alone, crawl, and stand assisted at eight months of age. Thereafter, her development regressed. She was observed to have unsteady sitting, and she startled easily to even soft sounds. At 11 months of age, she was referred to our hospital for evaluation of developmental regression. She smiled, tracked, and looked around. Neurological examination revealed spastic quadriplegia, involving the lower limbs predominantly, as well as exaggerated deep tendon reflexes.
Blood tests were all within the normal range. Protein in the cerebrospinal fluid was slightly elevated (52 mg/dL; normal: 10–40 mg/dL). Nerve conduction studies of the right median and right tibial nerves were within the normal ranges (median nerve: motor conduction velocity [MCV] 47.2 m/s, normal 42.3 ± 6.4 m/s; compound muscle action potential [CMAP] 10.45 mV, normal 4–25 mV; tibial nerve: MCV 35.2 m/s, normal 40.8 ± 6.2 m/s, CMAP: 19.8 mV, normal 7–40 mV). Brain stem auditory-evoked potentials revealed V-wave latency delays (6.52 milliseconds in the right ear and 6.60 milliseconds in the left ear, normal 6.16 ± 0.38 milliseconds).
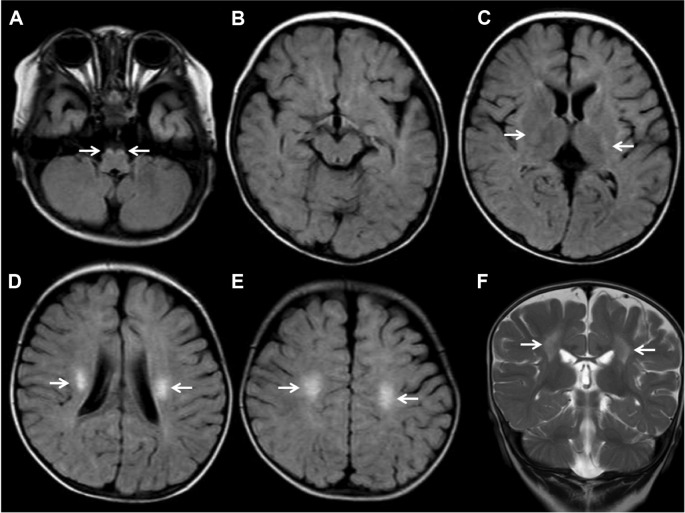
Brain MRI showed bilateral symmetric T2-weighted/fluid-attenuated inversion recovery (FLAIR) hyperintensities in bilateral corticospinal tracts (Fig. 1). There was no involvement of cerebellar white matter and the deep gray nuclei. Apparent diffusion coefficient (ADC) mapping revealed increased signal intensities in the bilateral corticospinal tracts (Fig. 2). The ADC value was 1.12 × 10−3 mm2/s in the right and 1.08 × 10−3 mm2/s in the left corticospinal tracts.
Figure 1.
Axial FLAIR (A–E) and coronal T2-weighted (F) MRI images.
Notes: Figure shows high intensities in the bilateral corticospinal tracts from the centrum semiovale through the posterior internal capsule to the brainstem (white arrows). There are no abnormalities of the dentate and cerebellar white matter.
Abbreviations: FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging.
Figure 2.

Axial ADC map images show high intensities in bilateral corticospinal tracts (arrow heads).
Note: The ADC value was 1.08 × 10−3 mm2/s in the left and 1.12 × 10−3 mm2/s in the right corticospinal tracts.
Abbreviation: ADC, apparent diffusion coefficient.
GALC activity in the patient’s lymphocytes and cultured fibroblasts was 0.1 nmol/mg protein/hour and 0.029 nmol/mg protein/hour, respectively; both were much lower than the normal range (0.75 ± 0.27 nmol/mg protein/hour and 0.55 ± 0.18 nmol/mg protein/hour, respectively). The clinical course and the laboratory findings confirmed the diagnosis of late-infantile Krabbe disease. Genetic analysis was performed after obtaining informed consent from her parents; compound heterozygous mutations, c.635_646delinsCTC and c.1901T>C [p.L618S] (old nomenclature), were identified.
The patient’s condition then worsened until she gradually became unable to control her head, and she had little visual attention. However, she could ingest orally and turn her attention to sounds at 29 months of age.
Discussion
This was a case of an eight-month-old child showing onset of Krabbe disease, classified as late-infantile-onset disease. The patient presented with progressive spastic quadriplegia without apparent cognitive impairment, and she had predominant corticospinal tract involvement on MRI three months after onset. The diagnosis was based on clinical evaluation, enzymatic findings, and genetic analysis. We searched PubMed MEDLINE for late-infantile Krabbe disease with predominant corticospinal tract involvement2–7 and concluded that this is the first report to include these reported gene mutations.
The MRI findings of Krabbe disease differ among clinical phenotypes.4 In the early-infantile form, MRI classically shows widespread supra- and infratentorial white matter signal changes, predominantly in the dentate/cerebellum and corticospinal tract, with sparing of subcortical U-fibers early in the disease. In the late-onset forms, MRI shows parieto-occipital periventricular white matter and posterior corpus callosal signal changes, with sparing of subcortical U-fibers and cerebellar white matter.7–9 Particularly, MRI in the late-infantile phenotype (onset 7–12 months) shows extensive signal abnormality in the deep cerebral white matter. Predominant corticospinal tract involvement has been reported as a characteristic pattern in adult-onset forms.4,10 Although rare, there have been reports of young children with Krabbe disease.5,6 Predominant corticospinal tract involvement could be an initial finding in late-infantile-onset forms, and this could spread to the parieto-occipital white matter with time.5 In the present case, in spite of an eight-month-old onset, MRI at three months after onset showed predominant corticospinal tract involvement, clinically corresponding to spastic quadriplegia without cognitive impairment. Because the patient’s development, including cognitive ability, then regressed slowly, it is possible that her MRI lesion had spread. Moreover, this corticospinal tract region showed hyperintensity on the ADC map, which suggests that the corticospinal tract was vulnerable. Based on diffusion-weighted images, active demyelinating areas usually show hyperintensity in the early stage of the disease, and demyelinated areas become hypointense in the more advanced stages. ADC reflects the mobility of water molecules in the tissue. Normal white matter is a highly structured tissue with a strong orientational bias. Therefore, diffusion of water in normal white matter is constrained by the molecular structure and content of that tissue and is anisotropic. In pathologic states, the molecular structure of white matter is altered, and thus the degree of diffusion anisotropy changes. In the early stage of leukodystrophies, the actively demyelinating areas display increased anisotropy due to myelin edema. In advanced stages, complete destruction of myelin areas leads to increased water in the extracellular space and decreased anisotropy, which lead to increased ADC values.11–13 The present patient carried two mutations. Genotype–phenotype correlations have been reported for Japanese patients with Krabbe disease.4,14 If a patient has one allele of the p.L618S mutations (old nomenclature), the phenotype will likely be a later-onset phenotype.3 A homozygous p.L618S mutation has been reported in a patient with the adult form, in which a brain MRI showed predominant corticospinal tract involvement.15 Previous studies have suggested that selective vulnerability of the corticospinal tracts might be attributable to their potentially greater myelin turnover compared with the other white matter regions, where the low GALC activity is sufficient for their maintenance.15 The molecular basis for the range of severity of the disease is not completely understood, but it is probably related to differences in the enzymatic effects of individual GALC mutations.3 The precursor form of GALC is processed into two fragments, an N-terminal fragment and a C-terminal fragment (active GALC), and enzyme activity depends on the amount of protein processed from the precursor. Late-onset mutations result in enzymes with higher GALC activity and somewhat-processed protein.3 The combination of mutations found in the present case might be related to predominant corticospinal involvement on MRI. Further case studies that include genetic mutation information are needed because the GALC mutation pattern in late-infantile Krabbe disease with predominant cortical lesions has not been reported.
In conclusion, it has been previously reported that 90% of Krabbe disease cases are of the early infantile-onset type; however, recent reports indicate that about half of Krabbe disease cases are later-onset forms.3 The two mutations in this case were common mutations in Japanese patients. If the mutation pattern is related to unusual images showing predominant corticospinal tract involvement without extensive deep cerebral white matter lesions during infancy, there may be more undiagnosed and late-diagnosed patients with late-infantile Krabbe disease. Krabbe disease is a progressive disease, and early diagnosis before symptoms progress may lead to better treatments.16 Performing MRI before the symptoms progress in the late-onset form might reveal a limited lesion. Corticospinal tract involvement is apparent in both early- and late-onset Krabbe disease.7,17 Late-infantile Krabbe disease should be considered even when the regressed infant shows predominant corticospinal tract involvement on brain MRI images.
Footnotes
ACADEMIC EDITOR: Yasuo Ito, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 511 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Examined the patient: AY, TK, and KY. Measured galactocerebrosidase activity: KI and NS. Performed genetic testing: KI and NS. Wrote the manuscript: AY. Critically reviewed the manuscript: TK and KY. All the authors read and approved the final manuscript.
REFERENCES
- 1.Suzuki K. Globoid cell leukodystrophy (Krabbe’s disease): update. J Child Neurol. 2003;18:595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- 2.Duffner PK, Barczykowski A, Kay DM, et al. Later onset phenotypes of Krabbe disease: results of the world-wide registry. Pediatr Neurol. 2012;46:298–306. doi: 10.1016/j.pediatrneurol.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Hossain MA, Otomo T, Saito S, et al. Late-onset Krabbe disease is predominant in Japan and its mutant precursor protein undergoes more effective processing than the infantile-onset form. Gene. 2014;534:144–154. doi: 10.1016/j.gene.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Abdelhalim AN, Alberico RA, Barczykowski AL, Duffner PK. Patterns of magnetic resonance imaging abnormalities in symptomatic patients with Krabbe disease correspond to phenotype. Pediatr Neurol. 2014;50:127–134. doi: 10.1016/j.pediatrneurol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kamate M, Hattiholi V. Predominant corticospinal tract involvement in early-onset Krabbe disease. Pediatr Neurol. 2011;44:155–156. doi: 10.1016/j.pediatrneurol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal R, Sharma S, Sankhyan N, Kumar A, Gulati S. Selective corticospinal tract involvement in late-onset Krabbe disease. Neurology. 2011;77:e20. doi: 10.1212/WNL.0b013e318225aaf5. [DOI] [PubMed] [Google Scholar]
- 7.Loes DJ, Peters C, Krivit W. Globoid cell leukodystrophy: distinguishing early-onset form late-onset disease using a brain MR imaging scoring method. Am J Neurol. 1996;20:316–323. [PMC free article] [PubMed] [Google Scholar]
- 8.Barone R, Bruhl K, Stoeter P, Fiumara A, Pavone L, Beck M. Clinical and neuroradiological findings in classic infantile and late-onset globoid-cell leukodystrophy [Krabbe’s disease] Am J Med Genet. 1996;63:2009–2017. doi: 10.1002/(SICI)1096-8628(19960503)63:1<209::AID-AJMG37>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Nagar VA, Ursekar MA, Krishnan P, Jankharia BG. Krabbe disease: unusual MRI findings. Pediatr Radiol. 2006;36:61–64. doi: 10.1007/s00247-005-0008-y. [DOI] [PubMed] [Google Scholar]
- 10.Tokushige S, Sonoo T, Maekawa R, et al. Isolated pyramidal tract impairment in the central nervous system of adult-onset Krabbe disease with novel mutations in the GALC gene. Brain Dev. 2013;35:579–581. doi: 10.1016/j.braindev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Patay Z. Diffusion-weighted MR imaging in leukodystrophies. Eur Radiol. 2005;15:2284–2303. doi: 10.1007/s00330-005-2846-2. [DOI] [PubMed] [Google Scholar]
- 12.Guo AC, Petrella JR, Kurtzberg J, Provenzale JM. Evaluation of white matter anisotropy in Krabbe disease with diffusion tensor MR imaging: initial experience. Radiology. 2001;218:809–815. doi: 10.1148/radiology.218.3.r01mr14809. [DOI] [PubMed] [Google Scholar]
- 13.Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Modde U. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–418. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Sakai N, Taniike M, Inui K, Ozono K. Six novel mutations detected in the GALC gene in 17 patients with 17 Japanese patients with Krabbe disease, and new genotype-phenotype correlation. J Hum Genet. 2006;51:548–554. doi: 10.1007/s10038-006-0396-3. [DOI] [PubMed] [Google Scholar]
- 15.Satoh J, Tokumoto H, Kurohara K, et al. Adult-onset Krabbe disease with homozygous T1853C mutation in the galactocerebrosidase gene. Unusual MRI findings of corticospinal tract demyelination. Neurology. 1997;49:1392–1399. doi: 10.1212/wnl.49.5.1392. [DOI] [PubMed] [Google Scholar]
- 16.Sakai N. Pathogenesis of leukodystrophy for Krabbe disease: molecular mechanism and clinical treatment. Brain Dev. 2009;31:485–487. doi: 10.1016/j.braindev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Barkovich AJ. An approach to MRI of metabolic disorders in children. J Neuroradiol. 2007;34:75–88. doi: 10.1016/j.neurad.2007.01.125. [DOI] [PubMed] [Google Scholar]